Beyond Earth, Beyond Time: Preserving Female Fertility in Space Missions
Abstract
1. Introduction
2. Composition and Sources of Space Radiation
- Protons (85%): High-energy hydrogen nuclei that make up the majority of cosmic radiation.
- Helium nuclei (14%): Energetic alpha particles with strong penetrative capacity.
3. Radiation Mitigation Strategies in Spacecraft Design
4. Cosmic Radiation Effects on Human Health
5. Studies on Cosmic Radiation on Reproductive Health
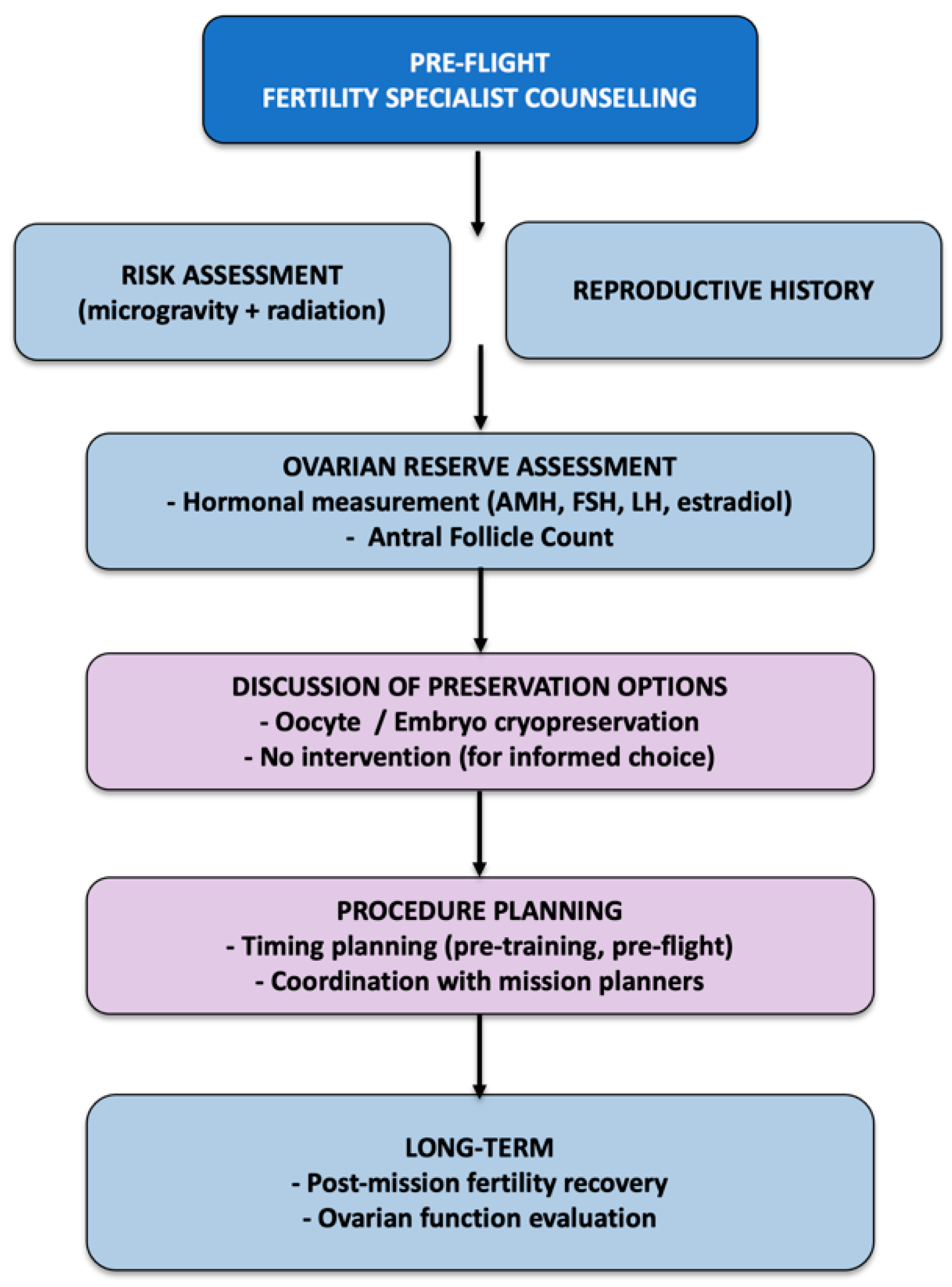
6. Fertility Counseling for Female Astronauts
7. Fertility Preservation for Female Astronauts
8. Future Studies
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPE | Solar Particle Events |
| GCR | Galactic Cosmic Ray |
| LET | Linear Energy Transfer |
| NSRL | NASA Space Radiation Laboratory |
| HZE | High atomic number and energy |
| OTT | Ovarian tissue transplantation |
References
- Betson, J.R.J.; Secrest, R.R. Prospective women astronauts selection program. Rationale and comments. Am. J. Obstet. Gynecol. 1964, 88, 421–423. [Google Scholar] [CrossRef]
- Mandt, K.E. Increasing Diversity on Spacecraft Mission Teams Reduces Risk. Science 2023, 382, eadk7373. [Google Scholar] [CrossRef]
- Chiu, S. Space Culture–Public Engagement in Space Through Culture. In Proceedings of the 73rd International Astronautical Congress, Paris, France, 18–22 September 2022. [Google Scholar]
- Crane, L. Meet Christina Koch, Who Will Be the First Woman to Go to the Moon. NewScientist. 2024. Available online: https://www.newscientist.com/article/mg26134730-100-meet-christina-koch-who-will-be-the-first-woman-to-go-to-the-moon/ (accessed on 24 May 2025).
- Drago-Ferrante, R.; Di Fiore, R.; Karouia, F.; Subbannayya, Y.; Das, S.; Aydogan Mathyk, B.; Arif, S.; Guevara-Cerdán, A.P.; Seylani, A.; Galsinh, A.S.; et al. Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review. Int. J. Mol. Sci. 2022, 23, 7465. [Google Scholar] [CrossRef]
- Gimunová, M.; Paludo, A.C.; Bernaciková, M.; Bienertova-Vasku, J. The Effect of Space Travel on Human Reproductive Health: A Systematic Review. npj Microgravity 2024, 10, 10. [Google Scholar] [CrossRef]
- Ronca, A.E.; Baker, E.S.; Bavendam, T.G.; Beck, K.D.; Miller, V.M.; Tash, J.S.; Jenkins, M. Effects of Sex and Gender on Adaptations to Space: Reproductive Health. J. Womens Health 2014, 23, 967–974. [Google Scholar] [CrossRef]
