Utilization of Child-Appropriate Medicines: Use of Pediatric Use Marketing Authorisation (PUMA) Products in Croatia
Abstract
1. Introduction
2. Materials and Methods
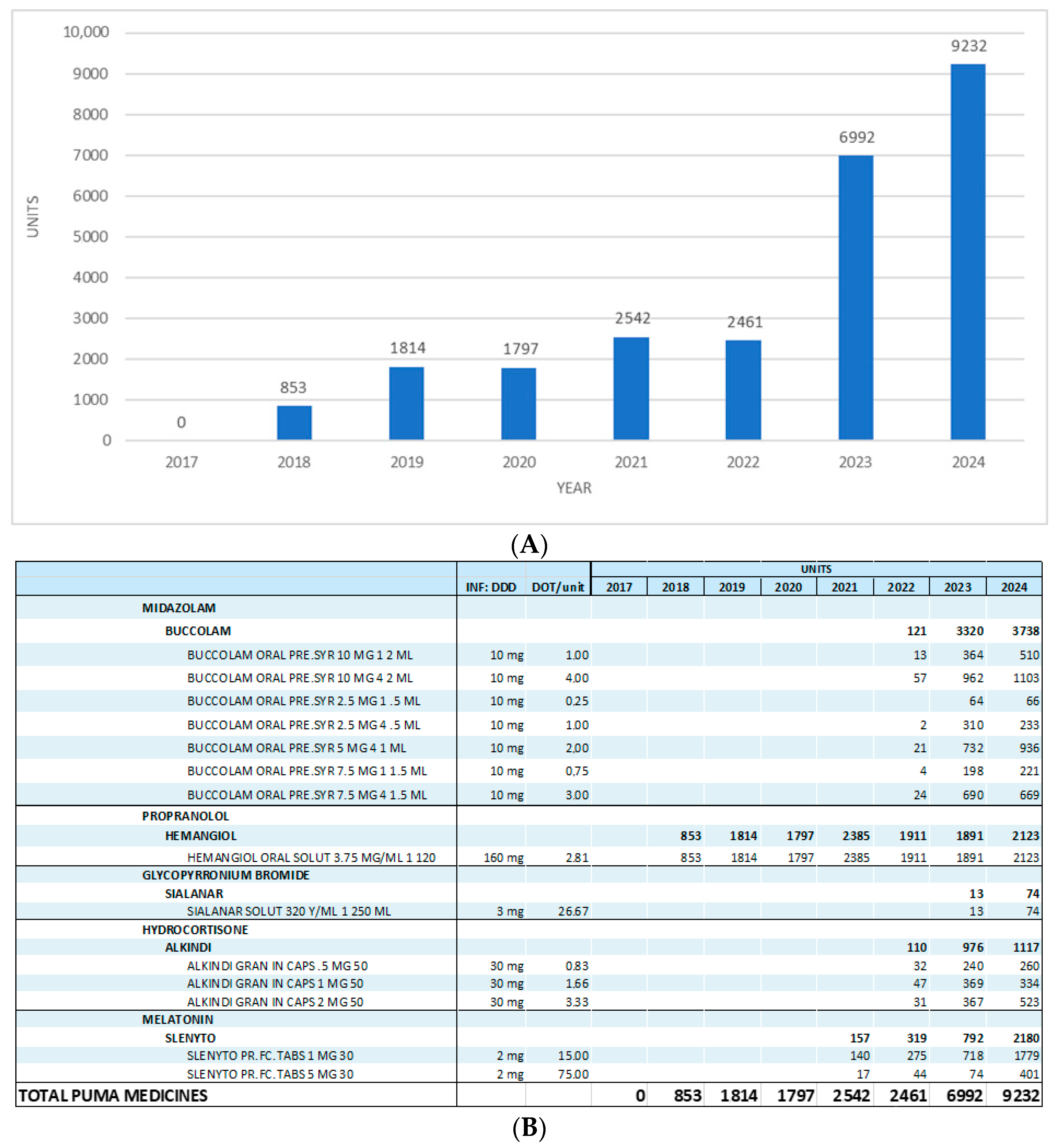
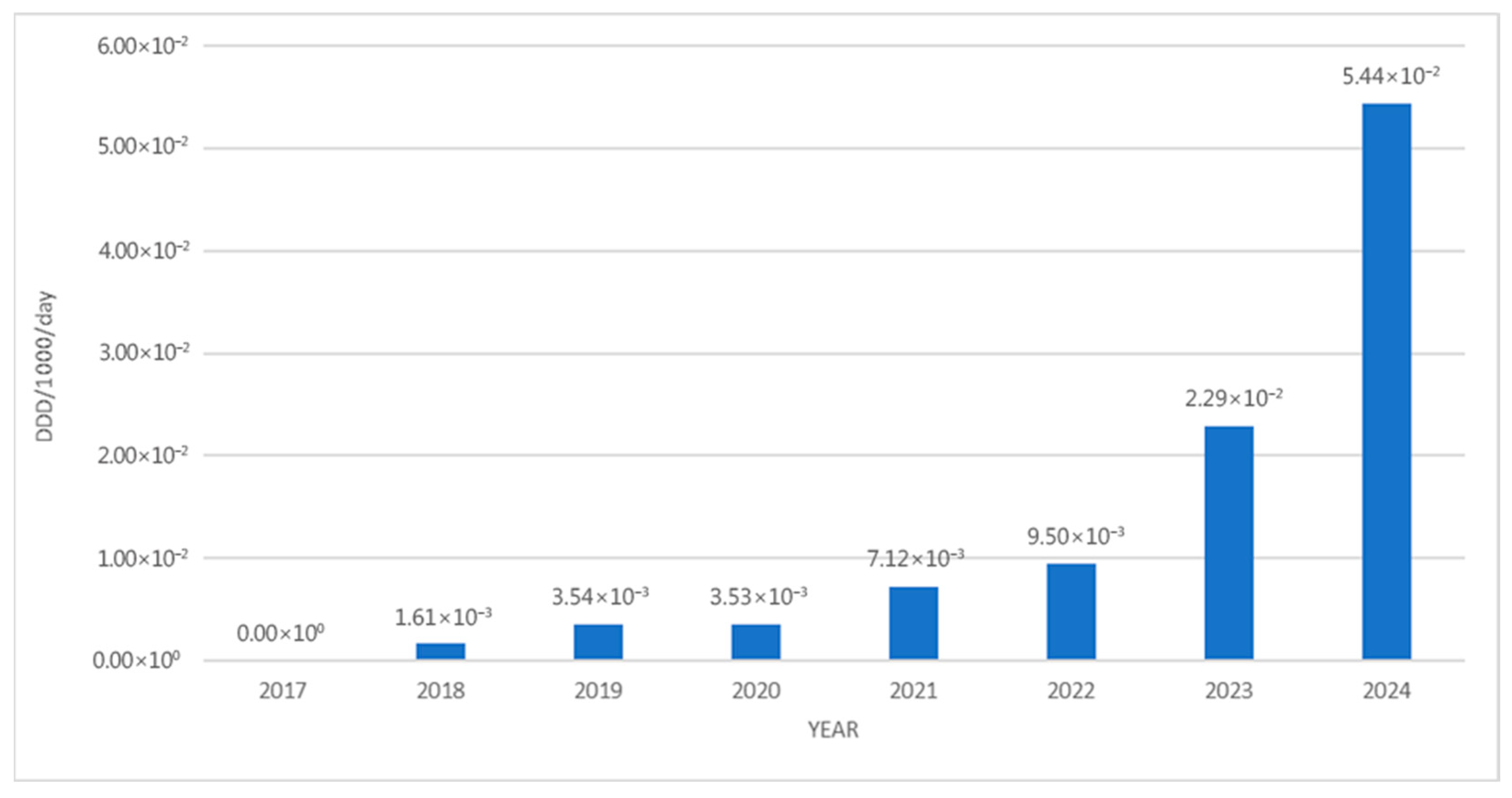
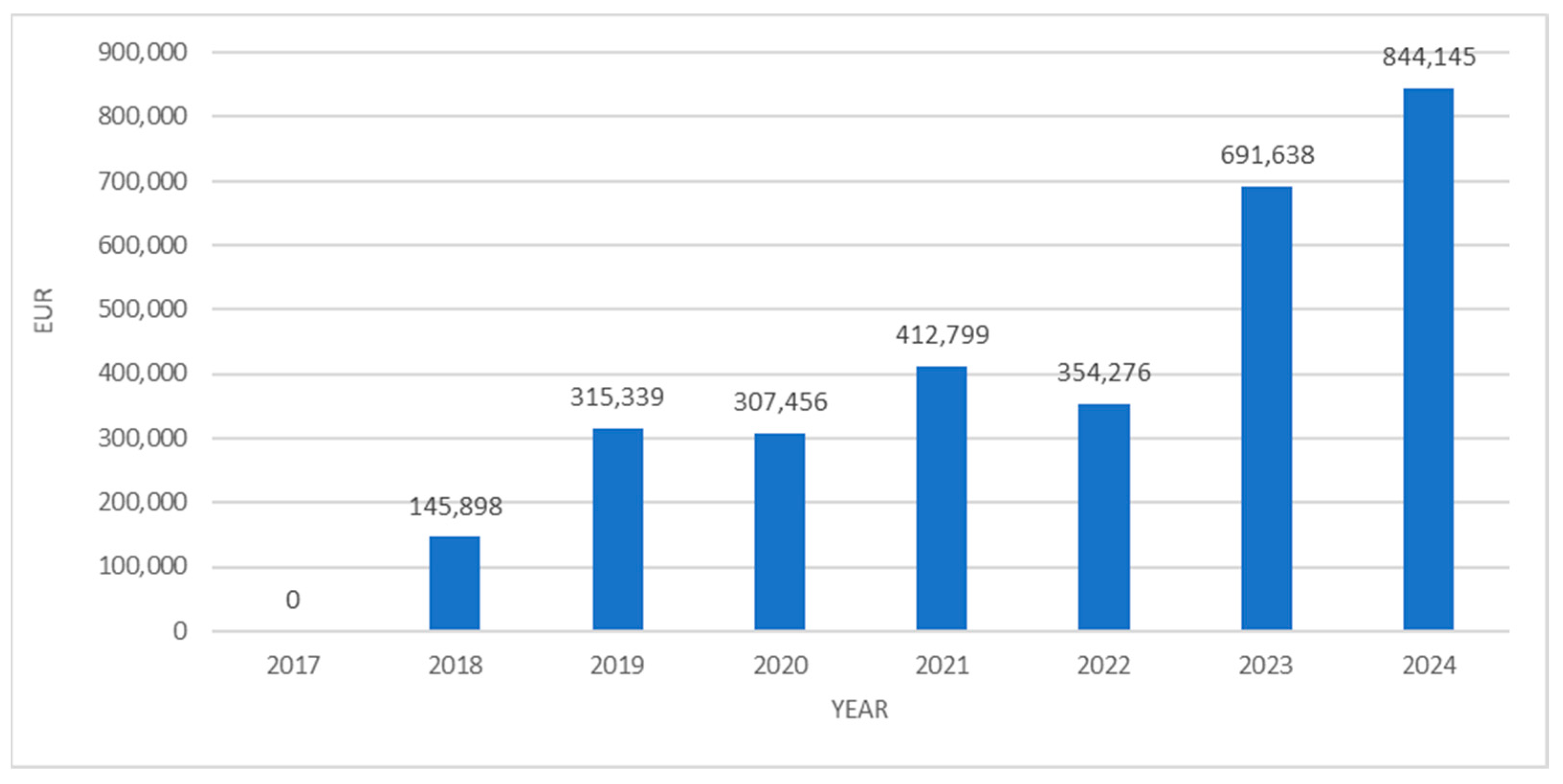
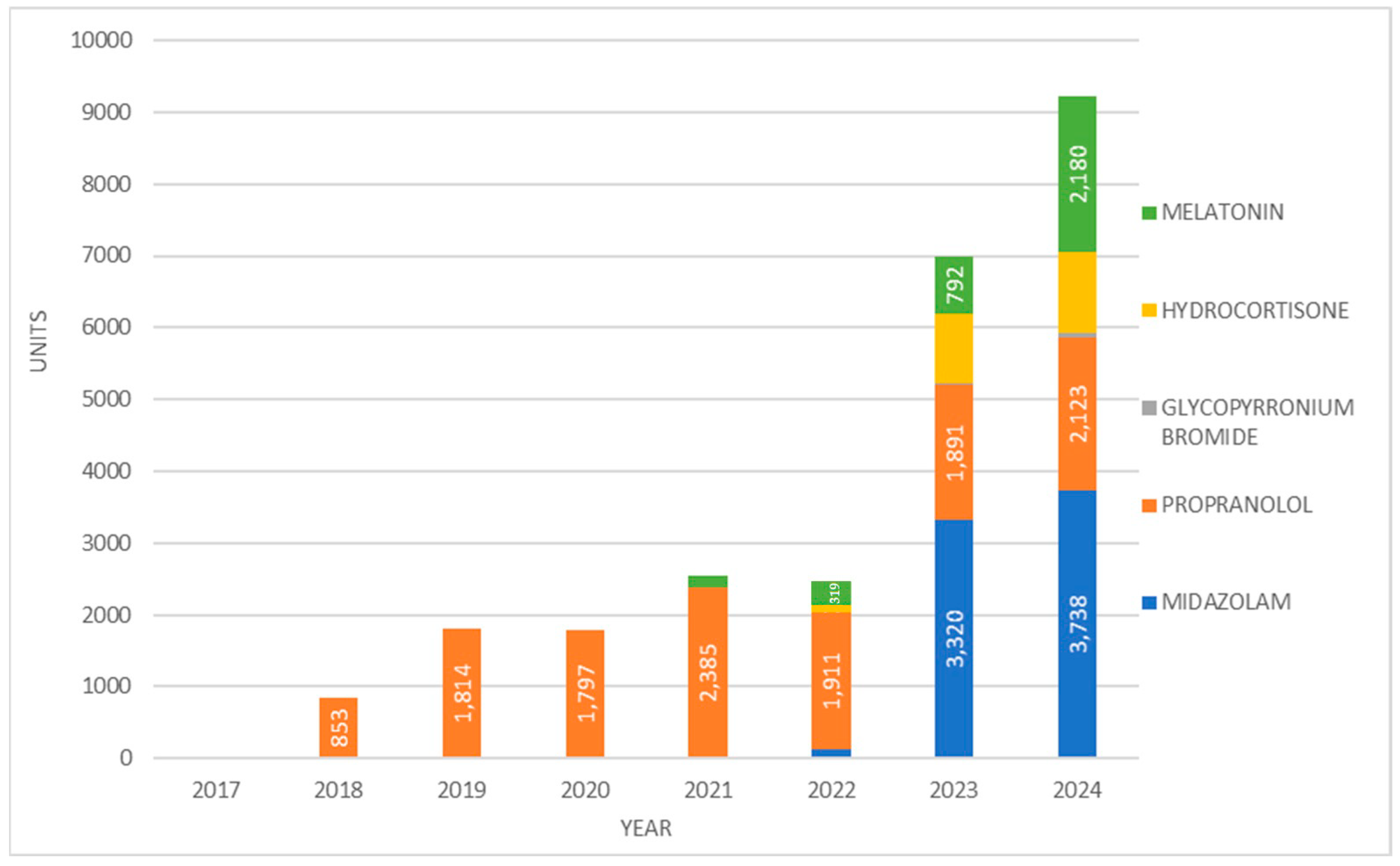
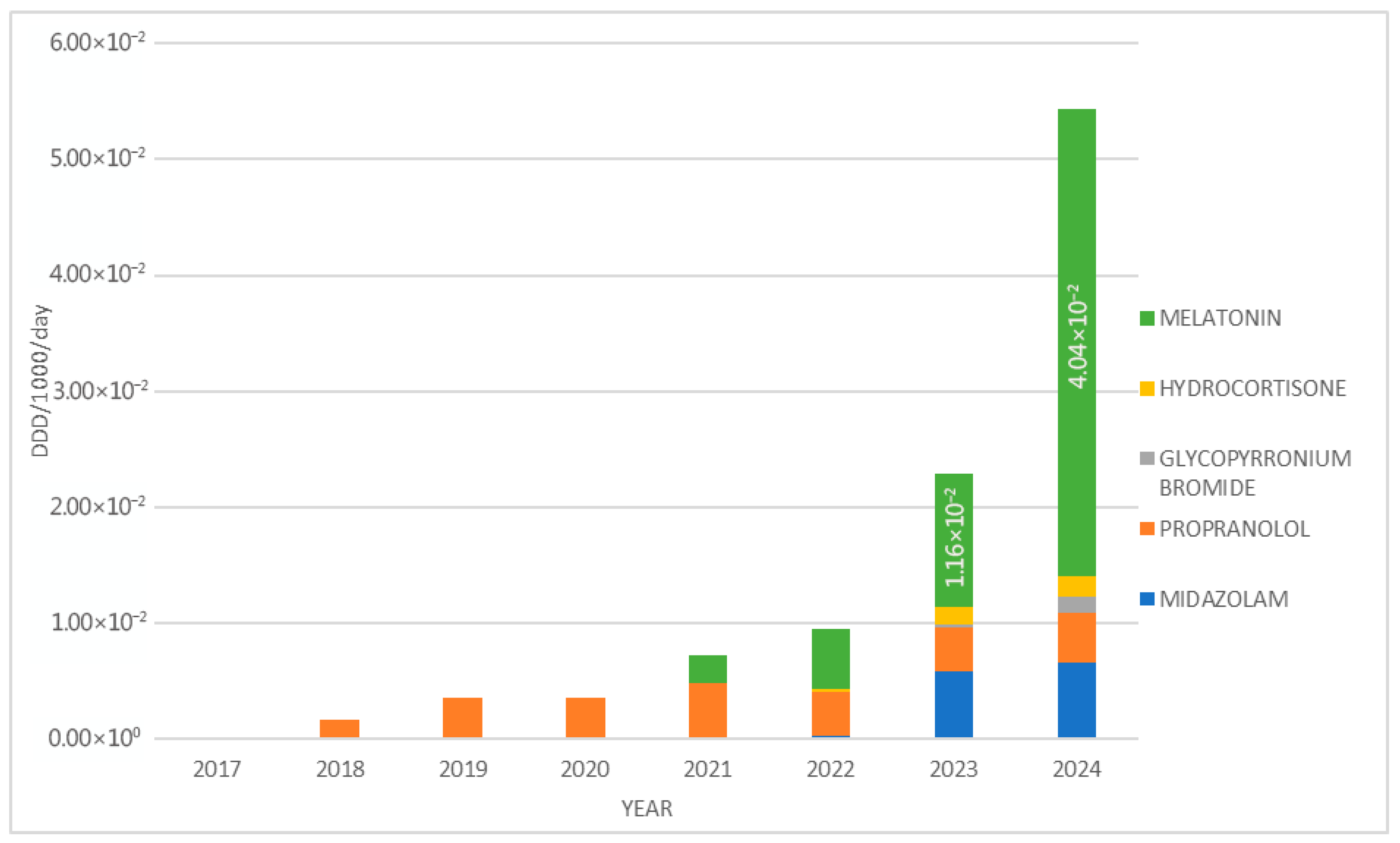
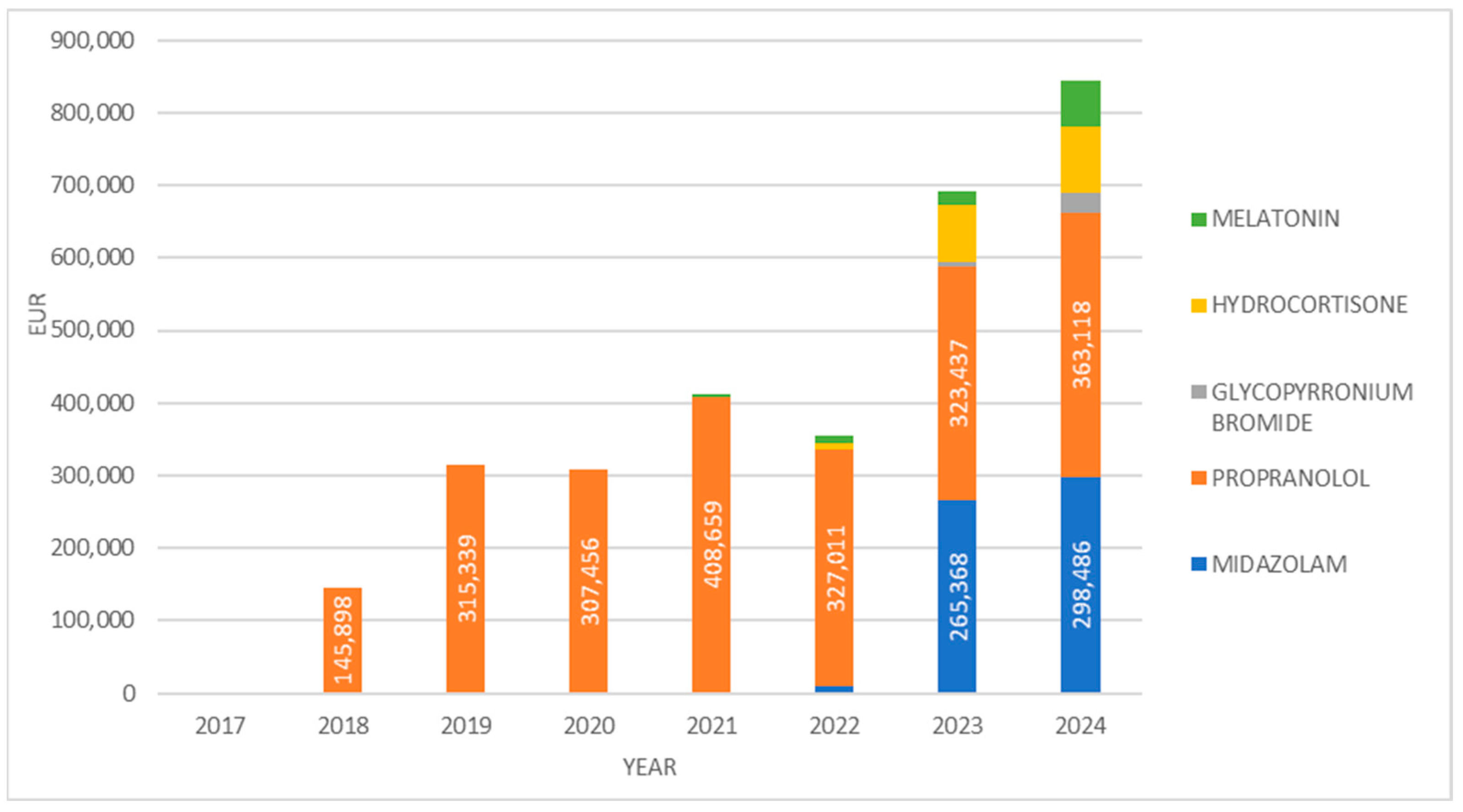
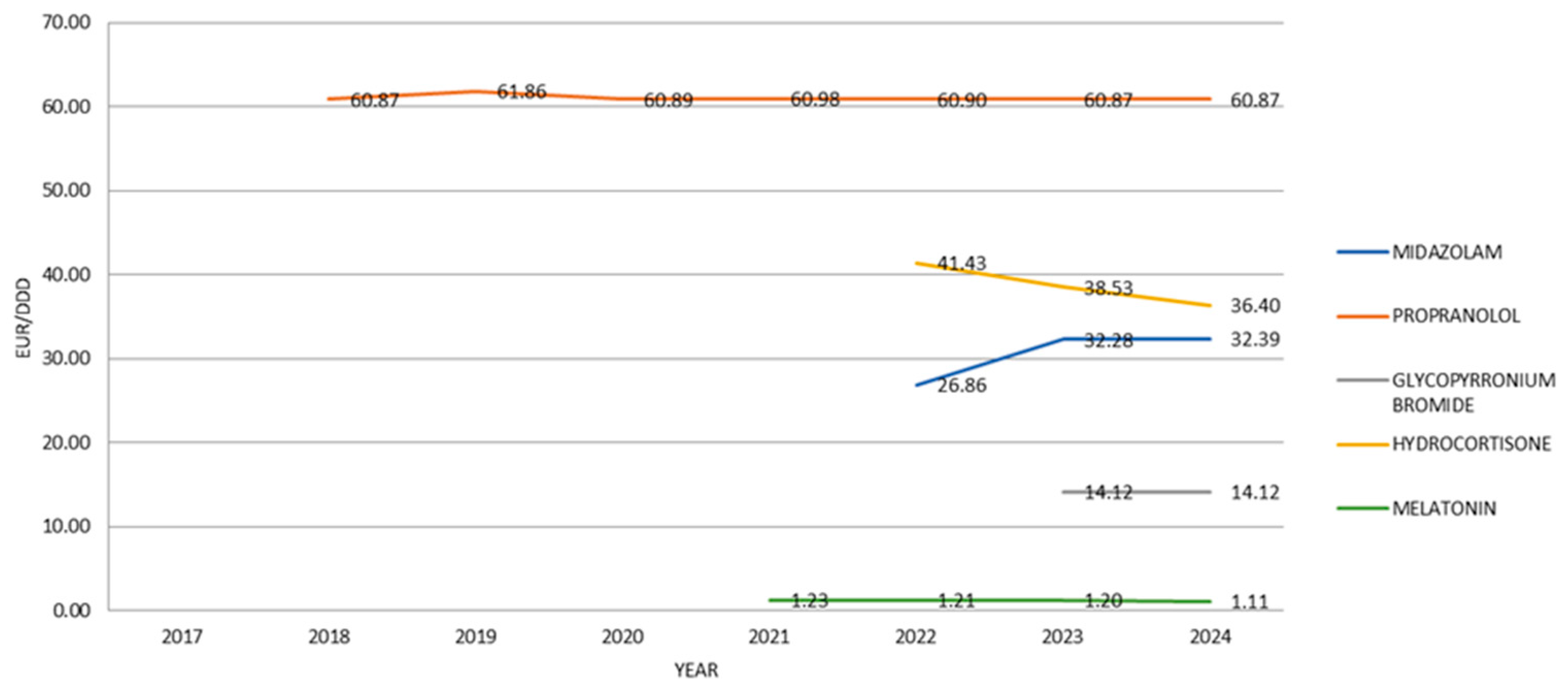
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juárez-Hernández, J.E.; Carleton, B.C. Paediatric oral formulations: Why don’t our kids have the medicines they need? Br. J. Clin. Pharmacol. 2022, 88, 4337–4348. [Google Scholar] [CrossRef]
