Review of Methods for Evaluating Changes in the Tension and Properties of the Gluteus Medius Muscle (GMED) and the Tensor Fascia Latae (TFL) as a Result of Hip Osteoarthritis (HOA) and After Total Hip Arthroplasty (THA)—Could MyotonPRO Assessment Be the New Standard?
Abstract
1. Introduction
2. Materials and Methods
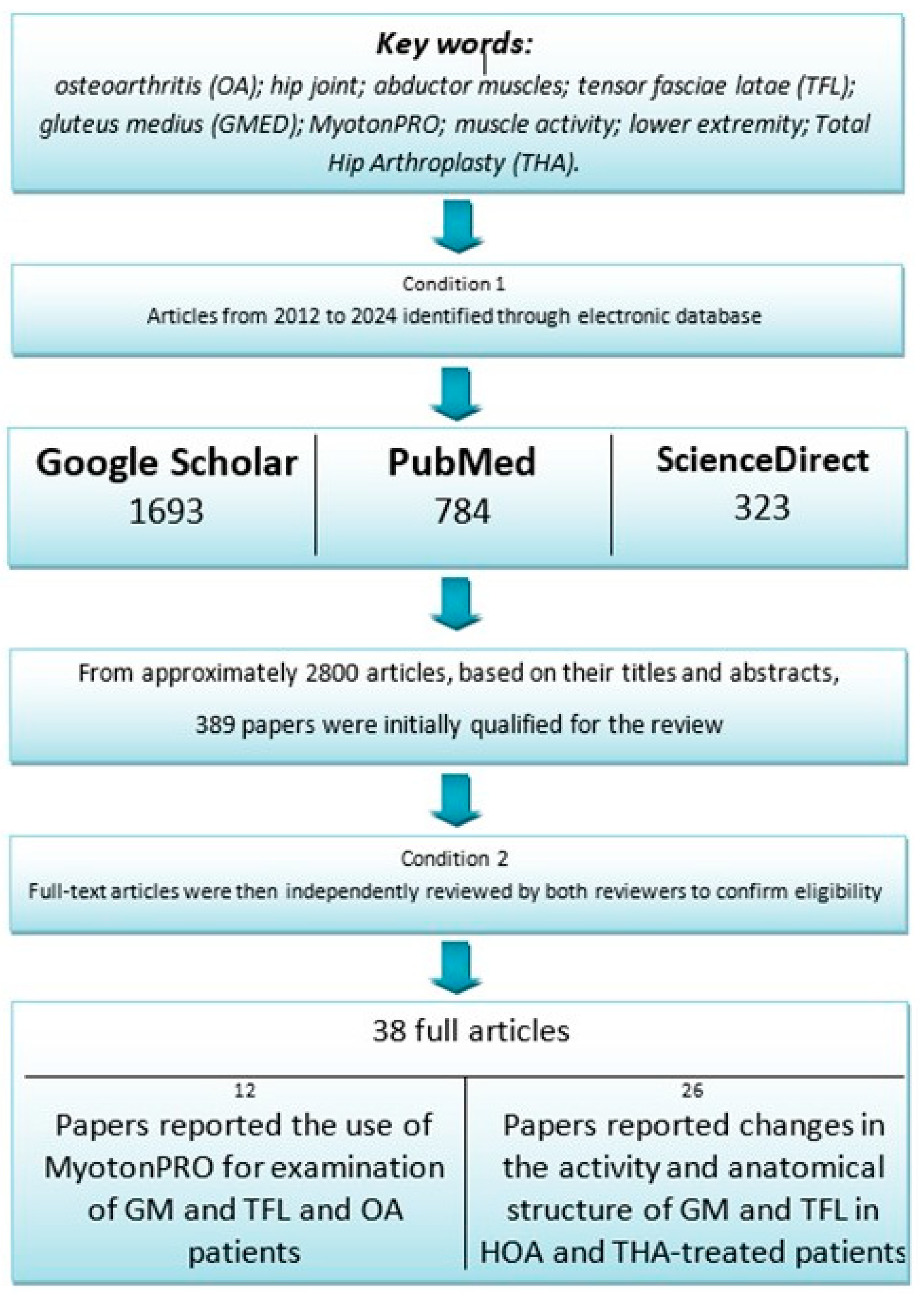
2.1. Literature Search: Databases and Keywords
2.2. Inclusion and Exclusion Criteria
2.3. Search Results
3. Results
3.1. Structure and Functions of GM and TFL
3.2. GMED and TFL in Hip Osteoarthritis (HOA)
3.2.1. Assessment of GMED and TFL Activity in HOA
GMED Activity in HOA
TFL Activity in HOA
3.2.2. Anatomical Structure of GMED and TFL
GMED Anatomical Structure in Patients with HOA
TFL Anatomical Structure in Patients with HOA
3.3. GMED and TFL Activity and Anatomical Structure in THA-Treated Patients
3.3.1. GMED and TFL Activity
GMED and TFL Examined with the Use of EMG
GMED and TFL Examined with the Use of SWE
3.3.2. GMED and TFL Anatomical Structure
GMED Anatomical Structure
TFL Anatomical Structure
3.4. MyotonPRO-Assisted Examinations
3.4.1. Assessment of GMED and TFL
3.4.2. Examination of Muscles in the OA-Affected Lower Limb
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| %C | muscle contribution to total muscle activity |
| ADL | activities of daily living |
| ASI | asymmetry index |
| CE | combined exercise |
| cm | centimeters |
| CMJ | countermovement jump |
| CPP | chronic pelvic pain |
| CSA | cross-sectional area of the muscle |
| CT | computer tomography |
| DOMS | delayed onset muscle soreness |
| EMG | Electromyography |
| F | female |
| GMAX | gluteus maximus |
| GMED | gluteus medius |
| GMIN | gluteus minimus |
| HHS | Harris Hip Score |
| kg | kilograms |
| LBP | low back pain |
| LDL | low-density lean tissue |
| LDL/TM | low-density lean tissue/total muscle area |
| LMM/TM | lean muscle mass area/total muscle area |
| M | male |
| MRI | magnetic resonance imaging |
| MUAP | motor unit action potentials |
| MVC | maximum voluntary contraction |
| N | number |
| OA | osteoarthritis |
| PLLD | perceived leg length discrepancy |
| PT | plyometric training |
| yrs | years |
| sEMG | surface electromyography |
| SMD | skeletal muscle density |
| SWE | shear wave elastography |
| TFL | tensor fasciae latae |
| THA | total hip arthroplasty |
| TTP | time to peak |
| TUG | Timed Up and Go test |
| VAS | Visual Analogue Scale |
| WHO | World Health Organization |
| KOA | knee osteoarthritis |
| RF | rectus femoris |
| VL | vastus lateralis |
| VM | vastus medialis |
| BF | bicep femoris |
| GL | gastrocnemius lateralis |
| GM | gastrocnemius medialis |
| RSL | relatively serious leg |
| RML | relatively moderate leg |
References
- Zacharias, A.; Green, R.A.; Semciw, A.; English, D.J.; Kapakoulakis, T.; Pizzari, T. Atrophy of Hip Abductor Muscles Is Related to Clinical Severity in a Hip Osteoarthritis Population. Clin. Anat. 2018, 31, 507–513. [Google Scholar] [CrossRef]
- Alkhamis, B.A.; Reddy, R.S.; Alahmari, K.A.; Alshahrani, M.S.; Koura, G.M.; Ali, O.I.; Mukherjee, D.; Elrefaey, B.H. Balancing Act: Unraveling the Link between Muscle Strength, Proprioception, and Stability in Unilateral Hip Osteoarthritis. PLoS ONE 2024, 19, e0298625. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, L.; Liu, H.; Li, X.; Fan, K.; Liu, Q.; Li, J.J.; Wang, B. The Prevalence of Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Res. Ther. 2023, 25, 51. [Google Scholar] [CrossRef]
- Nouman, M.; Shabnam, J.; Anwar, S.; Perveen, W.; Alexe, D.I.; Sánchez-Gómez, R.; Sava, M.A.; Alexe, C.I. Effect of Iliotibial Band Myofascial Release Combined with Valgus Correction Exercise on Pain, Range of Motion, Balance, and Quality of Life in Patients with Grade II Knee Osteoarthritis: A Randomized Clinical Trial. Life 2024, 14, 1379. [Google Scholar] [CrossRef]
- Zacharias, A.; Pizzari, T.; English, D.J.; Kapakoulakis, T.; Green, R.A. Hip Abductor Muscle Volume in Hip Osteoarthritis and Matched Controls. Osteoarthr. Cartil. 2016, 24, 1727–1735. [Google Scholar] [CrossRef]
- Arslan, I.G.; Damen, J.; de Wilde, M.; van den Driest, J.J.; Bindels, P.J.E.; van der Lei, J.; Bierma-Zeinstra, S.M.A.; Schiphof, D. Estimating Incidence and Prevalence of Hip Osteoarthritis Using Electronic Health Records: A Population-Based Cohort Study. Osteoarthr. Cartil. 2022, 30, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Peiris, W.L.; Cicuttini, F.M.; Constantinou, M.; Yaqobi, A.; Hussain, S.M.; Wluka, A.E.; Urquhart, D.; Barrett, R.; Kennedy, B.; Wang, Y. Association between Hip Muscle Cross-Sectional Area and Hip Pain and Function in Individuals with Mild-to-Moderate Hip Osteoarthritis: A Cross-Sectional Study. BMC Musculoskelet. Disord. 2020, 21, 316. [Google Scholar] [CrossRef]
- Marshall, A.R.; Noronha, M.D.; Zacharias, A.; Kapakoulakis, T.; Green, R. Structure and Function of the Abductors in Patients with Hip Osteoarthritis: Systematic Review and Meta-Analysis. J. Back. Musculoskelet. Rehabil. 2016, 29, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.J.; Vilella, R.C. Anatomy, Bony Pelvis and Lower Limb, Gluteus Minimus Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Dwyer, M.K.; Stafford, K.; Mattacola, C.G.; Uhl, T.L.; Giordani, M. Comparison of Gluteus Medius Muscle Activity during Functional Tasks in Individuals with and without Osteoarthritis of the Hip Joint. Clin. Biomech. 2013, 28, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Sims, K.J.; Richardson, C.A.; Brauer, S.G. Investigation of Hip Abductor Activation in Subjects with Clinical Unilateral Hip Osteoarthritis. Ann. Rheum. Dis. 2002, 61, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, D.J.; Moreside, J.; Wong, I. Hip Joint Motion and Gluteal Muscle Activation Differences between Healthy Controls and Those with Varying Degrees of Hip Osteoarthritis during Walking. J. Electromyogr. Kinesiol. 