- Steller, J.G.; Blue, R.S.; Burns, R.; Bayuse, T.M.; Antonsen, E.L.; Jain, V.; Blackwell, M.M.; Jennings, R.T. Gynecologic Risk Mitigation Considerations for Long-Duration Spaceflight. Aerosp. Med. Hum. Perform. 2020, 91, 543–564. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Wotring, V.E. Medically Induced Amenorrhea in Female Astronauts. npj Microgravity 2016, 2, 16008. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.T.; Baker, E.S. Gynecological and Reproductive Issues for Women in Space: A Review. Obstet. Gynecol. Surv. 2000, 55, 109–116. [Google Scholar] [CrossRef]
- Cheng, K.; Feng, X.; Yang, C.; Ma, C.; Niu, S.; Jia, L.; Yang, X.; Liang, J.; Bo, Y.; Geng, K.; et al. Simulated Microgravity Reduces Quality of Ovarian Follicles and Oocytes by Disrupting Communications of Follicle Cells. npj Microgravity 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Loomer, P.M. The Impact of Microgravity on Bone Metabolism in Vitro and In Vivo. Crit. Rev. Oral. Biol. Med. 2001, 12, 252–261. [Google Scholar] [CrossRef]
- Mishra, B.; Luderer, U. Reproductive Hazards of Space Travel in Women and Men. Nat. Rev. Endocrinol. 2019, 15, 713–730. [Google Scholar] [CrossRef]
- Restier-Verlet, J.; El-Nachef, L.; Ferlazzo, M.L.; Al-Choboq, J.; Granzotto, A.; Bouchet, A.; Foray, N. Radiation on Earth or in Space: What Does It Change? Int. J. Mol. Sci. 2021, 22, 3739. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Almurayshid, M.; Almasoud, F.I.; Alyahyawi, A.R.; Yasmin, S.; Elsafi, M. Developed a New Radiation Shielding Absorber Composed of Waste Marble, Polyester, PbCO(3), and CdO to Reduce Waste Marble Considering Environmental Safety. Materials 2022, 15, 8371. [Google Scholar] [CrossRef]
- Dobney, W.; Mols, L.; Mistry, D.; Tabury, K.; Baselet, B.; Baatout, S. Evaluation of Deep Space Exploration Risks and Mitigations against Radiation and Microgravity. Front. Nucl. Med. 2023, 3, 1225034. [Google Scholar] [CrossRef]
- Knipp, D.J.; Fraser, B.J.; Shea, M.A.; Smart, D.F. On the Little-Known Consequences of the 4 August 1972 Ultra-Fast Coronal Mass Ejecta: Facts, Commentary, and Call to Action. Space Weather 2018, 16, 1635–1643. [Google Scholar] [CrossRef]
- Guan, S.; Fu, G.; Wan, B.; Wang, X.; Chen, Z. Multi-Objective Optimization and Reliability Assessment of Multi-Layer Radiation Shielding for Deep Space Missions. Aerospace 2025, 12, 337. [Google Scholar] [CrossRef]
- Naito, M.; Kitamura, H.; Koike, M.; Kusano, H.; Kusumoto, T.; Uchihori, Y.; Endo, T.; Hagiwara, Y.; Kiyono, N.; Kodama, H.; et al. Applicability of Composite Materials for Space Radiation Shielding of Spacecraft. Life Sci. Space Res. 2021, 31, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Kausar, A.; Manzoor, S.; Rakha, S.A.; Uzair, A.; Sajid, M.; Arif, A.; Khan, A.F.; Diallo, A.; Ahmad, I. Views on Radiation Shielding Efficiency of Polymeric Composites/Nanocomposites and Multi-Layered Materials: Current State and Advancements. Radiation 2023, 3, 1–20. [Google Scholar] [CrossRef]
