Sport-Based Exercise in Pediatric Acquired Brain Injury: Protocol for a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
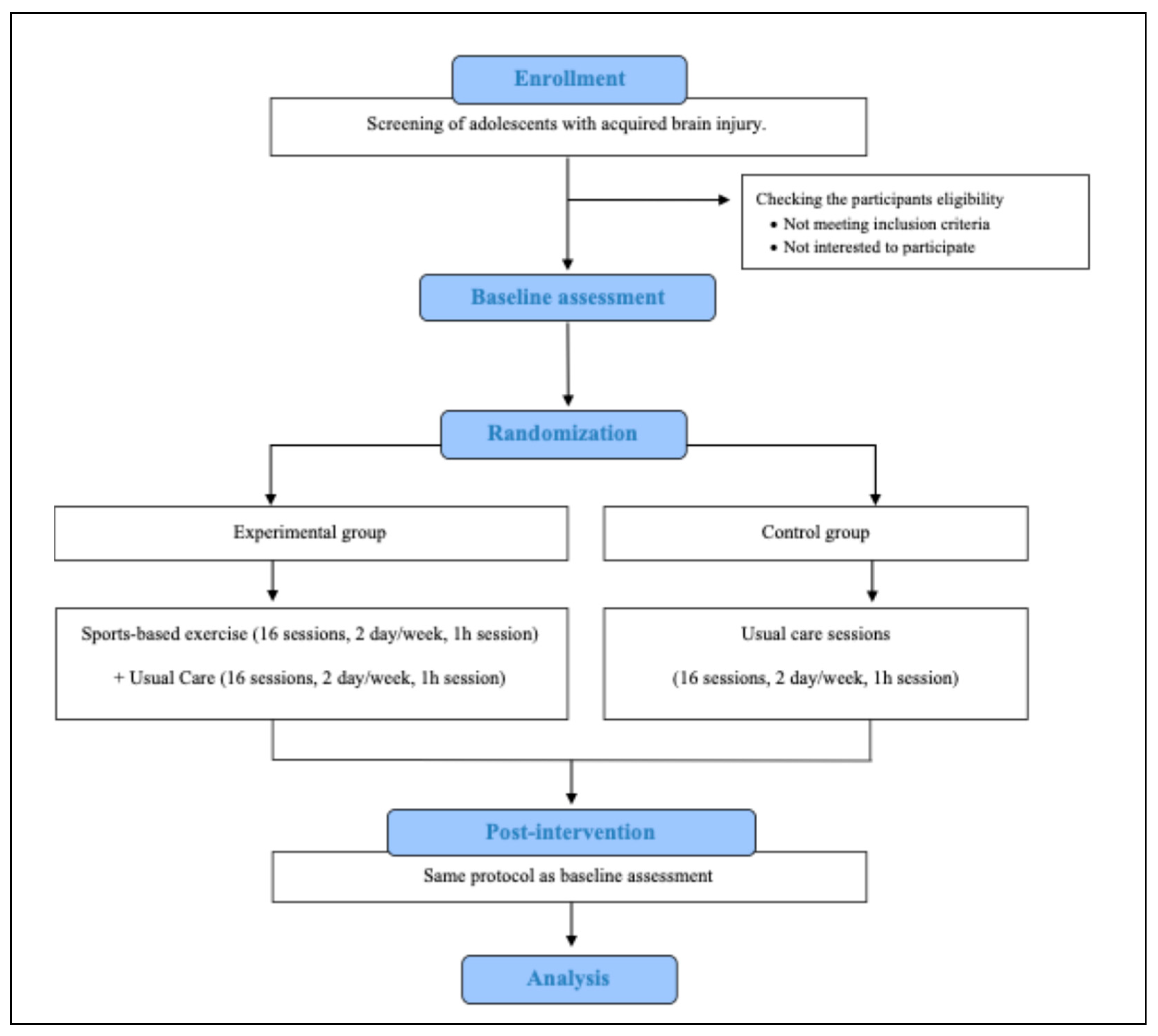
2.1. Trial Design and Setting
2.2. Eligibility and Recruitment
2.3. Allocation and Blinding
2.4. Interventions
Sport-Based Exercise and Usual Care (Experimental Group)
Usual Care (Control Group)
2.5. Harms
2.6. Outcome Measures
2.6.1. Sociodemographic, Clinical, and Anthropometric Data
2.6.2. Pediatric Quality of Life Inventory (PedsQL)
2.6.3. Child and Adolescent Scale of Participation (CASP)
2.6.4. Global Physical Activity Questionnaire (GPAQ)
2.6.5. Bruininks–Oseretsky Test of Motor Proficiency, Second Edition (BOT-2)
2.7. Study Timeline
2.8. Sample Size
2.9. Data Management and Monitoring
2.10. Statistical Methods
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohrer-Baumgartner, N.; Laberg Holthe, I.; Svendsen, E.J.; Dahl, H.M.; Borgen, I.M.H.; Hauger, S.L.; Thulesius, M.S.; Wade, S.L.; Røe, C.; Løvstad, M. Children and families with chronic pediatric acquired brain injury in need of rehabilitation: Characteristics and main challenges in daily life. Disabil. Rehabil. 2025, 47, 1543–1552. [Google Scholar] [CrossRef]
- Castellanos-Pinedo, F.; Cid-Gala, M.; Duque, P.; Ramírez-Moreno, J.; Zurdo-Hernández, J. Acquired brain injury: A proposal for its definition, diagnostic criteria and classification. Rev. Neurol. 2012, 54, 357–366. [Google Scholar]
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, e551–e567. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Neurological Disorders: Public Health Challenges; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. The International Classification Functioning, Disability and Health: ICF; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Lassaletta, A.; Bouffet, E.; Mabbott, D.; Kulkarni, A.V. Functional and neuropsychological late outcomes in posterior fossa tumors in children. Childs Nerv. Syst. 2015, 31, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.C.; Revill, G.; Blackman, G.; Shaikh, M.; Bell, V. Pediatric traumatic brain injury as a risk factor for psychosis and psychotic symptoms: A systematic review and meta-analysis. Psychol. Med. 2024, 54, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Bushmeneva, K.; Zagorski, B.; Colantonio, A.; Parsons, D.; Wodchis, W.P. Direct cost associated with acquired brain injury in Ontario. BMC Neurol. 2012, 12, 76. [Google Scholar] [CrossRef]