- Hoon, D.; Taylor, M.T.; Kapadia, P.; Gerhard, T.; Strom, B.L.; Horton, D.B. Trends in Off-Label Drug Use in Ambulatory Settings: 2006–2015. Pediatrics 2019, 144, e20190896. [Google Scholar] [CrossRef]
- Korth-Bradley, J.M. The Path to Perfect Pediatric Posology—Drug Development in Pediatrics. J. Clin. Pharmacol. 2018, 58 (Suppl. 10), S48–S57. [Google Scholar] [CrossRef]
- Carmack, M.; Hwang, T.; Bourgeois, F.T. Pediatric Drug Policies Supporting Safe And Effective Use Of Therapeutics In Children: A Systematic Analysis. Health Aff. 2020, 39, 1799–1805. [Google Scholar] [CrossRef]
- Riedel, C.; Lehmann, B.; Broich, K.; Sudhop, T. Arzneimittelzulassung für Kinder und Jugendliche verbessern: Positionspapier zum Kinderarzneimittel-Symposium am 8. Juni 2015 in Bonn [Improving Drug Licensing for Children and Adolescents: Position Paper from the More Medicines for Minors Symposium 8 June 2015 in Bonn]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2016, 59, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, L.; Torretta, S.; Giannuzzi, V.; Natale, A.; Felisi, M.; Ceci, A.; Bonifazi, F. Effects of the Paediatric Regulation Funding on the Development of Off-Patent Medicines in Children. Front. Med. 2025, 11, 1473862. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.W.M.; Maton, L.C.H.; van Dartel, M.; van den Heuvel, M.; den Otter, L.; Versantvoort, C.; Colin, P.J.; Koomen, J.V. A Review on the Role of Extrapolation as Basis for Paediatric Marketing Authorization Applications of Medicines in the EU. Br. J. Clin. Pharmacol. 