- Townsend, L.W. Implications of the Space Radiation Environment for Human Exploration in Deep Space. Radiat. Prot. Dosim. 2005, 115, 44–50. [Google Scholar] [CrossRef]
- Ferrone, K.; Willis, C.; Guan, F.; Ma, J.; Peterson, L.; Kry, S. A Review of Magnetic Shielding Technology for Space Radiation. Radiation 2023, 3, 46–57. [Google Scholar] [CrossRef]
- Adams, J.; Hathaway, D.; Grugel, R.; Watts, J.; Parnell, T.; Gregory, J.; Winglee, R. Revolutionary Concepts of Radiation Shielding for Human Exploration of Space. 2005. Available online: https://ntrs.nasa.gov/citations/20050180620 (accessed on 7 August 2024).
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Measurements of Energetic Particle Radiation in Transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef]
- Bannova, O.; Gulacsi, E. Architectural Approach for Evaluation of Radiation Shielding Integration in Space Habitats. Acta Astronaut. 2024, 220, 27–36. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Kim, M.-H.Y.; Chappell, L.J.; Huff, J.L. How Safe Is Safe Enough? Radiation Risk for a Human Mission to Mars. PLoS ONE 2013, 8, e74988. [Google Scholar] [CrossRef]
- Chancellor, J.C.; Scott, G.B.I.; Sutton, J.P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef]
- Chylack, L.T.J.; Peterson, L.E.; Feiveson, A.H.; Wear, M.L.; Manuel, F.K.; Tung, W.H.; Hardy, D.S.; Marak, L.J.; Cucinotta, F.A. NASA Study of Cataract in Astronauts (NASCA). Report 1: Cross-Sectional Study of the Relationship of Exposure to Space Radiation and Risk of Lens Opacity. Radiat. Res. 2009, 172, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Ong, J.; Brunstetter, T.; Gibson, C.R.; Macias, B.R.; Laurie, S.; Mader, T.; Hargens, A.; Buckey, J.C.; Lan, M.; et al. Spaceflight Associated Neuro-Ocular Syndrome (SANS) and Its Countermeasures. Prog. Retin. Eye Res. 2025, 106, 101340. [Google Scholar] [CrossRef]
- Galdamez, L.A.; Mader, T.H.; Ong, J.; Kadipasaoglu, C.M.; Lee, A.G. A Multifactorial, Evidence-Based Analysis of Pathophysiology in Spaceflight Associated Neuro-Ocular Syndrome (SANS). Eye 2025, 39, 700–709. [Google Scholar] [CrossRef]
- Mao, X.W.; Stanbouly, S.; Chieu, B.; Sridharan, V.; Allen, A.R.; Boerma, M. Low Dose Space Radiation-Induced Effects on the Mouse Retina and Blood-Retinal Barrier Integrity. Acta Astronaut. 2022, 199, 412–419. [Google Scholar] [CrossRef]
- Onorato, G.; Di Schiavi, E.; Di Cunto, F. Understanding the Effects of Deep Space Radiation on Nervous System: The Role of Genetically Tractable Experimental Models. Front. Phys. 2020, 8, 362. [Google Scholar] [CrossRef]