- Rimmer, J. Use of the ICF in identifying factors that impact participation in physical activity/rehabilitation among people with disabilities. Disabil. Rehabil. 2006, 15, 1087–1095. [Google Scholar] [CrossRef]
- Rimmer, J.; Lai, B. Framing new pathways in transformative exercise for individuals with existing and newly acquired disability. Disabil. Rehabil. 2015, 39, 173–180. [Google Scholar] [CrossRef]
- Karloh, M.; Barbosa, G.B.; Matias, T.S. The Unifying Theory of Physical Activity: A promising holistic perspective for physiotherapy and rehabilitation. Physiotherapy 2023, 120, 36–37. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; García-Hernández, J.; García-Gómez, S.; Pérez-Tejero, J. Exercise for people with acquired brain injury: An ICF perspective. Appl. Sci. 2022, 12, 3862. [Google Scholar] [CrossRef]
- Khaleqi-Sohi, M.; Sadria, G.; Ghalibafian, M.; Khademi-Kalantari, K.; Irannejad, S. The Effects of Physical Activity and Exercise Therapy on Pediatric Brain Tumor Survivors: A systematic review. J. Bodyw. Mov. Ther. 2022, 30, 1–9. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Gutiérrez-Suárez, A.; Arias, J.Á.R.; Andreu-Caravaca, L.; Pérez-Tejero, J. Effects of Exercise Programs on Functional Capacity and Quality of Life in People with Acquired Brain Injury: A Systematic Review and Meta-Analysis. Phys. Ther. 2022, 103, pzac153. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; García-Gómez, S.; Coterón, J.; García-Hernández, J.J.; Pérez-Tejero, J. Physical Activity and Sport for Acquired Brain Injury (PASABI): A Non-Randomized Controlled Trial. Medicina 2021, 57, 122. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Suárez, A.; Pérez-Rodríguez, M.; Silva-José, C.; Rodríguez-Romero, B. Effectiveness of an Exercise Therapy Program Based on Sports in Adults with Acquired Brain Injury: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2025, 106, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.; Franzén, E.; Johansson, A. A Pilot Study of the Feasibility and Effects of Table Tennis Training in Parkinson Disease. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100064. [Google Scholar] [CrossRef]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang Hear Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Cox, E.; Bells, S.; Timmons, B.W.; Laughlin, S.; Bouffet, E.; de Medeiros, C.; Beera, K.; Harasym, D.; Mabbott, D.J. A controlled clinical crossover trial of exercise training to improve cognition and neural communication in pediatric brain tumor survivors. Clin. Neurophysiol. 2020, 131, 1533–1547. [Google Scholar] [CrossRef]
