Rare and Aggressive Disease: Urinary Bladder Leiomyosarcoma
Abstract
1. Introduction
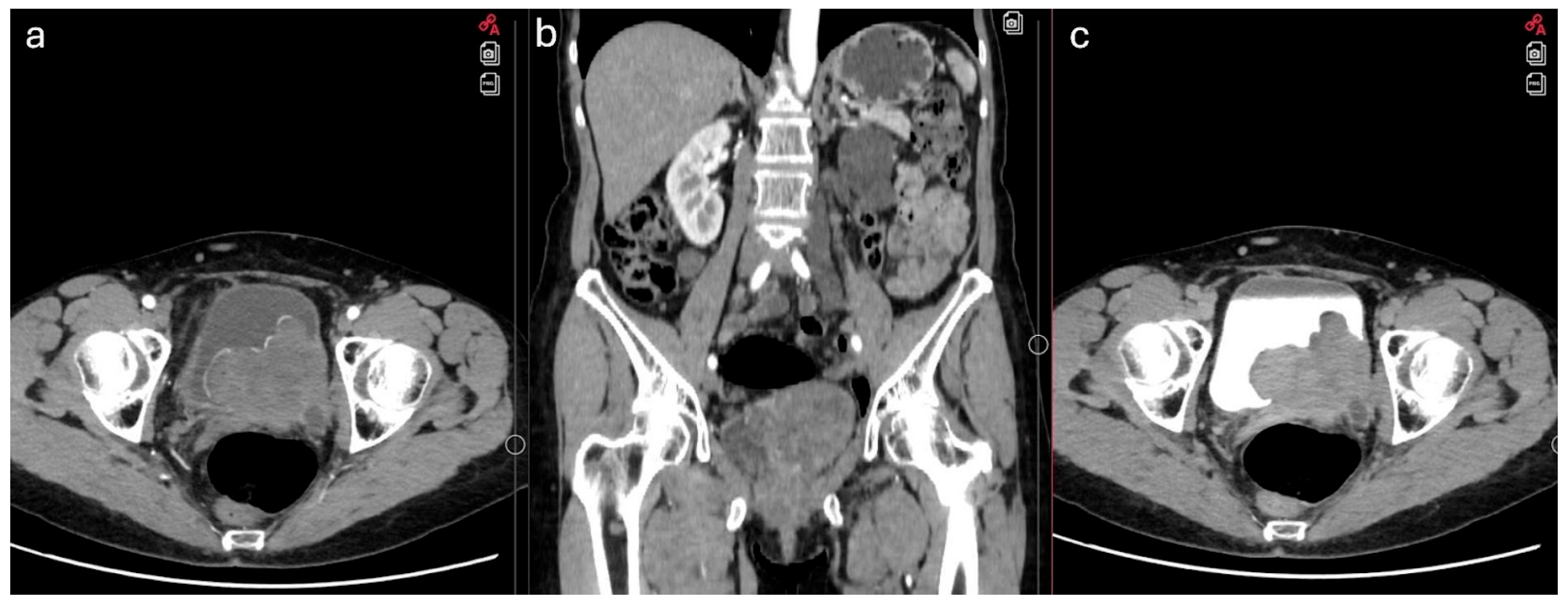
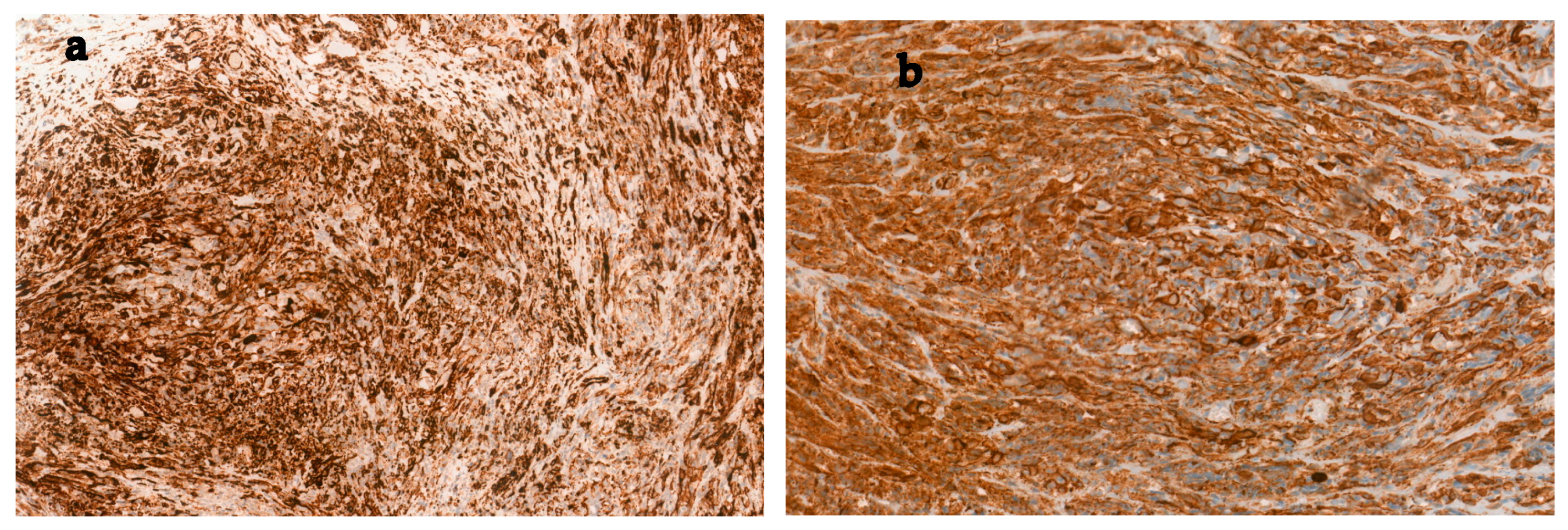
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, A.G.; Bruins, H.M.; Carrion, A.; Cathomas, R.; Compérat, E.; Dimitropoulos, K.; Efstathiou, J.A.; Fietkau, R.; Kailavasan, M.; Lorch, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2025 Guidelines. Eur. Urol. 2025, 87, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Fransen, M.; Wegelin, O.; Richardson, R. A rare bladder tumour: A case presentation of primary low-grade leiomyosarcoma of the urinary bladder in a female patient. Urol. Case Rep. 2025, 59, 102981. [Google Scholar] [CrossRef]
- Dahm, P.; Gschwend, J.E. Malignant Non-Urothelial Neoplasms of the Urinary Bladder: A Review. Eur. Urol. 2003, 44, 672–681. [Google Scholar] [CrossRef]
- Nelius, T.; Stevens, J.; Samathanam, C.; Filleur, S. Leiomyosarcoma of the urinary bladder presenting as life threatening gross hematuria. Med. Oncol. 2010, 27, 562–567. [Google Scholar] [CrossRef]
- Slaoui, H.; Sanchez-Salas, R.; Validire, P.; Barret, E.; Rozet, F.; Galiano, M.; Cathelineau, X. Urinary Bladder Leiomyosarcoma: Primary Surgical Treatment. Urol. Case Rep. 2014, 2, 137–138. [Google Scholar] [CrossRef]
- Kundlia, A.; Dharwadkar, A.; Gore, C.; Viswanathan, V.; Ingale, Y.; GORE, C. Exploring the Histopathological Landscape of Urinary Bladder Diseases: A Tertiary Care Center Study. Cureus 2024, 16, e64557. [Google Scholar] [CrossRef]
- Alamri, N.; Abdullah, H.; Alibrahim, F.; Eid, K.; Alshehri, M. Imaging findings of atypical leiomyoma of urinary bladder simulating ureterocele. J. Surg. Case Rep. 2022, 2022, rjac256. [Google Scholar] [CrossRef]
- Menon, G.; Mangla, A.; Yadav, U. Leiomyosarcoma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Martini, A.; Sfakianos, J.P.; Renström-Koskela, L.; Mortezavi, A.; Falagario, U.G.; Egevad, L.; Hosseini, A.; Mehrazin, R.; Galsky, M.D.; Steineck, G.; et al. The natural history of untreated muscle-invasive bladder cancer. BJU Int. 2020, 125, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.; Preston, M.A.; Barrisford, G.W.; Olumi, A.F.; Feldman, A.S. Clinical features of leiomyosarcoma of the urinary bladder: Analysis of 183 cases. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 958–965. [Google Scholar] [CrossRef]
- Gupta, D.K.; Singh, V.; Sinha, R.J.; Kumar, V.; Nagathan, D.S.; Sankhwar, S.N. Leiomyosarcoma, a Nonurothelial Bladder Tumor: A Rare Entity with Therapeutic Diversity. Korean J. Urol. 2013, 54, 409. [Google Scholar] [CrossRef] [PubMed]