2015, 25, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, A.; Pizzari, T.; Semciw, A.I.; English, D.J.; Kapakoulakis, T.; Green, R.A. Gluteus Medius and Minimus Activity during Stepping Tasks: Comparisons between People with Hip Osteoarthritis and Matched Control Participants. Gait Posture 2020, 80, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-T.; Zhu, Y.-C.; Li, Z.; Li, F.; Li, Y.-P.; Guo, J.-Y.; Wang, X.-Q.; Zhang, Z.-J. Modulation in the Stiffness of Specific Muscles of the Quadriceps in Patients With Knee Osteoarthritis and Their Relationship With Functional Ability. Front. Bioeng. Biotechnol. 2021, 9, 781672. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, X.; Shen, Z.; Wang, Y.; Wu, Z.; Chen, G.; Guan, Y.; Wu, J.; Jiang, T.; Wu, H.; et al. Comparison of the Asymmetries in Foot Posture and Properties of Gastrocnemius Muscle and Achilles Tendon Between Patients With Unilateral and Bilateral Knee Osteoarthritis. Front. Bioeng. Biotechnol. 2021, 9, 636571. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, S.; Chen, J.; Li, J.; Li, C.; Xiang, R.; Zhao, C.; Xu, X. Inter-Rater Reliability and Test-Retest Reliability of the Foot Posture Index (FPI-6) for Assessing Static Foot Posture in Elderly Female Patients with Knee Osteoarthritis and Its Association with Quadriceps Muscle Tone and Stiffness. Front. Bioeng. Biotechnol. 2024, 12, 1385986. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Lu, B.; Li, C.; Wang, S.; Zhang, J.; Shen, X.; Xiang, R.; Chen, J.; Jiang, T.; et al. The Differences in Parameters in Ultrasound Imaging and Biomechanical Properties of the Quadriceps Femoris with Unilateral Knee Osteoarthritis in the Elderly: A Preliminary Observational Study. Clin. Interv. Aging 2024, 19, 1479–1491. [Google Scholar] [CrossRef]
- Moerenhout, K.; Derome, P.; Laflamme, G.Y.; Leduc, S.; Gaspard, H.S.; Benoit, B. Direct Anterior versus Posterior Approach for Total Hip Arthroplasty: A Multicentre, Prospective, Randomized Clinical Trial. Can. J. Surg. 2020, 63, E412–E417. [Google Scholar] [CrossRef]
- Weißenberger, M.; Heinz, T.; Rak, D.; Stratos, I.; Anderson, P.M.; Lüdemann, M.; Horas, K.; Jakuscheit, A.; Rudert, M. Does Body Mass Index (BMI) Affect the Reconstruction of Biomechanical Parameters in Patients Undergoing Total Hip Replacement (THR) through the Direct Anterior Approach (DAA)? J. Clin. Med. 2024, 13, 467. [Google Scholar] [CrossRef]
- Arokoski, M.H.; Arokoski, J.P.A.; Haara, M.; Kankaanpää, M.; Vesterinen, M.; Niemitukia, L.H.; Helminen, H.J. Hip Muscle Strength and Muscle Cross Sectional Area in Men with and without Hip Osteoarthritis. J. Rheumatol. 2002, 29, 2185–2195. [Google Scholar]
- Jacobs, C.A.; Lewis, M.; Bolgla, L.A.; Christensen, C.P.; Nitz, A.J.; Uhl, T.L. Electromyographic Analysis of Hip Abductor Exercises Performed by a Sample of Total Hip Arthroplasty Patients. J. Arthroplast. 2009, 24, 1130–1136. [Google Scholar] [CrossRef]
- Kopeć, K.; Bereza, P.; Sobota, G.; Hajduk, G.; Kusz, D. The Electromyographic Activity Characteristics of the Gluteus Medius Muscle before and after Total Hip Arthroplasty. Acta Bioeng. Biomech. 2021, 23, 187–195. [Google Scholar] [CrossRef]
- Chang, T.-T.; Feng, Y.-N.; Zhu, Y.; Liu, C.-L.; Wang, X.-Q.; Zhang, Z.-J. Objective Assessment of Regional Stiffness in Achilles Tendon in Different Ankle Joint Positions Using the MyotonPRO. Med. Sci. Monit. 2020, 26, e926407. [Google Scholar] [CrossRef]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the Elastic Properties of Skeletal Muscle and Tendon Using Shearwave Ultrasound Elastography and MyotonPRO. Sci. Rep. 2018, 8, 17064. [Google Scholar] [CrossRef]
- Liu, C.L.; Feng, Y.N.; Zhang, H.Q.; Li, Y.P.; Zhu, Y.; Zhang, Z.J. Assessing the Viscoelastic Properties of Upper Trapezius Muscle: Intra- and Inter-Tester Reliability and the Effect of Shoulder Elevation. J. Electromyogr. Kinesiol. 2018, 43, 226–229. [Google Scholar] [CrossRef]
- Chen, G.; Wu, J.; Chen, G.; Lu, Y.; Ren, W.; Xu, W.; Xu, X.; Wu, Z.; Guan, Y.; Zheng, Y.; et al. Reliability of a Portable Device for Quantifying Tone and Stiffness of Quadriceps Femoris and Patellar Tendon at Different Knee Flexion Angles. PLoS ONE 2019, 14, e0220521. [Google Scholar] [CrossRef]
- Morgan, G.E.; Martin, R.; Williams, L.; Pearce, O.; Morris, K. Objective Assessment of Stiffness in Achilles Tendinopathy: A Novel Approach Using the MyotonPRO. BMJ Open Sport Exerc. Med. 2018, 4, e000446. [Google Scholar] [CrossRef]
- Ge, J.-S.; Chang, T.-T.; Zhang, Z.-J. Reliability of Myotonometric Measurement of Stiffness in Patients with Spinal Cord Injury. Med. Sci. Monit. 2020, 26, e924811. [Google Scholar] [CrossRef] [PubMed]
- Drenth, H.; Zuidema, S.U.; Krijnen, W.P.; Bautmans, I.; van der Schans, C.; Hobbelen, H. Psychometric Properties of the MyotonPRO in Dementia Patients with Paratonia. Gerontology 2018, 64, 401–412. [Google Scholar] [CrossRef]
- Marusiak, J.; Jaskólska, A.; Koszewicz, M.; Budrewicz, S.; Jaskólski, A. Myometry Revealed Medication-Induced Decrease in Resting Skeletal Muscle Stiffness in Parkinson’s Disease Patients. Clin. Biomech. 2012, 27, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.L.A.; Zhao, J.L.; Li, L.; Mao, Y.R.; Huang, D.F. Relative and Absolute Interrater Reliabilities of a Hand-Held Myotonometer to Quantify Mechanical Muscle Properties in Patients with Acute Stroke in an Inpatient Ward. Biomed. Res. Int. 2017, 2017, 4294028. [Google Scholar] [CrossRef] [PubMed]
- Lukas, K.; Gutschmidt, K.; Schoser, B.; Wenninger, S. Evaluation of Myotonometry for Myotonia, Muscle Stiffness and Elasticity in Neuromuscular Disorders. J. Neurol. 2023, 270, 5398–5407. [Google Scholar] [CrossRef]
- Chuang, L.; Wu, C.; Lin, K. Reliability, Validity, and Responsiveness of Myotonometric Measurement of Muscle Tone, Elasticity, and Stiffness in Patients with Stroke. Arch. Phys. Med. Rehabil. 2012, 93, 532–540. [Google Scholar] [CrossRef]
- How to Measure. Available online: https://myoton.com/quick-instructions-for-use/ (accessed on 6 May 2025).