- Yeiser, L.A.; Villasana, L.E.; Raber, J. ApoE Isoform Modulates Effects of Cranial 56Fe Irradiation on Spatial Learning and Memory in the Water Maze. Behav. Brain Res. 2013, 237, 207–214. [Google Scholar] [CrossRef]
- Raber, J.; Yamazaki, J.; Torres, E.R.S.; Kirchoff, N.; Stagaman, K.; Sharpton, T.; Turker, M.S.; Kronenberg, A. Combined Effects of Three High-Energy Charged Particle Beams Important for Space Flight on Brain, Behavioral and Cognitive Endpoints in B6D2F1 Female and Male Mice. Front. Physiol. 2019, 10, 179. [Google Scholar] [CrossRef]
- Parihar, V.K.; Allen, B.D.; Caressi, C.; Kwok, S.; Chu, E.; Tran, K.K.; Chmielewski, N.N.; Giedzinski, E.; Acharya, M.M.; Britten, R.A.; et al. Cosmic Radiation Exposure and Persistent Cognitive Dysfunction. Sci. Rep. 2016, 6, 34774. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Kiffer, F.C.; Bancroft, G.L.; Guzman, C.S.; Soler, I.; Haas, H.A.; Shi, R.; Patel, R.; Lara-Jiménez, J.; Kumar, P.L.; et al. The Longitudinal Behavioral Effects of Acute Exposure to Galactic Cosmic Radiation in Female C57BL/6J Mice: Implications for Deep Space Missions, Female Crews, and Potential Antioxidant Countermeasures. J. Neurochem. 2025, 169, e16225. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.M.; Baulch, J.E.; Klein, P.M.; Baddour, A.A.D.; Apodaca, L.A.; Kramár, E.A.; Alikhani, L.; Garcia, C.J.; Angulo, M.C.; Batra, R.S.; et al. New Concerns for Neurocognitive Function during Deep Space Exposures to Chronic, Low Dose-Rate, Neutron Radiation. eNeuro 2019, 6, ENEURO.0094-19.2019. [Google Scholar] [CrossRef] [PubMed]
- Kiffer, F.; Boerma, M.; Allen, A. Behavioral Effects of Space Radiation: A Comprehensive Review of Animal Studies. Life Sci. Space Res. 2019, 21, 1–21. [Google Scholar] [CrossRef]
- Miller, K.B.; Mi, K.L.; Nelson, G.A.; Norman, R.B.; Patel, Z.S.; Huff, J.L. Ionizing Radiation, Cerebrovascular Disease, and Consequent Dementia: A Review and Proposed Framework Relevant to Space Radiation Exposure. Front. Physiol. 2022, 13, 1008640. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Wang, X.; Chen, J.; Du, C.; Wang, J.; Liao, W. Insights into Ionizing Radiation-Induced Bone Marrow Hematopoietic Stem Cell Injury. Stem Cell Res. Ther. 2024, 15, 222. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Heavy Ion Carcinogenesis and Human Space Exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef]
- Man, J.; Graham, T.; Squires-Donelly, G.; Laslett, A.L. The Effects of Microgravity on Bone Structure and Function. npj Microgravity 2022, 8, 9. [Google Scholar] [CrossRef]
- Alwood, J.S.; Kumar, A.; Tran, L.H.; Wang, A.; Limoli, C.L.; Globus, R.K. Low-Dose, Ionizing Radiation and Age-Related Changes in Skeletal Microarchitecture. J. Aging Res. 2012, 2012, 481983. [Google Scholar] [CrossRef] [PubMed]