- Rosser, C.J.; Slaton, J.W.; Izawa, J.I.; Levy, L.B.; Dinney, C.P.N. Clinical presentation and outcome of high-grade urinary bladder leiomyosarcoma in adults. Urology 2003, 61, 1151–1155. [Google Scholar] [CrossRef]
- Spiess, P.E.; Kassouf, W.; Steinberg, J.R.; Tuziak, T.; Hernandez, M.; Tibbs, R.F.; Czerniak, B.; Kamat, A.M.; Dinney, C.P.; Grossman, H.B. Review of the M.D. Anderson experience in the treatment of bladder sarcoma. Urol. Oncol. Semin. Orig. Investig. 2007, 25, 38–45. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Cilio, S.; Aveta, A.; Wu, Z.; Cerrato, C.; Napolitano, L.; Lasorsa, F.; Lucarelli, G.; Verze, P.; Siracusano, S.; et al. Upper Tract Urothelial Cancer: Guideline of Guidelines. Cancers 2024, 16, 1115. [Google Scholar] [CrossRef]
- Lee, T.K.; Miyamoto, H.; Osunkoya, A.O.; Guo, C.C.; Weiss, S.W.; Epstein, J.I. Smooth muscle neoplasms of the urinary bladder: A clinicopathologic study of 51 cases. Am. J. Surg. Pathol. 2010, 34, 502–509. [Google Scholar] [CrossRef]
- Wong-You-Cheong, J.J.; Woodward, P.J.; Manning, M.A.; Sesterhenn, I.A. From the archives of the AFIP: Neoplasms of the urinary bladder: Radiologic-pathologic correlation. Radiographics 2006, 26, 553–580. [Google Scholar] [CrossRef]
- Mazurczyk, Ł.; Czarnogórski, M.; Czernicka, A.; Lipowski, P.; Ostrowski, A.; Juszczak, K.; Adamowicz, J.; Drewa, T. Urinary incontinence as the first clinical symptom of urinary bladder leiomyosarcoma. Cent. Eur. J. Urol. 2025, 78, 103–108. [Google Scholar]
- Ferro, M.; Tataru, O.S.; Fallara, G.; Fiori, C.; Manfredi, M.; Claps, F.; Hurle, R.; Buffi, N.M.; Lughezzani, G.; Lazzeri, M.; et al. Assessing the influence of smoking on inflammatory markers in bacillus Calmette Guérin response among bladder cancer patients: A novel machine-learning approach. Minerva Urol. Nephrol. 2024, 77, 338–346. [Google Scholar] [CrossRef]
- Penel, N.; Italiano, A.; Isambert, N.; Bompas, E.; Bousquet, G.; Duffaud, F. Factors affecting the outcome of patients with metastatic leiomyosarcoma treated with doxorubicin-containing chemotherapy. Ann. Oncol. 2009, 21, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.; Fianchi, L.; Pagano, L.; Voso, M.T. Incidence and susceptibility to therapy-related myeloid neoplasms. Chem.-Biol. Interact. 2010, 184, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Uhlman, M.A.; Gibbons, E.P.; Gasana, A.; Rwamasirabo, E.; Ngendahayo, E.; West, J.M.; Uhlman, M.S. A 26-year old female with leiomyosarcoma of the bladder and a serendipitous cystectomy—A success story from International Volunteers in Urology. Urol. Case Rep. 2018, 21, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Vias, P.; Goyal, S.; Periasamy, K.; Madan, R.; Devana, S.K.; Bal, A.; Kundu, R. Leiomyosarcoma of urinary bladder with unusual recurrence in intestinal mucosa and peritoneum: A case report. J. Egypt. Natl. Cancer Inst. 2021, 33, 38. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, H.; Taguchi, S.; Suyama, H.; Kishitani, K.; Akiyama, Y.; Yamada, Y.; Sato, Y.; Yamada, D.; Akiba, N.; Kumasawa, K.; et al. Surgical resection of retinoblastoma-associated bladder leiomyosarcoma during pregnancy: A case report. BMC Urol. 2023, 23, 125. [Google Scholar] [CrossRef]
- Anakievski, D.; Kalchev, K. Bladder leiomyosarcoma with total laparoscopic intracoropreal orthotopic management-case report. Urol. Case Rep. 2019, 28, 101019. [Google Scholar]
- Tan, Z.; Ning, L.; Wang, J.; Huang, Y.; Wang, H.; Zuo, Y. Leiomyosarcoma of the bladder: A case report. Cancer Plus 2021, 3, 48–50. [Google Scholar] [CrossRef]
- Hart, A.A.; Schlaepfer, C.H.; Dhungana, N.; Milhem, M.M.; Packiam, V.T. Surgical management for giant bladder leiomyosarcoma. Urol. Case Rep. 2022, 42, 101995. [Google Scholar] [CrossRef] [PubMed]
- Doddamani, S.C.; Bhat, S.; Jacob, A. Urinary bladder leiomyosarcoma following radiotherapy in a patient with bilateral retinoblastoma: A case report. Indian J. Urol. 2015, 31, 366–368. [Google Scholar] [CrossRef]
- Fiorentino, V.; Pierconti, F.; Lenci, N.; Calicchia, M.; Palermo, G.; Bassi, P.; Larocca, L.M.; Martini, M. Urinary bladder leiomyosarcoma with osteoclast-like multinucleated giant cells: A case report. BMC Cancer 2019, 19, 763. [Google Scholar] [CrossRef]
- Patnayak, R.; Jena, A.; Rambabu, S.; Reddy, M.K. Leiomyosarcoma of urinary bladder-potential mimicker of carcinoma: Case report and short review of literature. Indian J. Cancer 2015, 52, 573–574. [Google Scholar] [CrossRef]
- Ribeiro, J.G.; Klojda, C.A.; Araújo, C.P.; Pires, L.A.; Babinski, M.A. Giant Leiomyosarcoma of the Urinary Bladder. J. Clin. Diagn. Res. 2016, 10, PD14–PD15. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, X.; Peng, K.; Ren, D.; Zhu, X.; Zhang, L. Robot-assisted laparoscopic enucleation in the treatment of leiomyosarcoma of urinary bladder: A case report. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2023, 48, 782–788. [Google Scholar]
- Ishizaki, A.; Okuwaki, K.; Kida, M.; Imaizumi, H.; Iwai, T.; Yamauchi, H.; Kaneko, T.; Hasegawa, R.; Masutani, H.; Tadehara, M.; et al. The First Case of Metastatic Pancreatic Leiomyosarcoma Derived from the Urinary Bladder Diagnosed Using an Endoscopic Ultrasound-guided Fine-needle Biopsy. Intern. Med. 2021, 60, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Watanabe, D.; Yonemasu, H.; Imagawa, M. Total endoscopic management of a patient with urinary bladder leiomyosarcoma presenting with dysuria: A case report. Urol. Case Rep. 2018, 20, 45–47. [Google Scholar] [CrossRef]