- Nguyen, A.P.; Detrembleur, C.; Fisette, P.; Selves, C.; Mahaudens, P. MyotonPro Is a Valid Device for Assessing Wrist Biomechanical Stiffness in Healthy Young Adults. Front. Sports Act. Living 2022, 4, 797975. [Google Scholar] [CrossRef]
- Usgu, S.; Ramazanoğlu, E.; Yakut, Y. The Relation of Body Mass Index to Muscular Viscoelastic Properties in Normal and Overweight Individuals. Medicina 2021, 57, 1022. [Google Scholar] [CrossRef] [PubMed]
- Mencel, J.; Jaskólska, A.; Marusiak, J.; Kisiel-Sajewicz, K.; Siemiatycka, M.; Kaminski, L.; Jaskólski, A. Effect of Gender, Muscle Type and Skinfold Thickness on Myometric Parameters in Young People. PeerJ 2021, 9, e12367. [Google Scholar] [CrossRef] [PubMed]
- Trammell, A.P.; Nahian, A.; Pilson, H. Anatomy, Bony Pelvis and Lower Limb: Tensor Fasciae Latae Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Magrath, W.J.; Qiu, C.S.; Hanwright, P.J.; Tuffaha, S.H.; Khavanin, N. A Systematic Review of Muscle Synergies during a Walking Gait to Define Optimal Donor-Recipient Pairings for Lower Extremity Functional Reconstruction. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4438. [Google Scholar] [CrossRef]
- 3D Model: Muscular System-MSD Manual Consumer Version. Available online: https://www.msdmanuals.com/home/multimedia/3dmodel/muscular-system (accessed on 6 May 2025).
- Weskamp, K.; Olwin, B.B.; Parker, R. Post-Transcriptional Regulation in Skeletal Muscle Development, Repair, and Disease. Trends Mol. Med. 2021, 27, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Jin, Z.W.; Abe, H.; Wilting, J.; Murakami, G.; Rodríguez-Vázquez, J.F. Tensor Fasciae Latae Muscle in Human Embryos and Foetuses with Special Reference to Its Contribution to the Development of the Iliotibial Tract. Folia Morphol. 2018, 77, 703–710. [Google Scholar] [CrossRef]
- Bergmann, G.; Graichen, F.; Rohlmann, A. Hip Joint Loading during Walking and Running, Measured in Two Patients. J. Biomech. 1993, 26, 969–990. [Google Scholar] [CrossRef]
- Zacharias, A.; Pizzari, T.; Semciw, A.I.; English, D.J.; Kapakoulakis, T.; Green, R.A. Comparison of Gluteus Medius and Minimus Activity during Gait in People with Hip Osteoarthritis and Matched Controls. Scand. J. Med. Sci. Sports 2019, 29, 696–705. [Google Scholar] [CrossRef]
- Schmidt, A.; Stief, F.; Lenarz, K.; Froemel, D.; Lutz, F.; Barker, J.; Meurer, A. Unilateral Hip Osteoarthritis: Its Effects on Preoperative Lower Limb Muscle Activation and Intramuscular Coordination Patterns. Gait Posture 2016, 45, 187–192. [Google Scholar] [CrossRef]
- Kawano, T.; Nankaku, M.; Murao, M.; Goto, K.; Kuroda, Y.; Kawai, T.; Ikeguchi, R.; Matsuda, S. Functional Characteristics Associated with Hip Abductor Torque in Severe Hip Osteoarthritis. Musculoskelet. Sci. Pract. 2021, 55, 102431. [Google Scholar] [CrossRef]
- Homma, D.; Minato, I.; Imai, N.; Miyasaka, D.; Sakai, Y.; Horigome, Y.; Suzuki, H.; Dohmae, Y.; Endo, N. Relationship between the Hip Abductor Muscles and Abduction Strength in Patients with Hip Osteoarthritis. Acta Med. Okayama 2023, 77, 461–469. [Google Scholar] [CrossRef]
- Loureiro, A.; Constantinou, M.; Diamond, L.E.; Beck, B.; Barrett, R. Individuals with Mild-to-Moderate Hip Osteoarthritis Have Lower Limb Muscle Strength and Volume Deficits. BMC Musculoskelet. Disord. 2018, 19, 303. [Google Scholar] [CrossRef] [PubMed]
- Momose, T.; Inaba, Y.; Choe, H.; Kobayashi, N.; Tezuka, T.; Saito, T. CT-Based Analysis of Muscle Volume and Degeneration of Gluteus Medius in Patients with Unilateral Hip Osteoarthritis. BMC Musculoskelet. Disord. 2017, 18, 457. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Ota, S.; Yamashita, S.; Tsukamoto, Y.; Onishi, E. Association of Preoperative Variables of Ipsilateral Hip Abductor Muscles with Gait Function after Total Hip Arthroplasty: A Retrospective Study. Arthroplasty 2022, 4, 23. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, N.; Hsu, W.-C.; Zhang, J.; Li, H.; Chen, Y.; Dai, K.; Tsai, T.-Y. Adverse Effects of Total Hip Arthroplasty on the Hip Abductor and Adductor Muscle Lengths and Moment Arms during Gait. J. Orthop. Surg. Res. 2020, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.; Razanabola, F.; Beldame, J.; Van Driessche, S.; Brunel, H.; Poirier, T.; Matsoukis, J.; Billuart, F. Electromyographic Study of Hip Muscles Involved in Total Hip Arthroplasty: Surprising Results Using the Direct Anterior Minimally Invasive Approach. Orthop. Traumatol. Surg. Res. 2018, 104, 1137–1142. [Google Scholar] [CrossRef]
- Manafi Rasi, A.; Zandi, R.; Qoreishi, M.; Habibollahzadeh, A. Magnetic Resonance Imaging Assessment of Hip Abductor after Total Hip Arthroplasty Using a Direct Lateral Approach. Arch. Bone Jt. Surg. 2020, 8, 83–88. [Google Scholar] [CrossRef]
- Kinoshita, K.; Kimura, K.; Miyamoto, S.; Takata, Y.; Kodama, Y.; Ieiri, A.; Ishida, K.; Inoue, M.; Abe, S.; Mikami, T.; et al. Relationship between Perceived Leg Length Discrepancy at One Month and Preoperative Hip Abductor Muscle Elasticity in Patients after Total Hip Arthroplasty. Phys. Ther. Res. 2021, 24, 232–239. [Google Scholar] [CrossRef]
- Nankaku, M.; Tsuboyama, T.; Aoyama, T.; Kuroda, Y.; Ikeguchi, R.; Matsuda, S. Preoperative Gluteus Medius Muscle Atrophy as a Predictor of Walking Ability after Total Hip Arthroplasty. Phys. Ther. Res. 2016, 19, 8–12. [Google Scholar] [CrossRef]
- Horstmann, T.; Listringhaus, R.; Haase, G.-B.; Grau, S.; Mündermann, A. Changes in Gait Patterns and Muscle Activity Following Total Hip Arthroplasty: A Six-Month Follow-Up. Clin. Biomech. 2013, 28, 762–769. [Google Scholar] [CrossRef]
- Yasuda, T.; Ota, S.; Mitsuzawa, S.; Yamashita, S.; Tsukamoto, Y.; Takeuchi, H.; Onishi, E. Preoperative Lower-Limb Muscle Predictors for Gait Speed Improvement after Total Hip Arthroplasty for Patients with Osteoarthritis. J. Pers. Med. 2023, 13, 1279. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, Y.E.; Hamed, M.; Abdelhameed, M.A.; El-Hamed, M.A.A.; Bakr, H.; Rageh, T.; Abdelnasser, M.K. Hip Abductor Dysfunction Following Total Hip Arthroplasty by Modified Direct Lateral Approach: Assessment by Quantitative Electromyography. Egypt. Orthop. J. 2023, 58, 295. [Google Scholar] [CrossRef]
- Damm, P.; Zonneveld, J.; Brackertz, S.; Streitparth, F.; Winkler, T. Gluteal Muscle Damage Leads to Higher in Vivo Hip Joint Loads 3 Months after Total Hip Arthroplasty. PLoS ONE 2018, 13, e0190626. [Google Scholar] [CrossRef]
- Kawakami, T.; Imagama, T.; Matsuki, Y.; Okazaki, T.; Kaneoka, T.; Yamazaki, K.; Ueda, M.; Sakai, T. Preoperative Abductor Muscle Strength on the Healthy Side Affects the Timed Up and Go Test after Total Hip Arthroplasty in Women. BMC Musculoskelet. Disord. 2024, 25, 881. [Google Scholar] [CrossRef]
- Kovalak, E.; Özdemir, H.; Ermutlu, C.; Obut, A. Assessment of Hip Abductors by MRI after Total Hip Arthroplasty and Effect of Fatty Atrophy on Functional Outcome. Acta Orthop. Traumatol. Turc. 2018, 52, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Takao, M.; Sakai, T.; Nishii, T.; Sugano, N. Volume Increases of the Gluteus Maximus, Gluteus Medius, and Thigh Muscles After Hip Arthroplasty. J. Arthroplast. 2016, 31, 906–912.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, H.-Y. Effects of Combined Hip Exercise and Passive Stretching on Muscle Stiffness, Pain Perception and Painrelated Disability, and Physical Function in Older Adults with Low Back Pain. Phys. Act. Nutr. 2022, 26, 16–24. [Google Scholar] [CrossRef]
- Proulx, L.; Brizzolara, K.; Thompson, M.; Wang-Price, S.; Rodriguez, P.; Koppenhaver, S. Characterizing Chronic Pelvic Pain: The Relationship Between Extrapelvic Muscle Stiffness, Pain Level, Health History, and Pelvic Floor Symptoms in Women With Chronic Pelvic Pain. J. Women’s Pelvic Health Phys. Ther. 2024, 48, 165. [Google Scholar] [CrossRef]