- Matheson, B.E.; Walle, M.; Liphardt, A.-M.; Hulme, P.A.; Heer, M.; Zwart, S.R.; Sibonga, J.D.; Smith, S.M.; Gabel, L.; Boyd, S.K. Recovery of Bone Microarchitecture and Density Four Years after Spaceflight: Two Case Studies. npj Microgravity 2025, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.C.; Slaba, T.C.; Guida, P.; Rusek, A. NASA’s First Ground-Based Galactic Cosmic Ray Simulator: Enabling a New Era in Space Radiobiology Research. PLoS Biol. 2020, 18, e3000669. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red Risks for a Journey to the Red Planet: The Highest Priority Human Health Risks for a Mission to Mars. npj Microgravity 2020, 6, 33. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, G.; Hu, W. Carcinogenesis Induced by Space Radiation: A Systematic Review. Neoplasia 2022, 32, 100828. [Google Scholar] [CrossRef] [PubMed]
- Sishc, B.J.; Zawaski, J.; Saha, J.; Carnell, L.S.; Fabre, K.M.; Elgart, S.R. The Need for Biological Countermeasures to Mitigate the Risk of Space Radiation-Induced Carcinogenesis, Cardiovascular Disease, and Central Nervous System Deficiencies. Life Sci. Space Res. 2022, 35, 4–8. [Google Scholar] [CrossRef]
- Mishra, B.; Lawson, G.W.; Ripperdan, R.; Ortiz, L.; Luderer, U. Charged-Iron-Particles Found in Galactic Cosmic Rays Are Potent Inducers of Epithelial Ovarian Tumors. Radiat. Res. 2018, 190, 142–150. [Google Scholar] [CrossRef]
- Ijiri, K. Development of Space-Fertilized Eggs and Formation of Primordial Germ Cells in the Embryos of Medaka Fish. Adv. Space Res. 1998, 21, 1155–1158. [Google Scholar] [CrossRef]
- Aimar, C.; Bautz, A.; Durand, D.; Membre, H.; Chardard, D.; Gualandris-Parisot, L.; Husson, D.; Dournon, C. Microgravity and Hypergravity Effects on Fertilization of the Salamander Pleurodeles waltl (Urodele Amphibian). Biol. Reprod. 2000, 63, 551–558. [Google Scholar] [CrossRef]
- Souza, K.A.; Black, S.D.; Wassersug, R.J. Amphibian Development in the Virtual Absence of Gravity. Proc. Natl. Acad. Sci. USA 1995, 92, 1975–1978. [Google Scholar] [CrossRef]
- Megory, E.; Oyama, J. Hypergravity Induced Prolactin Surge in Female Rats. Aviat. Space Environ. Med. 1985, 56, 415–418. [Google Scholar]
- Megory, E.; Konikoff, F.; Ishay, J.S.; Lelyveld, J. Hypergravity: Its Effect on the Estrous Cycle and Hormonal Levels in Female Rats. Life Sci. Space Res. 1979, 17, 213–218. [Google Scholar] [CrossRef]
- Masini, M.A.; Prato, P.; Scarabelli, L.; Lanza, C.; Palmero, S.; Pointis, G.; Ricci, F.; Strollo, F. In Vitro Effects of Simulated Microgravity on Sertoli Cell Function. Adv. Space Res. 2011, 47, 575–581. [Google Scholar] [CrossRef]