- Anastasiou, A.; Katafigiotis, I.; Skoufias, S.; Anastasiou, I.; Constantinides, C. Conservative management of a bladder leiomyosarcoma in a 43-year-old patient. Arch. Ital. Di Urol. E Androl. 2018, 90, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M.; Hwang, R.R.; Silletti, J.; Bjurlin, M.A. Bladder Leiomyosarcoma: A Rare, but Aggressive Diagnosis. Curr. Urol. 2016, 9, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Wang, Z.; Shi, H.; Nie, L.; Zhao, R. A brief review and case report of urothelial carcinoma and metachronous leiomyosarcoma of the bladder at the same anatomic region. Urol. Case Rep. 2021, 40, 101931. [Google Scholar] [CrossRef]
- Sevilla, C.R.; Admella-Salvador, C.; Romero-Martin, J.A.; Llopis-Manzanera, J.; Barranco-Sanz, M.A. Bladder Leiomyosarcoma 25 Years after Treatment with Cyclophosphamide in Patient with History of Retinoblastoma. Urol. Int. 2018, 100, 119–121. [Google Scholar] [CrossRef]
- Ohan, H.; Minassian, G.; Minassian, H.; Farooq, T.; Zdilla, M.J. Retinoblastoma in Infancy with Subsequent Bladder Leiomyosarcoma in Adulthood: Genomic Considerations. Urology 2020, 140, 38–40. [Google Scholar] [CrossRef]
- Boutaggount, F.; Maskrout, M.; Mokfi, R.; Rais, G. Synchronous primary tumors of the bladder: Successfully managed case. Radiol Case Rep. 2022, 17, 3982–3986. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venclovas, Z.; Simkunaite, K.; Pijadin, V.; Auskalnis, S.; Jievaltas, M.; Navickis, T.; Milonas, D. Rare and Aggressive Disease: Urinary Bladder Leiomyosarcoma. J. Clin. Med. 2025, 14, 5999. https://doi.org/10.3390/jcm14175999
Venclovas Z, Simkunaite K, Pijadin V, Auskalnis S, Jievaltas M, Navickis T, Milonas D. Rare and Aggressive Disease: Urinary Bladder Leiomyosarcoma. Journal of Clinical Medicine. 2025; 14(17):5999. https://doi.org/10.3390/jcm14175999
Chicago/Turabian StyleVenclovas, Zilvinas, Kotryna Simkunaite, Vaidas Pijadin, Stasys Auskalnis, Mindaugas Jievaltas, Tomas Navickis, and Daimantas Milonas. 2025. "Rare and Aggressive Disease: Urinary Bladder Leiomyosarcoma" Journal of Clinical Medicine 14, no. 17: 5999. https://doi.org/10.3390/jcm14175999
APA StyleVenclovas, Z., Simkunaite, K., Pijadin, V., Auskalnis, S., Jievaltas, M., Navickis, T., & Milonas, D. (2025). Rare and Aggressive Disease: Urinary Bladder Leiomyosarcoma. Journal of Clinical Medicine, 14(17), 5999. https://doi.org/10.3390/jcm14175999