2025, 91, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.Z.; Elagouza, I.; El Gaafary, M.; El-Garhy, R.; El-Rashidy, O. Intranasal Versus Buccal Versus Intramuscular Midazolam for the Home and Emergency Treatment of Acute Seizures in Pediatric Patients: A Randomized Controlled Trial. Pediatr. Neurol. 2024, 158, 135–143. [Google Scholar] [CrossRef]
- Ueda, T.; Nishiyama, M.; Yamaguchi, H.; Soma, K.; Ishida, Y.; Maruyama, A.; Nozu, K.; Nagase, H. Efficacy and safety of buccal midazolam for seizures outside the hospital: Real-world clinical experience. Brain Dev. 2024, 46, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Socchi, F.; Bigorre, M.; Normandin, M.; Captier, G.; Bessis, D.; Mondain, M.; Blanchet, C.; Akkari, M.; Amedro, P.; Gavotto, A. Hemangiol in Infantile Haemangioma: A Paediatric Post-Marketing Surveillance Drug Study. Br. J. Clin. Pharmacol. 2021, 87, 1970–1980. [Google Scholar] [CrossRef]
- Fayoux, P.; Dinomais, M.; Shaw, H.; Villain, F.; Schwartz, D.; Gautheron, V.; Letellier, G.; Auvin, S. Glycopyrronium 320 μg/mL in Children and Adolescents with Severe Sialorrhoea and Neurodisabilities: An Open-Label Study Extension of the SALIVA Trial. Dev. Med. Child Neurol. 2025, 67, 1085–1094. [Google Scholar] [CrossRef]
- Wollmer, E.; Neal, G.; Whitaker, M.J.; Margetson, D.; Klein, S. Biorelevant In Vitro Assessment of Dissolution and Compatibility Properties