- Ahrari, K.; Omolaoye, T.S.; Goswami, N.; Alsuwaidi, H.; du Plessis, S.S. Effects of Space Flight on Sperm Function and Integrity: A Systematic Review. Front. Physiol. 2022, 13, 904375. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; Maya, W.D.C.; Plessis, S.S. du Could Exposure to Spaceflight Cause Mutations in Genes That Affect Male Fertility? Life Sci. Space Res. 2023, 37, 15–17. [Google Scholar] [CrossRef]
- Holets, L.M.; Gupta, V.; Roby, K.F.; Tash, J.S. Spaceflight Inhibits Ovarian Follicle Development, Induces Down Regulation of Estrogen Receptor Alpha, and Alters Metabolic Pathways and Gene Expression in Mouse Uterus. Biol. Reprod. 2012, 87, 18. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, D.; Wu, Y.; Lin, W.; Chen, Z.; Meng, L.; Liu, J.; Zhou, Y. Simulated Microgravity Using a Rotary Culture System Compromises the In Vitro Development of Mouse Preantral Follicles. PLoS ONE 2016, 11, e0151062. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Ortiz, L.; Luderer, U. Charged Iron Particles, Components of Space Radiation, Destroy Ovarian Follicles. Hum. Reprod. 2016, 31, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Ripperdan, R.; Ortiz, L.; Luderer, U. Very Low Doses of Heavy Oxygen Ion Radiation Induce Premature Ovarian Failure. Reproduction 2017, 154, 123–133. [Google Scholar] [CrossRef]
- Baird, D.T.; Webb, R.; Campbell, B.K.; Harkness, L.M.; Gosden, R.G. Long-Term Ovarian Function in Sheep after Ovariectomy and Transplantation of Autografts Stored at -196 C. Endocrinology 1999, 140, 462–471. [Google Scholar] [CrossRef]
- Delp, M.D.; Charvat, J.M.; Limoli, C.L.; Globus, R.K.; Ghosh, P. Apollo Lunar Astronauts Show Higher Cardiovascular Disease Mortality: Possible Deep Space Radiation Effects on the Vascular Endothelium. Sci. Rep. 2016, 6, 29901. [Google Scholar] [CrossRef]
- George, S.P.; Gaza, R.; Matthiä, D.; Laramore, D.; Lehti, J.; Campbell-Ricketts, T.; Kroupa, M.; Stoffle, N.; Marsalek, K.; Przybyla, B.; et al. Space Radiation Measurements during the Artemis I Lunar Mission. Nature 2024, 634, 48–52. [Google Scholar] [CrossRef]
- Ogilvy-Stuart, A.L.; Shalet, S.M. Effect of Radiation on the Human Reproductive System. Env. Health Perspect 1993, 101 (Suppl. 2), 109–116. [Google Scholar] [CrossRef]