- Mroczek, D.; Maćkała, K.; Chmura, P.; Superlak, E.; Konefał, M.; Seweryniak, T.; Borzucka, D.; Rektor, Z.; Chmura, J. Effects of Plyometrics Training on Muscle Stiffness Changes in Male Volleyball Players. J. Strength. Cond. Res. 2019, 33, 910–921. [Google Scholar] [CrossRef]
- Wolska, B.; Domagała, Ł.; Kisilewicz, A.; Hassanlouei, H.; Makar, P.; Kawczyński, A.; Klich, S. Multiple Cryosauna Sessions for Post-Exercise Recovery of Delayed Onset Muscle Soreness (DOMS): A Randomized Control Trial. Front. Physiol. 2023, 14, 1253140. [Google Scholar] [CrossRef]
- Mackala, K.; Mroczek, D.; Chmura, P.; Konefał, M.; Pawlik, D.; Ochman, B.; Chmura, J.; Paleczny, B.; Seredyński, R.; Wyciszkiewicz, M.; et al. Impact of Marathon Performance on Muscles Stiffness in Runners over 50 Years Old. Front. Psychol. 2023, 14, 1069774. [Google Scholar] [CrossRef]
- Murillo, B.C.; Johnson, A.J.; Peterson, J.A.; Cruz-Almeida, Y. The Relationship Between Muscle Mechanical Properties And Mechanical Pain Sensitivity In Older Adults With Knee Osteoarthritis. J. Pain 2023, 24, 48. [Google Scholar] [CrossRef]
- Ohko, H.; Ota, S.; Imai, Y.; Kawasaki, S. Limiting Factors of Knee Flexion Angle and Inferior Patellar Mobility in Patients with Knee Osteoarthritis. Osteoarthr. Cartil. 2020, 28, S162–S163. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Ikezoe, T.; Tateuchi, H.; Tsukagoshi, R.; Akiyama, H.; So, K.; Kuroda, Y.; Yoneyama, T.; Ichihashi, N. Muscle Mass and Composition of the Hip, Thigh and Abdominal Muscles in Women with and without Hip Osteoarthritis. Ultrasound Med. Biol. 2012, 38, 1540–1545. [Google Scholar] [CrossRef]
- Dombrowski, M.E.; Klatt, B.A. Abductor Deficiency in Total Hip Arthroplasty: Anatomy, Diagnosis, and Treatment Strategies. Oper. Tech. Orthop. 2019, 29, 100727. [Google Scholar] [CrossRef]
- Andersson, E.A.; Nilsson, J.; Thorstensson, A. Intramuscular EMG from the Hip Flexor Muscles during Human Locomotion. Acta Physiol. Scand. 1997, 161, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Yagi, R.; Akasaka, K.; Momose, K.; Ihashi, K.; Handa, Y. Relationship between EMG Signals and Force in Human Vastus Lateralis Muscle Using Multiple Bipolar Wire Electrodes. J. Electromyogr. Kinesiol. 2000, 10, 59–67. [Google Scholar] [CrossRef]
- Péter, A.; Andersson, E.; Hegyi, A.; Finni, T.; Tarassova, O.; Cronin, N.; Grundström, H.; Arndt, A. Comparing Surface and Fine-Wire Electromyography Activity of Lower Leg Muscles at Different Walking Speeds. Front. Physiol. 2019, 10, 1283. [Google Scholar] [CrossRef]
- Minetto, M.A.; Botter, A.; Šprager, S.; Agosti, F.; Patrizi, A.; Lanfranco, F.; Sartorio, A. Feasibility Study of Detecting Surface Electromyograms in Severely Obese Patients. J. Electromyogr. Kinesiol. 2013, 23, 285–295. [Google Scholar] [CrossRef] [PubMed]
- De la Barrera, E.J.; Milner, T.E. The Effects of Skinfold Thickness on the Selectivity of Surface EMG. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 91–99. [Google Scholar] [CrossRef]
- Lenchik, L.; Mazzoli, V.; Cawthon, P.M.; Hepple, R.T.; Boutin, R.D. Muscle Steatosis and Fibrosis in Older Adults, From the AJR Special Series on Imaging of Fibrosis. AJR Am. J. Roentgenol. 2024, 222, e2329742. [Google Scholar] [CrossRef]
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle Tissue Changes with Aging. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Engelke, K.; Museyko, O.; Wang, L.; Laredo, J.-D. Quantitative Analysis of Skeletal Muscle by Computed Tomography Imaging-State of the Art. J. Orthop. Translat 2018, 15, 91–103. [Google Scholar] [CrossRef]
- Pons, C.; Borotikar, B.; Garetier, M.; Burdin, V.; Ben Salem, D.; Lempereur, M.; Brochard, S. Quantifying Skeletal Muscle Volume and Shape in Humans Using MRI: A Systematic Review of Validity and Reliability. PLoS ONE 2018, 13, e0207847. [Google Scholar] [CrossRef]
- Ogawa, T.; Takao, M.; Otake, Y.; Yokota, F.; Hamada, H.; Sakai, T.; Sato, Y.; Sugano, N. Validation Study of the CT-Based Cross-Sectional Evaluation of Muscular Atrophy and Fatty Degeneration around the Pelvis and the Femur. Orthop. Sci. 2020, 25, 139–144. [Google Scholar] [CrossRef]
- Mak, M.S.; Teh, J. Magnetic Resonance Imaging of the Hip: Anatomy and Pathology. Pol. J. Radiol. 2020, 85, e489–e508. [Google Scholar] [CrossRef]
- Ranganathan, P. An Introduction to Statistics: Choosing the Correct Statistical Test. Indian. J. Crit. Care Med. 2021, 25, S184–S186. [Google Scholar] [CrossRef]
- Ismailidis, P.; Kvarda, P.; Vach, W.; Cadosch, D.; Appenzeller-Herzog, C.; Mündermann, A. Abductor Muscle Strength Deficit in Patients After Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2021, 36, 3015–3027. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.L.; Gandhi, R.; Mahomed, S.; Mahomed, N. Patient Satisfaction after Total Knee and Hip Arthroplasty. Clin. Geriatr. Med. 2012, 28, 349–365. [Google Scholar] [CrossRef]
- Cinnamon, C.C.; Longworth, J.A.; Brunner, J.H.; Chau, V.K.; Ryan, C.A.; Dapiton, K.R.; Chmell, S.J.; Foucher, K.C. Static and Dynamic Abductor Function Are Both Associated with Physical Function 1 to 5 years after Total Hip Arthroplasty. Clin. Biomech. 2019, 67, 127–133. [Google Scholar] [CrossRef]
- Judd, D.L.; Dennis, D.A.; Thomas, A.C.; Wolfe, P.; Dayton, M.R.; Stevens-Lapsley, J.E. Muscle Strength and Functional Recovery During the First Year After THA. Clin. Orthop. Relat. Res. 2014, 472, 654–664. [Google Scholar] [CrossRef]
- Rizzuto, E.; Pisu, S.; Nicoletti, C.; Prete, Z.D.; Musarò, A. Measuring Neuromuscular Junction Functionality. JoVE J. Vis. Exp. 2017, 126, e55227. [Google Scholar] [CrossRef]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms Regulating Muscle Regeneration: Insights into the Interrelated and Time-Dependent Phases of Tissue Healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Miano, C.; Pelosi, L.; Musarò, A. An Overview about the Biology of Skeletal Muscle Satellite Cells. Curr. Genom. 2019, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D. Complexity of Extracellular Matrix and Skeletal Muscle Regeneration. In Skeletal Muscle Repair and Regeneration; Schiaffino, S., Partridge, T., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2008; pp. 269–302. ISBN 978-1-4020-6768-6. [Google Scholar]
- Tatsumi, R.; Sankoda, Y.; Anderson, J.E.; Sato, Y.; Mizunoya, W.; Shimizu, N.; Suzuki, T.; Yamada, M.; Rhoads, R.P.; Ikeuchi, Y.; et al. Possible Implication of Satellite Cells in Regenerative Motoneuritogenesis: HGF Upregulates Neural Chemorepellent Sema3A during Myogenic Differentiation. Am. J. Physiol. Cell Physiol. 2009, 297, C238–C252. [Google Scholar] [CrossRef]
- Rivera, F.; Comba, L.C.; Bardelli, A. Direct Anterior Approach Hip Arthroplasty: How to Reduce Complications—A 10-Years Single Center Experience and Literature Review. World J. Orthop. 2022, 13, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Mjaaland, K.E.; Kivle, K.; Svenningsen, S.; Nordsletten, L. Do Postoperative Results Differ in a Randomized Trial Between a Direct Anterior and a Direct Lateral Approach in THA? Clin. Orthop. Relat. Res. 2019, 477, 145–155. [Google Scholar] [CrossRef]
- Spaans, A.J.; van den Hout, J.A.A.M.; Bolder, S.B.T. High Complication Rate in the Early Experience of Minimally Invasive Total Hip Arthroplasty by the Direct Anterior Approach. Acta Orthop. 2012, 83, 342–346. [Google Scholar] [CrossRef]
- Wojciechowski, P.; Kusz, D.; Kopeć, K.; Borowski, M. Minimally Invasive Approaches in Total Hip Arthroplasty. Ortop. Traumatol. Rehabil. 2007, 9, 1–7. [Google Scholar]