of a Novel Paediatric Hydrocortisone Drug Product Following Exposure of the Drug Product to Child-Appropriate Administration Fluids. Eur. J. Pharm. Biopharm. 2018, 133, 277–284. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Wang, Z.; Sun, C.; Zhang, X.; Liu, C. Adjunctive Treatment for Pediatric Focal Epilepsy: A Systematic Review. Eur. J. Clin. Pharmacol. 2025, 81, 507–523. [Google Scholar] [CrossRef]
- Schroder, C.M.; Banaschewski, T.; Fuentes, J.; Hill, C.M.; Hvolby, A.; Posserud, M.B.; Bruni, O. Pediatric Prolonged-Release Melatonin for Insomnia in Children and Adolescents with Autism Spectrum Disorders. Expert Opin. Pharmacother. 2021, 22, 2445–2454. [Google Scholar] [CrossRef]
- European Commission. State of Paediatric Medicines in the EU: 10 Years of the EU Paediatric Regulation; European Commission: Brussels, Belgium, 2017; Available online: https://ec.europa.eu/health/system/files/2017-11/2017_childrensmedicines_report_en_0.pdf (accessed on 2 July 2025).
- Wan, M.; Houshian, A.; Tomlin, S.; Rashed, A.N. Access to Child-Appropriate Medicines: An Exploratory Survey of the Use of Paediatric Use Marketing Authorisation Products in the UK. Eur. J. Pediatr. 2025, 184, 154. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2024; WHO: Oslo, Norway, 2023. [Google Scholar]
- Croatian Bureau of Statistics. Population—Statistical Data. Available online: https://podaci.dzs.hr/hr/podaci/stanovnistvo/ (accessed on 31 July 2025).