- Marci, R.; Mallozzi, M.; Di Benedetto, L.; Schimberni, M.; Mossa, S.; Soave, I.; Palomba, S.; Caserta, D. Radiations and Female Fertility. Reprod. Biol. Endocrinol. 2018, 16, 112. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian Aging: Mechanisms and Clinical Consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Wang, B.; Yasuda, H. Relative Biological Effectiveness of High LET Particles on the Reproductive System and Fetal Development. Life 2020, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Cedars, M.I. Evaluation of Female Fertility-AMH and Ovarian Reserve Testing. J. Clin. Endocrinol. Metab. 2022, 107, 1510–1519. [Google Scholar] [CrossRef]
- Mahajan, N.K. Optimizing Advice and Approaches for Elective Fertility Preservation. Best Pract. Res. Clin. Obstet. Gynaecol. 2025, 99, 102591. [Google Scholar] [CrossRef]
- van de Loo, L.E.X.M.; van den Berg, M.H.; Overbeek, A.; van Dijk, M.; Damen, L.; Lambalk, C.B.; Ronckers, C.M.; van den Heuvel-Eibrink, M.M.; Kremer, L.C.M.; van der Pal, H.J.; et al. Uterine Function, Pregnancy Complications, and Pregnancy Outcomes among Female Childhood Cancer Survivors. Fertil. Steril. 2019, 111, 372–380. [Google Scholar] [CrossRef]
- Teh, W.T.; Stern, C.; Chander, S.; Hickey, M. The Impact of Uterine Radiation on Subsequent Fertility and Pregnancy Outcomes. Biomed. Res. Int. 2014, 2014, 482968. [Google Scholar] [CrossRef]
- Buonomo, B.; Orecchia, R.; Tomao, F.; Pup, L.D.; Garcia-Faura, A.; Peccatori, F.A. Uterine Irradiation as a Determinant of Infertility and Pregnancy Losses in Young Cancer Survivors. ecancermedicalscience 2020, 14, 1032. [Google Scholar] [CrossRef]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubert, J.; Agrawal, D.K. Radioprotective Agents to Prevent Cellular Damage Due to Ionizing Radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef]
- Jit, B.P.; Pattnaik, S.; Arya, R.; Dash, R.; Sahoo, S.S.; Pradhan, B.; Bhuyan, P.P.; Behera, P.K.; Jena, M.; Sharma, A.; et al. Phytochemicals: A Potential next Generation Agent for Radioprotection. Phytomedicine 2022, 106, 154188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Li, Z.; Wu, H.; Zou, B.; Xu, Y. Exploring Natural Products as Radioprotective Agents for Cancer Therapy: Mechanisms, Challenges, and Opportunities. Cancers 2023, 15, 3585. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef]
- Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; Maslin, C.; et al. ESHRE Guideline: Female Fertility Preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [CrossRef]
- Ethics Committee of the American Society for Reproductive Medicine. Planned Oocyte Cryopreservation to Preserve Future Reproductive Potential: An Ethics Committee Opinion. Fertil. Steril. 2024, 121, 604–612. [Google Scholar] [CrossRef]
- Marin, L.; Turan, V.; Oktay, K. Fertility Preservation in Breast Cancer Patients. In Female and Male Fertility Preservation; Grynberg, M., Patrizio, P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 185–198. ISBN 978-3-030-47767-7. [Google Scholar]