- Piech, P.; Kuroska-Walczyna, G.; Samczuk, M.; Sowińska-Pelak, A.; Węgłowski, R.; Pelak, J.; Staśkiewicz, G. Unveiling the Tensor Vastus Intermedius—A Distinct Anatomical Phenomenon or a Standard Variation? A Comparative Analysis of Comprehensive Literature and Original Cadaveric Studies. Ann. Agric. Environ. Med. 2024, 31, 410–416. [Google Scholar] [CrossRef]
- Bizzini, M.; Mannion, A.F. Reliability of a New, Hand-Held Device for Assessing Skeletal Muscle Stiffness. Clin. Biomech. 2003, 18, 459–461. [Google Scholar] [CrossRef]
- Leonard, C.T.; Brown, J.S.; Price, T.R.; Queen, S.A.; Mikhailenok, E.L. Comparison of Surface Electromyography and Myotonometric Measurements during Voluntary Isometric Contractions. J. Electromyogr. Kinesiol. 2004, 14, 709–714. [Google Scholar] [CrossRef]
- Lidström, A.; Ahlsten, G.; Hirchfeld, H.; Norrlin, S. Intrarater and Interrater Reliability of Myotonometer Measurements of Muscle Tone in Children. J. Child. Neurol. 2009, 24, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lettner, J.; Królikowska, A.; Ramadanov, N.; Oleksy, Ł.; Hakam, H.T.; Becker, R.; Prill, R. Evaluating the Reliability of MyotonPro in Assessing Muscle Properties: A Systematic Review of Diagnostic Test Accuracy. Medicina 2024, 60, 851. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
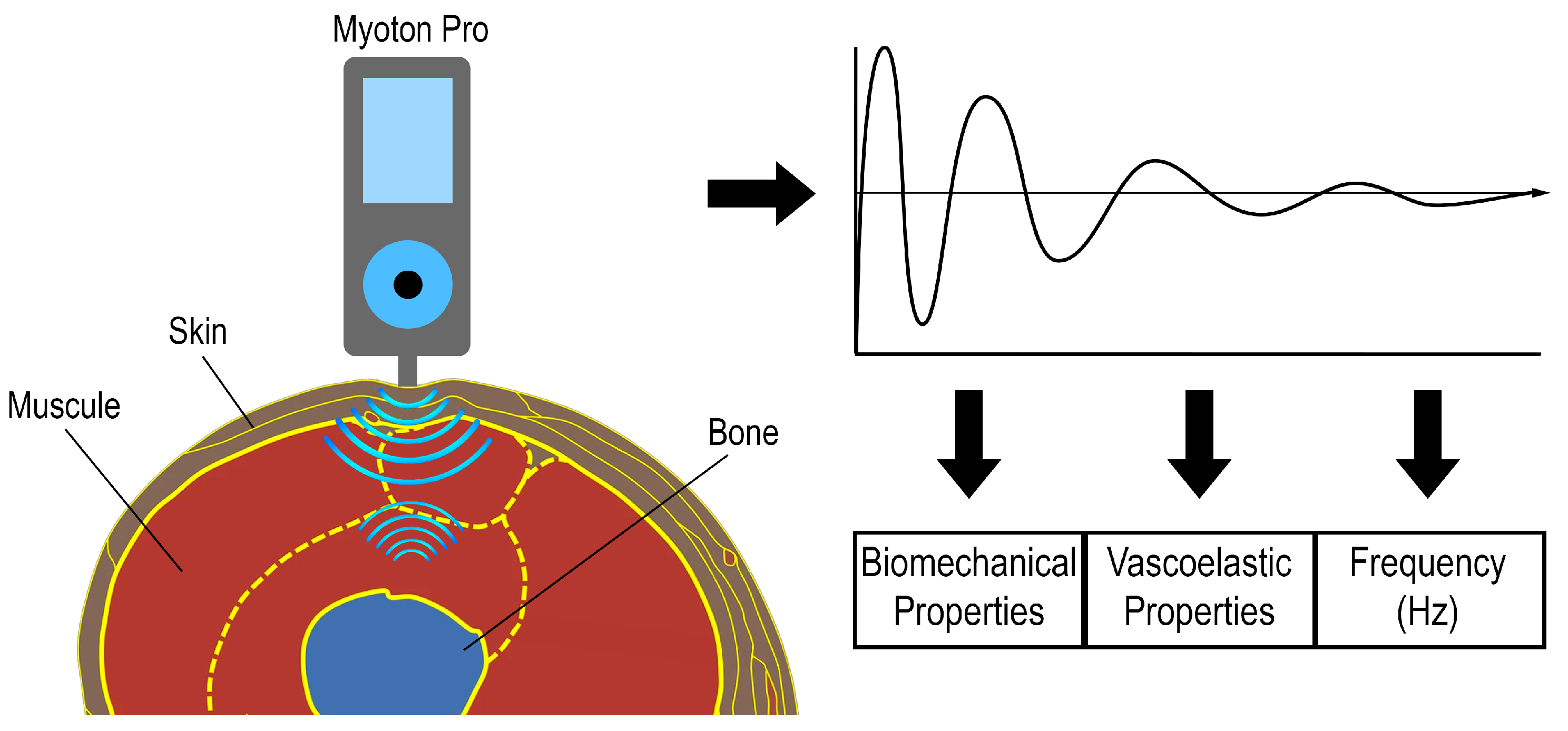

| MyotonPRO | |
|---|---|
| Tension | |
| Frequency | [Hz] natural oscillation characterizing intracellular muscle tension |
| Biomechanical properties | |
| Stiffness | [N/m] tissue resistance to contraction or to the action of applied force |
| Deformation/elasticity | [-] tissue’s ability to return to its original state after cessation of the action of force or cessation of contraction |
| Viscoelastic properties | |
| Relaxation | [ms] time between the maximum deformation of muscle tissue and its return to the initial state |
| Creep | [-] gradual elongation of muscle tissue during the action of tensile stress (tensile force) |
| Authors | TFL/GMED | Participants | Examination | Results |
|---|---|---|---|---|
| [1] | GMED TFL | Total N–40: HOA group N–20 patients with unilateral HOA were divided into two subgroups based on OA severity: Mild N—10 (5F/5M): -age 62.7 ± 5.9 yrs; -height: 164.2 ± 8.4 cm; -weight: 81.4 ± 18.1 kg; -BMI: 30.1 ± 5.6 kg/m2. Moderate-Severe N–10 (6F/4M): -age: 62.7 ± 5.9 yrs; -height: 167.6 ± 8.3 cm; -weight: 84.6 ± 18.9 kg; -BMI: 29.8 ± 5.1 kg/m2. Control N—20 (11F/9M): asymptomatic volunteers; -age: 62.1 ± 5.6 yrs; -height: 167.5 ± 9.6 cm; -weight: 69.7 ± 9.7 kg; -BMI: 24.8 ± 2.8 kg/m2. | - the Minnesota Leisure Time Physical Activity; Questionnaire - hand dynamometer; - MRI. | 1. The statistical analysis showed that the GMED asymmetry observed in MRI was correlated with OA severity. 2. The Moderate-Severe clinical OA group exhibited greater GMED asymmetry than the asymptomatic group. 3. No such differences were noted in TFL. |
| [44] | GMED | Total N – 40 divided into two groups: Group HOA N—20 (11F/9M) patients with unilateral HOA: -age: 63.4 ± 5.4 yrs; -height: 165.8 ± 8.3 cm; -weight: 83.0 ± 18 kg; -BMI: 30.0 ± 5.2 kg/m2. Control N—20 (11F/9M): -age: 62.1 ± 5.6 yrs; -height: 167.5 ± 9.6 cm; -weight: 69.7 ± 9.7 kg; -BMI: 24.8 ± 2.8 kg/m2. | - Needle EMG; - Functional assessment. | 1. The statistical analysis showed no significant differences in the variability in GMED muscle activation regardless of the tested parts (anterior, middle, and posterior). 2. During gait, the variability in the anterior hip GMED segments was significantly lower in the OA group in the early and entire stance. 3. However, the value of the average or peak GMED activity of all tested parts did not differ significantly between the groups. |
| [13] | GMED | Total N—40 divided into two groups: Group HOA N—20 (11F/9M) patients with unilateral HOA: -age: 63.4 ± 5.4 yrs; -height: 165.8 ± 8.3 cm; -weight: 83.0 ± 18 kg; -BMI: 30.0 ± 5.2 kg/m2. Control N—20 (11FK/9M): -age: 62.1 ± 5.6 yrs; -height: 167.5 ± 9.6 cm; -weight: 69.7 ± 9.7 kg; -BMI: 24.8 ± 2.8 kg/m2. | - Needle EMG; - Footswitches (Interlink Electronics FSRTM 402); - Step-up, step-down, and side-step tasks. | 1. Step-up and step-down task test—reduced activity of the middle and posterior GMED was observed in the OA patients, compared to the control group. 2. Side-step task test—the anterior and posterior GM were characterized by later TTP. |
| [10] | GMED | Total N—30 Group HOA N—13 patients with unilateral HOA: -age: 51.1 ± 2.3 yrs; -height: 178.2 ± 4.3 cm; -weight: 84.2 ± 6.8 kg. Control N—17 asymptomatic volunteers: -age: 50.8 ± 1.4 yrs; -height: 173.1 ± 2.5 cm; -weight: 77.3 ± 3.8 kg. | - Force platform; - sEMG; - Step-up and step-down task; - gait assessment. | 1. Higher GMED amplitude was observed during the step-up and step-down tasks in both the involved and uninvolved limbs in the OA group, compared to the control group. 2. The sEMG examination during gait exhibited significantly higher GMED amplitude in the dominant limb during the stance and swing phases in the OA group, compared to the control group. 3. The sEMG examination of the non-dominant limb during gait showed a significant increase in the amplitude only in the stance phase in the OA group, compared to the asymptomatic participants. |
| [45] | GMED TFL | Total N—34 Group HOA N—17 (7F/10M) patients with unilateral HOA: -age: 5.3 ± 8.3 yrs; -height: 170.2 ± 7.8 cm; -weight: 79.7 ± 14.8 kg; -BMI: 27.4 ± 3.5 kg/m2. Control N—17 (7F/10M) -age: 61.2 ± 7.6 yrs; -height: 172.0 ± 9.3 cm; -weight: 72.2 ± 14.5 kg; -BMI: 24.1 ± 3.3 kg/m2. | - VICON - motion capture system – gait analysis; - sEMG. | 1. Higher activity of the TFL muscle was noted on the OA-affected side than on the unaffected side. 2. No differences in GMED activity were observed between the OA-affected and unaffected limbs. 3. No differences were found in the ASI values of GM and TFL between the OA patients and the control group. 4. TFL was shown to have a significant effect on total muscle activity. A higher %C of TFL was observed on the affected than the unaffected side. Differences in the %C of TFL were also found between the limbs of patients in the control group. |
| [5] | GMED TFL | Total N—40 Group HOA N—20 (11F/9M) patients with unilateral HOA: -age: 63.4 ± 5.4 yrs; -height: 165.8 ± 8.3 cm; -weight: 83.0 ± 18 kg; -BMI: 30.0 ± 5.2. Control N—20 (11F/9M) -age: 62.1 ± 5.6 yrs; -height: 167.5 ± 9.6 cm; -weight: 69.7 ± 9.7 kg; -BMI: 24.8 ± 2.8 kg/m2. | - hand dynamometer; - MRI. | 1. No differences in the TFL volume were found between the OA-affected and unaffected sides. 2. Asymmetry was observed in the GMED volume. 3. No significant increase in fatty infiltration in GMED and TFL was found in the OA group, compared to the control group. 4. Hip abduction force was reduced in the OA group. |
| [46] | GMED | Total N—108 females with advanced unilateral HOA: -age: 65.4 ± 10.5 yrs; -BMI: 23.9 ± 3.6 kg/m2. | - hand dynamometer; - VAS; - CT. | 1. The regression analysis showed that CSA and SMD of GMED were significantly correlated with the hip abduction torque in the healthy limb. 2. This correlation was not observed in the OA-affected limb. |
| [47] | TFL GMED | Total N—28 (7M/21F): -age: 62.32 ± 7.41 yrs; -height: 158.02 ± 6.45 cm; -weight: 55.03 ± 10.09 kg. | - MRI; - Goniometer; - hand dynamometer. | 1. TFL exhibited more advanced fatty degeneration than GMAX. 2. A correlation was found between the volume and CSA of TFL. 3. The TFL strength was not correlated with CSA. |