- Toma, M.; Felisi, M.; Bonifazi, D.; Bonifazi, F.; Giannuzzi, V.; Reggiardo, G.; de Wildt, S.; Ceci, A. TEDDY European Network of Excellence for Paediatric Research. Paediatric Medicines in Europe: The Paediatric Regulation-Is It Time for Reform? Front. Med. 2021, 8, 593281. [Google Scholar] [CrossRef]
- Bachhav, S.S.; Kshirsagar, N.A. Systematic Review of Drug Utilization Studies & the Use of the Drug Classification System in the WHO-SEARO Region. Indian J. Med. Res. 2015, 142, 120–129. [Google Scholar] [CrossRef]
- Domingues, C.; Jarak, I.; Veiga, F.; Dourado, M.; Figueiras, A. Pediatric Drug Development: Reviewing Challenges and Opportunities by Tracking Innovative Therapies. Pharmaceutics 2023, 15, 2431. [Google Scholar] [CrossRef]
- Dahlén, E.; Kindblom, J.M.; Kimland, E.E. Defined Daily Doses in Pediatric Dosing—A Theoretical Example. Pharmacol. Res. Perspect. 2023, 11, e01061. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, C.; Richardson, J.; Bint, L.; Parsons, R.; Sunderland, B.; Czarniak, P. Cross-sectional survey of off-label and unlicensed prescribing for inpatients at a paediatric teaching hospital in Western Australia. PLoS ONE 2019, 14, e0210237. [Google Scholar] [CrossRef] [PubMed]
- Guidi, B.; Parziale, A.; Nocco, L.; Maiese, A.; La Russa, R.; Di Paolo, M.; Turillazzi, E. Regulating pediatric off-label uses of medicines in the EU and USA: Challenges and potential solutions: Comparative regulation framework of off label prescriptions in pediatrics: A review. Int. J. Clin. Pharm. 2022, 44, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, M.; Santi Laurini, G.; Motola, D.; Valpiani, G.; Sapigni, E.; Pompilio, A.; Arzenton, E.; Marra, A. PAttern of drug use in PEdiatrics: An observational study in Italian hOSpitals (the PAPEOS study). Br. J. Clin. Pharmacol. 2024, 90, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.C.; Garbe, M.C.; Lees, J.; Aziz, N.; Chaaban, H.; Miller, J.L.; Johnson, P.; DeLeon, S. Off-Label Medication Use in Children, More Common than We Think: A Systematic Review of the Literature. J. Okla. State Med. Assoc. 2018, 111, 776–783. [Google Scholar] [PubMed]
- Schrier, L.; Hadjipanayis, A.; Stiris, T.; Ross-Russell, R.I.; Valiulis, A.; Turner, M.A.; Zhao, W.; De Cock, P.; de Wildt, S.N.; Allegaert, K.; et al. Off-label use of medicines in neonates, infants, children, and adolescents: A joint policy statement by the European Academy of Paediatrics and the European society for Developmental Perinatal and Pediatric Pharmacology. Eur. J. Pediatr. 2020, 179, 839–847. [Google Scholar] [CrossRef]
- Lehmann, B. Reflections on the regulatory field covering the development of paediatric medicinal products: A brief overview of current status and challenges. Front. Pharmacol. 2024, 15, 1375988. [Google Scholar] [CrossRef]
| Medicine | Approved Indication/Use | Company | Year PUMA Was Obtained | References |
|---|---|---|---|---|
| Buccolam (midazolam) | The treatment of prolonged, acute, convulsive seizures in children aged 3 months to <18 years. | Shire | 2011 | [8,9] |
| Hemangiol (oral propranolol solution) | Proliferating infantile hemangioma requiring systemic therapy in infants aged 5 weeks to 5 months. | Pierre Fabre | 2014 | [10] |
| Sialanar (oral glycopyrronium bromide solution) | Severe sialorrhea (drooling) in children and adolescents with neurodisabilities (typically 3–17 years). | (Proveca) | 2016 | [11] |
| Alkindi (hydrocortisone granules) | Replacement therapy in pediatric patients with adrenocortical insufficiency (e.g., congenital adrenal hyperplasia, Addison’s disease). | Diurnal | 2018 | [12] |
| Kigabeq (vigabatrin) | West syndrome (infantile spasms) and refractory complex partial seizures in children and adolescents aged 1 month to 18 years. | Lundbeck | 2018 | [13] |
| Slenyto (prolonged-release melatonin) | Insomnia in children and adolescents aged 2–18 years with autism spectrum disorder and/or Smith–Magenis syndrome. | Neurim | 2018 | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belančić, A.; Kučan Štiglić, M.; Rešić, A.; Fajkić, A.; Vitezić, D. Utilization of Child-Appropriate Medicines: Use of Pediatric Use Marketing Authorisation (PUMA) Products in Croatia. J. Clin. Med. 2025, 14, 5994. https://doi.org/10.3390/jcm14175994
Belančić A, Kučan Štiglić M, Rešić A, Fajkić A, Vitezić D. Utilization of Child-Appropriate Medicines: Use of Pediatric Use Marketing Authorisation (PUMA) Products in Croatia. Journal of Clinical Medicine. 2025; 14(17):5994. https://doi.org/10.3390/jcm14175994
Chicago/Turabian StyleBelančić, Andrej, Marta Kučan Štiglić, Arnes Rešić, Almir Fajkić, and Dinko Vitezić. 2025. "Utilization of Child-Appropriate Medicines: Use of Pediatric Use Marketing Authorisation (PUMA) Products in Croatia" Journal of Clinical Medicine 14, no. 17: 5994. https://doi.org/10.3390/jcm14175994
APA StyleBelančić, A., Kučan Štiglić, M., Rešić, A., Fajkić, A., & Vitezić, D. (2025). Utilization of Child-Appropriate Medicines: Use of Pediatric Use Marketing Authorisation (PUMA) Products in Croatia. Journal of Clinical Medicine, 14(17), 5994. https://doi.org/10.3390/jcm14175994