- Oktay, K.H.; Marin, L.; Petrikovsky, B.; Terrani, M.; Babayev, S.N. Delaying Reproductive Aging by Ovarian Tissue Cryopreservation and Transplantation: Is It Prime Time? Trends Mol. Med. 2021, 27, 753–761. [Google Scholar] [CrossRef]
- Marin, L.; Ambrosini, G.; Esposito, F.; Capobianco, G.; Lagana, A.S.; Vio, C.; Nuzzi, L.; Rossato, M.; Andrisani, A. Fertility Preservation in Girls and Women: State of Art and Future Possibilities. Clin. Exp. Obstet. Gynecol. 2022, 49, 206. [Google Scholar] [CrossRef]
- Oktay, K.H.; Marin, L. Comparison of Orthotopic and Heterotopic Autologous Ovarian Tissue Transplantation Outcomes. Fertil. Steril. 2024, 121, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Marin, L.; Bedoschi, G.; Pacheco, F.; Sugishita, Y.; Kawahara, T.; Taylan, E.; Acosta, C.; Bang, H. Ovarian Transplantation with Robotic Surgery and a Neovascularizing Human Extracellular Matrix Scaffold: A Case Series in Comparison to Meta-Analytic Data. Fertil. Steril. 2022, 117, 181–192. [Google Scholar] [CrossRef] [PubMed]
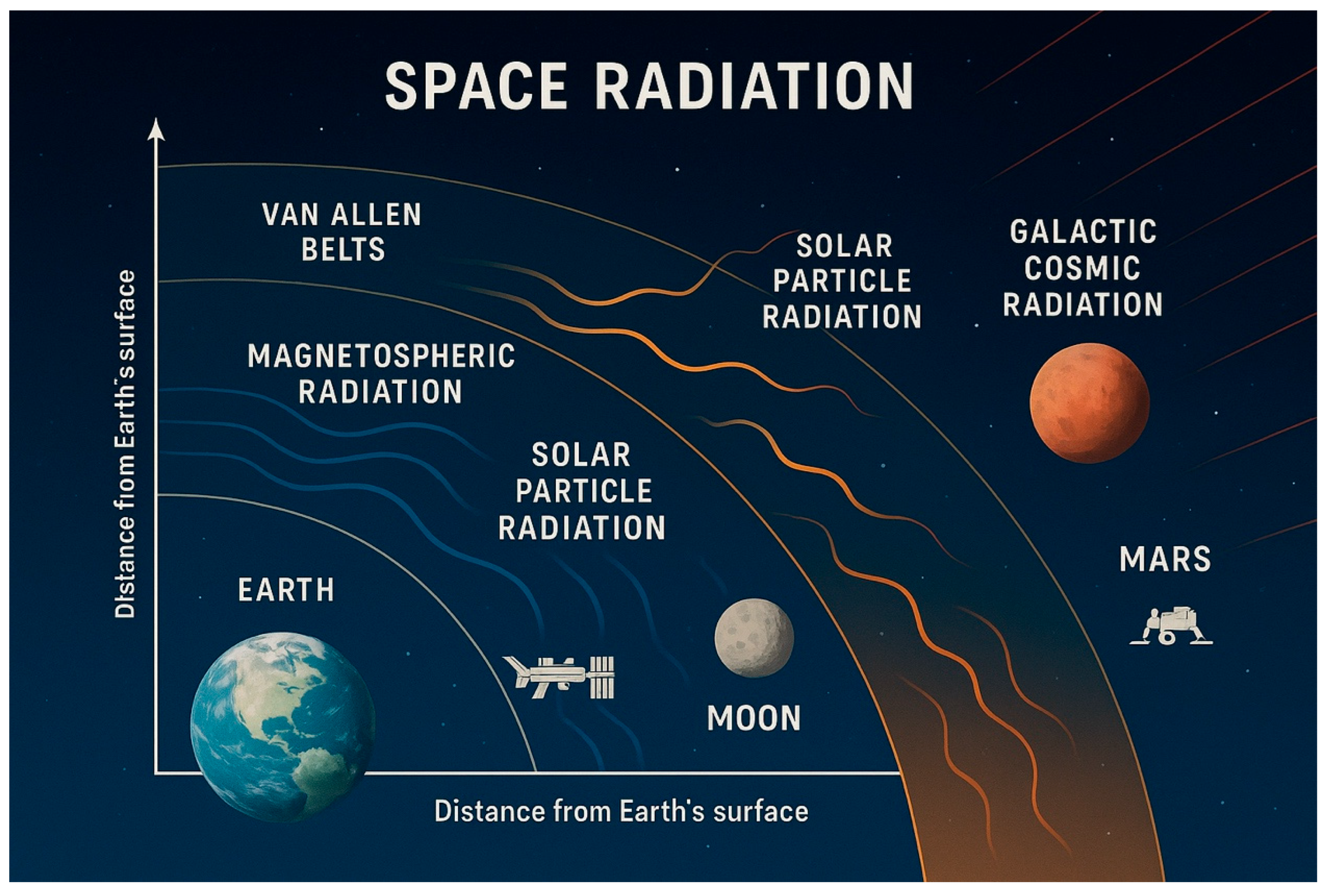
| Radiation Type | Distance from Earth | Main Particles | Exposed Missions | Biological Concern |
|---|---|---|---|---|
| Van Allen Belts | 1000–60,000 km | Protons, electrons | GEO satellites, Apollo, Artemis (transit) | Acute/prolonged exposure risk |
| Solar Particle Events | >60,000 km and interplanetary | High-energy protons | Moon, Mars, deep-space missions | Acute, unpredictable exposure |
| Galactic Cosmic Rays | >60,000 km to interstellar space | Protons, heavy ions | All beyond LEO missions (ISS partially) | Chronic exposure, high LET damage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, L.; Bordin, L.; Sabbadin, C.; Ambrosini, G.; Andrisani, A. Beyond Earth, Beyond Time: Preserving Female Fertility in Space Missions. J. Clin. Med. 2025, 14, 5975. https://doi.org/10.3390/jcm14175975
Marin L, Bordin L, Sabbadin C, Ambrosini G, Andrisani A. Beyond Earth, Beyond Time: Preserving Female Fertility in Space Missions. Journal of Clinical Medicine. 2025; 14(17):5975. https://doi.org/10.3390/jcm14175975
Chicago/Turabian StyleMarin, Loris, Luciana Bordin, Chiara Sabbadin, Guido Ambrosini, and Alessandra Andrisani. 2025. "Beyond Earth, Beyond Time: Preserving Female Fertility in Space Missions" Journal of Clinical Medicine 14, no. 17: 5975. https://doi.org/10.3390/jcm14175975
APA StyleMarin, L., Bordin, L., Sabbadin, C., Ambrosini, G., & Andrisani, A. (2025). Beyond Earth, Beyond Time: Preserving Female Fertility in Space Missions. Journal of Clinical Medicine, 14(17), 5975. https://doi.org/10.3390/jcm14175975