| [48] | TFL GMED | - Total N—42 Group HOA (N—19) was divided into unilateral and bilateral HOA groups: Unilateral HOA group N—12 (3M/9F): -age: 62.9 ± 10.0 yrs; -height: 1.65 ± 0.09 m; -weight: 77.3 ± 14.0 kg; -BMI: 28.2 ± 3.5 kg/m2. Bilateral HOA group N—7 (3M/4F): -age: 63.0 ± 6.4 yrs; -height: 1.69 ± 0.14 m; -weight: 77.2 ± 15.0 kg; -BMI: 27.1 ± 3.5 kg/m2. Control N—23 (8M/11F): -age: 58.2 ± 8.6 yrs; -height: 1.69 ± 0.08 m; -weight: 69.9 ± 10.0 kg; -BMI: 24.4 ± 3.0 kg/m2. | - Isokinetic dynamometer; - MRI. | 1. A significant decrease in the abduction force was observed in the HOA patients, compared to the controls. 2. No significant decrease in the TFL and GMED volume was observed. |
| [7] | GMED | Total N—27 (9M/18F): -age: 63.2 ± 7.6 yrs; -BMI: 28.0 ± 4.1 kg/m2. | - MRI; - HOOS; | CSA was not correlated with HOOS (quality of life and hip function) scores. |
| [49] | GMED | Total N—50 (12M/38F) with unilateral HOA: -age: 62 yrs; -BMI: 23.3 ± 4.1 kg/m2. | - CT; - hand dynamometer. | 1. A relationship between CSA and 3D muscle volume, hip abductor muscle strength, and CT measurements was found. 2. A significant effect of GMED on hip abduction was shown. |
| [12] | GMED | Total N—60 HOA group divided into subgroups based on OA severity: Moderate OA: -age: 59 ± 8 yrs; -BMI: 28.7 ± 4.3 kg/m2. Severe OA: -age: 63 ± 8 yrs; -BMI: 30.0 ± 4.4 kg/m2. Control: -age: 62 ± 6 yrs; -BMI: 25.6 ± 5.0 kg/m2. | - sEMG; - gait analysis. | 1. A reduced range of motion and increased GMED activity were demonstrated in severe HOA. 2. The hip joint function during gait deteriorated with the severity of OA. |
| Authors | GMED/ TFL | Participants | Examination | Results |
|---|---|---|---|---|
| [50] | GMED | Total N—42 (9F/33M); -age: 70.9 yrs; -BMI: 22.8 kg/m2. | - retrospective study; - THA was performed through a lateral approach; - CT assessment of the GM composition before the procedure; - gait speed before and 6 months after the procedure; - TUG before and 6 months after the procedure; - dynamometer before and 6 months after the procedure. | 1. Changes in the GMED composition were shown to affect postoperative outcomes in patients. 2. The LDL of the gluteal muscle was shown to exert a significant effect on TUG results after 6 months. |
| [51] | GMED | Total N—10 (8F/2M); -age: 61.9 ± 9.4 yrs. | - posterior approach; - MRI assessment of changes in muscle length; - dynamometry during gait. | 1. The length of abductor muscles, including GMED, in the THA-treated limb was shortened significantly, compared to the non-operated side. 2. The data suggest that THA may have an adverse effect on the stability of the joint. |
| [52] | GMED TFL | Total N—22: Group THA N—11: -age: 64.55 ± 9.53 yrs; -height: 1.67 ± 0.08 m; -weight: 74.1 ± 4.97 kg; -BMI 26.46 ± 4.49 kg/m2; Healthy control N—11: -age: 62.36 ± 8.15 yrs; -height: 1.68 ± 0.08 m; -BMI: 23.76 ± 1.73 kg/m2. | - anterior approach; - estimation of the sufficiency of a single sEMG test for assessment of the MVC of hip abductors; - sEMG; - AMTI AccuGait force plate; - examination in 2 sessions at a 1-week interval; - balance test; - examination at 45–60 days after the procedure. | 1. No differences in GMED and TFL were found between the sEMG sessions. 2. Higher GMED activity was observed in the Unipodal and Bipodal balance test on both the operated and non-operated sides, compared to the healthy control. 3. Simultaneous disturbances in postural parameters were observed. |
| [53] | GMED | Total N—88 (49M/39F); -age: 47.3 ± 1.574 yrs; Division into three groups based on Trendelenburg test results: Normal N—23 (11M/12F) Mild N—61 (36M/25F) Severe N—4 (2M/2F) | - Hardinge access; - Trendelenburg test; - MRI; - examination before and 6 months after the procedure. | 1. The GMED diameter was reduced after THA at 6-month follow-up. 2. Improved function after THA, i.e., reduction of Trendelenburg gait, was observed. |
| [54] | GMED TFL | Total N—73 divided into groups based on PLLD: Group with PLLD N—22 (21F/1M) -age: 66.6 ± 3.61 yrs; -height: 1.53 ± 0.05 m; -weight: 53.2 ± 8.4 kg Group without PLLD N—51 (43F/8M) -age: 67.3 ± 3.92 yrs; -height: 1.56 ± 0.06 m; -weight: 53.6 ± 9.2 kg. | - posterolateral approach; - PLLD measurement; - SWE; - VAS; - dynamometer; - assessment of pelvic position; - ROM measured using a goniometer. | 1. The elasticity of the abductor muscle was significantly higher prior to THA in patients from the PLLD group. 2. The multiple regression analysis showed that the preoperative elastic modulus of abductor muscles had an impact on PLLD. |
| [55] | GMED | Total N—74 Division into two groups based on the occurrence of limping in patients at 6 months post THA Non-limping group N—37 -age: 56.7 ± 9.3 yrs; -BMI: 23.5 ± 4.3 kg/m2. Limping group N—37: -age: 64.5 ± 9.6 yrs; -BMI: 21.7 ± 2.7 kg/m2. | - anterolateral approach; - dynamometer; - TUG test; - CT–CSA of gluteus medius. | 1. The statistical analysis showed a reduction in the CSA of GMED in patients from the limping group before THA. 2. The results suggest that the preoperative CSA of the GMED muscle predicts limping at 6 months post THA. |
| [56] | GMED TFL | Total N—76 THA group N—52 (28F/27M); -age: 58 ± 9.0 yrs; -weight: 77.4 ± 14.2 kg; -height: 169 ± 9 cm. Healthy control N—24 (8F/16M); -age: 54.0 ± 6.6 yrs; -weight: 75.5 ± 11.3 kg; -height: 172 ± 7 cm. | - standard lateral transgluteal (Bauer) approach; - three-dimensional ultrasonic motion analysis system (CMS S50, zebris Medical GmbH, Isny, Germany); - sEMG; - before and at 6 months post THA. | 1. The motion range, bioelectrical activity, and duration and distance of gait at 6 months post THA were comparable with those of the healthy control. 2. The TFL activity in EMG was higher during loading in the THA-treated group. 3. The GMED activity in EMG was higher in the initial phase of gait in the THA-treated group. 4. Higher EMG results were observed during the first 40% and last 10% of the gait cycle. |
| [57] | GMED | Total N—58 (45F/13M); -age: 70.9 ± 9.5 yrs; -BMI: 23.0 ± 3.4 kg/m2. | - lateral approach; - CT prior to THA; - dynamometer before and at 6 months post THA; - gait speed before and at 6 months post THA | 1. In the entire study group, the LMM/TM value in GMED was negatively correlated with the postoperative gait speed. 2. The LDL/TM value in GMED showed a positive relationship with postoperative improvement in gait speed. 3. The LDL value was positively correlated with postoperative improvement in gait speed. 4. In the female group, the values of LMM and LMM/TM in GMED were positively correlated with postoperative improvement in gait speed. |
| [58] | GMED TFL | Total N—40 (23M/17F); - age 56.35 ± 12.9 yrs. | - lateral approach; - needle EMG; - MUAP analysis; - preoperative examination and at 6- and 12 weeks post THA | 1. At 6 weeks post THA, the EMG examination showed denervation of abductor muscles in 15 patients. 2. After 12 weeks, spontaneous healing was observed in 8 patients from this group. 3. The MUAP analysis showed persistent denervation in the other seven patients 4. The duration of MUAP increased in the GMED and TFL muscles after 6 weeks. 5. Significant reduction in MUAP amplitude was observed only in the GMED muscle. 6. After 12 weeks, the values were slightly different from the results obtained before THA, which indicated restoration of normal muscle activity |
| [62] | GMED | Total N—40 (6M/34F); - age 58 yrs. | - posterolateral approach; - CT assessment of GM volume changes at 3 weeks and 2 years post THA | The regression analysis showed that the increase in the cross-sectional area of GMED assessed by CT at 2 years post THA was associated with improved functional test results. |
| [60] | GMED | Total N—124F Division into two groups based on TUG results Fast Group N—103: -age: 65.3 ± 10.5 yrs; -BMI: 24.0 ± 5.1 kg/m2. Slow Group N—21: -age 74.4 ± 7.8 yrs; -BMI 25.3 ± 5.6 kg/m2. | Posterolateral modified Watson-Jones approach and direct anterior approach; - hand dynamometer before and at 1 year post THA; - CT before and at 2 weeks post THA; - TUG before and at 1 year post | 1. Significantly lower abductor muscle strength was observed on the non-operated side in the slow group 2. One year after THA, the abductor muscle strength was significantly lower on the operated and non-operated side in the slow group. 3. Before THA, the CT of GMED showed significantly lower density on the non-operated and operated side in the slow group. 4. The strength and quality of GMED muscle on the healthy side, but not on the THA-treated side, are important for the outcomes of the TUG test performed at 1 year post THA. |
| [61] | GMED | Total N—22 (8M/14F) -age: 60 ± 14.4 yrs. Mean THA follow-up period: 13.8 ± 2.3 months | - posterolateral approach; - MRI; - HHS | 1. Significant GMED fatty degeneration was observed on the operated versus the non-operated side. 2. The age correlated positively with fatty atrophy. 3. Steatosis exerts a negative effect on HHS results. |
| [59] | GMED TFL | Total N—10 (2F/8M); -age: 57.3 yrs. | - CT; - hip joint contact forces; - ADL—lateral approach | Compared to preoperative results, the findings at 3 months post THA were as follows: 1. An insignificant increase in total TFL volume 2. Insignificant changes in the decrease in the lean muscle volume of GMED and an increase in the lean muscle volume of TFL 3. Fatty degeneration—an insignificant increase in the GMED 4. Fatty degeneration—a significant decrease in the TFL muscle 5. The correlation suggests that fatty degeneration in TFL and GMED has a significant effect on hip joint contact forces. 6. GMED steatosis strongly correlates with hip joint contact forces while sitting down and getting up from a chair. |
| Authors | Aim of Study | Study Design | Muscles | Contraction/Rest | Conclusion |
|---|---|---|---|---|---|
| [63] | 1. Assessment of differences in the effectiveness of standard LSE used in the treatment of LBP, compared with an exercise program based on a combination of LSE with muscle strengthening and stretching exercises—CE 2. Assessment of the effectiveness of new exercise programs that may be used in the treatment of LBP | The study included elderly patients suffering from LBP for at least 3 months; Total N—20 (6M/14F) (mean age 67.5 ± 5.8 yrs); the study lasted 8 weeks; The participants were divided into two 10-person groups based on the type of exercises performed 3 times a week: LSE—patients performing lumbar spine stabilization exercises: -average age (yrs)—67.3 ± 5.92; -average BMI (kg/m2)—23.31; CE—patients performing combined exercises: -average age (yrs)—67.7 ± 5.37; -average BMI (kg/m2)—22.37. | GMED | rest | 1. Both groups of patients (CE and LSE) exhibited a decrease in muscle stiffness, including GMED, after the exercise period. 2. The study showed that both programs were effective in the treatment of LBP in elderly patients. 3. The comparison of the CE and LSE programs showed that the inclusion of additional programs of stretching and strengthening of the lower limb was a more effective method for LBP treatment in elderly patients. |
| [64] | Assessment of the relationship between lower limb muscle stiffness and CPP symptoms in females. | Patients suffering from CPP for at least 3 months were qualified for the study. The study included an asymptomatic control group. Total N—197F (age range 18–50 years). The study group was divided into two CPP and an asymptomatic group: CPP group—N—149 -average age (yrs)—35.7 ± 7.6; -average BMI (kg/m2)—25.71 ± 3.63. Asymptomatic—N—48 average age (yrs)—34.9 ± 9.2; average BMI (kg/m2)—25.1 ± 3.7. | TFL GMED | rest | 1. A slight correlation was found between muscle stiffness measurements and clinical CPP indices. 2. Significantly increased stiffness was found in 5 of the 11 muscles examined in the CPP group. In the case of TFL and GMED, no statistically significant differences were observed between the groups. |
| [65] | Assessment of the potential effect of 6-week specialist PT on changes in muscle stiffness and the ability to perform CMJ. | Professional volleyball players were qualified for the study, which lasted 6 weeks and included a 4-week preparatory period and a 2-week period before the tournament. Total N—16M - experience—4–5 years of consistent training: -average age (yrs)—21.12 ± 1.67; -average height (cm)—191.60 ± 5.74; -average weight (kg)—86.30 ± 6.66 kg. The examination was conducted two days before the start of training and at the end of each study week. | TFL | rest | 1. The study showed that the 6-week PT program did not induce a statistically significant increase in lower limb muscle stiffness, except for the tibialis anterior muscle. 2. The statistically insignificant increase in muscle stiffness was sufficient for a significant improvement in CMJ. |
| [66] | Assessment of the effectiveness of cryosauna in preventing DOMS. Biochemical tests of participants’ blood were performed, and markers of muscle tissue damage were determined. The stiffness of selected lower limb muscles was also examined using the MyotonPRO device. | The study included healthy individuals training in martial arts. The participants were asked to give up training 48 hours prior to the study. Total N—31 was divided into two groups: Experimental group—CRYO N—16—cryosauna was used after a series of exercises in this group: -average age (yrs)—22.1 ± 1.8; -average height (cm)—176.3 ± 8.3 cm; -average weight (kg)—76.1 ± 17.1 kg. Control group—CON N—15—participants performed the same series of exercises without cryosauna: -average age (yrs)—21.8 ± 1.6; -average height (cm)—175.3 ± 11.5 m; -average weight (kg)—76.2 ± 17.2. The participants were examined (blood sampling and MyotonPRO examination) before, immediately after, and 24, 48, 72, and 96 h after the exercise. | TFL | rest | 1. The study showed that cryosauna caused a decrease in the concentration of blood biomarkers of muscle tissue damage (creatine kinase) and muscle stiffness following DOMS. 2. The stiffness degree did not change in any muscles. No differences in the stiffness of TFL were observed in the analyzed periods. |
| [67] | Assess the impact of outcomes of marathon running and long-term endurance training on lower limb muscle stiffness in middle-aged marathon runners. | Long-distance runners aged 50–73 years were qualified for the study. The participants reported no illnesses and were not taking any medications regularly. All participants had been training actively for at least a year. Total N—31 All male group: -average age (yrs)—57.32 ± 6.25; -average height (cm)—175.61 ± 5.74; average weight (kg)—75.36 ± 7.89 k. Stiffness was measured using a MyotonPRO device. The examination was performed three times: twice before the start: 1 day and 1–2 h, and immediately after the marathon (up to 30 min after the run). Both limbs were measured. | TFL | rest | The study did not confirm the hypothesis that muscle biomechanical properties and resting tension may change after long-term exercise. |
| Authors | Aim of Study | Study Design | Muscles | Contraction/Rest | Conclusion |
|---|---|---|---|---|---|
| [16] | 1. Assessment of the properties of quadriceps muscles in dominant and non-dominant limbs in elderly female KOA patients 2. Determination of the correlation between quadriceps muscle characteristics and abnormal foot posture | Only females more than 50 years old with unilateral or bilateral KOA were qualified for the study. Total N—40 F: -average age (yrs)—65.28 ± 5.93; -average height (cm)—163.03 ± 6.37; -average weight (kg)—59.25 ± 7.31; -average BMI (kg/m2)—22.27 ± 2.19; unilateral KOA—N—25; bilateral KOA—N –15. The MyotonPRO examination was performed in the supine position. | RF VL VM | contraction | 1. Significantly greater tension and stiffness of the quadriceps muscle were observed in the non-dominant leg, compared to the dominant limb. 2. A relationship was found between changes in quadriceps muscle efficiency and static foot posture in the elderly female patients with KOA. |
| [17] | 1. Assessment of differences in the biomechanical parameters of quadriceps muscles in patients with knee osteoarthritis (KOA) in ultrasound examination, SWE, and MyotonPRO, compared to healthy controls 2. Identification of relationships between the examined muscle tissue and KOA severity | The study included over 60-year-old patients with unilateral KOA and healthy volunteers. Total N - 80, including: KG N—40 (13M/27F)—unilateral knee osteoarthritis; -average age (yrs)—66.78 ± 3.56; -average height (cm)—159.63 ± 6.17; -average weight (kg)—61.25 ± 4.34 -average BMI (kg/m2)—24.13 ± 2.39. CG N—40 (15F/25M) - control group of healthy volunteers: -average age (yrs)—66.38 ± 3.19; -average height (cm)—160.15 ± 6.08; -average weight (kg)—60.90 ± 4.62 -average BMI (kg/m2)—23.85 ± 2.57. The MyotonPRO examination was conducted in a neutral, relaxed supine position. | RF VM VL | rest | 1. The MyotonPro examination showed asymmetry of quadriceps biomechanical parameters between the sides with and without OA in patients with unilateral KOA. 2. Quadriceps muscle stiffness was higher in the KOA patients than in the healthy controls. 3. It was observed in the study group that tension was dependent on the intensity of pain sensation (VAS) and the disability degree (WOMAC). |
| [68] | Assessment of the relationship between muscle tissue properties and mechanical pain sensitivity in adults with KOA | Total N—42; -average age (yrs)—67.5 ± 8.5. | RF BF | rest | The study showed that changes in the biomechanical properties of BF (weakening) may impair joint loading, which causes greater pain during movement. |
| [15] | Assessment of the relationship between biomechanical properties of GM and foot posture asymmetry in patients with unilateral and bilateral KOA | Over 45-year-old patients with diagnosed KOA were qualified for the study. Total N—62, including: UG (unilateral group)—N—30 (13F/17M) -average age (yrs)—62.97 ± 6.96; -average height (cm)—156.13 ± 27.20; -average weight (kg)—63.30 ± 8.42; BG (bilateral group)—N—32 (15F/17M) -average age (yrs)—60.09 ± 6.12; -average height (cm)—161.84 ± 6.27; -average weight (kg)—64.44 ± 7.40. The MyotonPRO examination was conducted in the relaxed supine position. | GL GM | rest | 1. Higher stiffness and tension were observed in GL in RSL than in RML in the KOA patients. 2. Greater asymmetry in foot posture was found in the patients with unilateral KOA, compared to those with bilateral KOA. 3. The foot posture was significantly correlated with the biomechanical properties of GM and KOA severity. |
| [14] | 1. Comparison of RF, VL, and VM stiffness between KOA patients and healthy individuals 2. Determination of potential differences in stiffness between the study groups, depending on the range of knee joint motion 3. Identification of a relationship between the stiffness of the studied muscles and impaired knee joint function in KOA patients | The study involved KOA patients and healthy controls. Both study groups were age-matched. Total N—50, including: KOA group—N—25 (18/7): -average age (yrs) 62.2 ± 8.3; -average BMI (kg/m2)—24.22 ± 1.96; Healthy group – N—25 (18/7): -average age (yrs)—59.44 ± 5.33; -average BMI (kg/m2)—23.67 ± 2.67. The measurements were taken in a sitting position with 60° and 90° flexion. The angle was controlled by an orthosis. | RF VL VM | contraction | 1. Greater VL stiffness was observed in the KOA-affected patients than in the healthy controls. No significant differences in the stiffness of the other muscles were found between the groups. 2. The statistical analysis showed that the stiffness of the muscles increased with the motion range in both groups. 3. A correlation was found between the stiffness of the muscles and the disability degree in the KOA patients. This correlation was observed in particular between the VL stiffness and the disability degree determined with the WOMAC score. |
| [69] | Assessment of the effect of a reduced knee flexion angle on patella mobility in OA patients. | Patients with knee OA were qualified for the study. The study included two control groups: healthy elderly and healthy young individuals. Total N—50, including: KOA (OA-affected) patients—N—23: -average age (yrs)—71.7 ± 8.3; -average height (cm)—152 ± 7.4; -average weight (kg)—57.2 ± 9; -average BMI (kg/m2)—24.8 ± 3.7. Healthy elderly individuals—N—17: -average age (yrs) 69.7 ± 4.0; -average height (cm)—149.7 ± 4.4; -average weight (kg)—48.7 ± 6.3; -average BMI (kg/m2)—21.7 ± 2.6. Healthy young individuals—N—10: -average age (yrs)—21.7 ± 0.7; -average height (cm)—161.1 ± 5.1; -average weight (kg)—57.5 ± 8; -average BMI (kg/m2)—22.3 ± 3.9. The examination was performed in the supine position with 45° knee joint flexion. Stiffness, flexibility, and tension were measured using MyotonPRO. Patellar mobility was assessed with the use of PFA. | RF | contraction | 1. Patella mobility was found to change in the OA patients. 2. There was a relationship between reduced patella mobility and reduced knee motion range. 3. Reduced flexibility was shown to affect knee mobility in OA. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posturzyńska, A.; Łazorko, A.; Cukierman, B.; Tomczyk-Warunek, A.; Winiarska, A.; Skrzypek, T.; Lis, M.; Jarecki, J. Review of Methods for Evaluating Changes in the Tension and Properties of the Gluteus Medius Muscle (GMED) and the Tensor Fascia Latae (TFL) as a Result of Hip Osteoarthritis (HOA) and After Total Hip Arthroplasty (THA)—Could MyotonPRO Assessment Be the New Standard? J. Clin. Med. 2025, 14, 5982. https://doi.org/10.3390/jcm14175982
Posturzyńska A, Łazorko A, Cukierman B, Tomczyk-Warunek A, Winiarska A, Skrzypek T, Lis M, Jarecki J. Review of Methods for Evaluating Changes in the Tension and Properties of the Gluteus Medius Muscle (GMED) and the Tensor Fascia Latae (TFL) as a Result of Hip Osteoarthritis (HOA) and After Total Hip Arthroplasty (THA)—Could MyotonPRO Assessment Be the New Standard? Journal of Clinical Medicine. 2025; 14(17):5982. https://doi.org/10.3390/jcm14175982
Chicago/Turabian StylePosturzyńska, Agnieszka, Artur Łazorko, Bartosz Cukierman, Agnieszka Tomczyk-Warunek, Anna Winiarska, Tomasz Skrzypek, Magdalena Lis, and Jaromir Jarecki. 2025. "Review of Methods for Evaluating Changes in the Tension and Properties of the Gluteus Medius Muscle (GMED) and the Tensor Fascia Latae (TFL) as a Result of Hip Osteoarthritis (HOA) and After Total Hip Arthroplasty (THA)—Could MyotonPRO Assessment Be the New Standard?" Journal of Clinical Medicine 14, no. 17: 5982. https://doi.org/10.3390/jcm14175982
APA StylePosturzyńska, A., Łazorko, A., Cukierman, B., Tomczyk-Warunek, A., Winiarska, A., Skrzypek, T., Lis, M., & Jarecki, J. (2025). Review of Methods for Evaluating Changes in the Tension and Properties of the Gluteus Medius Muscle (GMED) and the Tensor Fascia Latae (TFL) as a Result of Hip Osteoarthritis (HOA) and After Total Hip Arthroplasty (THA)—Could MyotonPRO Assessment Be the New Standard? Journal of Clinical Medicine, 14(17), 5982. https://doi.org/10.3390/jcm14175982