- El-Sayes, J.; Harasym, D.; Turco, C.V.; Locke, M.B.; Nelson, A.J. Exercise-induced neuroplasticity: A mechanistic model and prospects for promoting plasticity. Neuroscientist 2019, 25, 65–85. [Google Scholar] [CrossRef]
- West, K.; Hassett, L.; Oliveira, J.S.; Kwok, W.S.S.; Geerts, M.; Gilchrist, H.; Gilbert, S.; Anderson, R.; Dario, A.B.; Robertson, G.J.; et al. Effects of sport and physical recreation on health-related outcomes among children and young people with physical disability: Systematic review with meta-analysis. BMJ Open Sport Exerc. Med. 2025, 11, e002350. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, G.L.; Auld, M.L.; Johnston, L.M. SPORTS STARS study protocol: A randomised, controlled trial of the effectiveness of a physiotherapist-led modified sport intervention for ambulant school-aged children with cerebral palsy. BMC Pediatr. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Lankhorst, K.; Takken, T.; Zwinkels, M.; van Gaalen, L.; Velde, S.T.; Backx, F.; Verschuren, O.; Wittink, H.; de Groot, J. Sports Participation, Physical Activity, and Health-Related Fitness in Youth with Chronic Diseases or Physical Disabilities: The Health in Adapted Youth Sports Study. J. Strength Cond. Res. 2021, 35, 2327–2337. [Google Scholar] [CrossRef]
- Zwinkels, M.; Verschuren, O.; Balemans, A.; Lankhorst, K.; Te Velde, S.; van Gaalen, L.; de Groot, J.; Visser-Meily, A.; Takken, T. Effects of a School-Based Sports Program on Physical Fitness, Physical Activity, and Cardiometabolic Health in Youth with Physical Disabilities: Data From the Sport-2-Stay-Fit Study. Front. Pediatr. 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 8, 346–7586. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Rode, C.A. The PedsQL: Measurement model for the pediatric quality of life inventory. Med. Care 1999, 37, 126–139. [Google Scholar] [CrossRef]
- Bedell, G. Further validation of the Child and Adolescent Scale of Participation (CASP). Dev. Neurorehabil. 2009, 12, 342–351. [Google Scholar] [CrossRef]
- Chu, A.H.Y.; Ng, S.H.X.; Koh, D.; Müller-Riemenschneider, F.; Brucki, S. Reliability and validity of the self- and interviewer- administered versions of the global physical activity questionnaire (GPAQ). PLoS ONE 2015, 10, e0136944. [Google Scholar] [CrossRef]
- Deitz, J.C.; Kartin, D.; Kopp, K. Review of the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition (BOT-2). Phys. Occup. Ther. Pediatr. 2007, 27, 87–102. [Google Scholar] [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Berrin, S.J.; Sherman, S.A.; Artavia, K.; Malcarne, V.L.; Chambers, H.G. The PedsQL in pediatric cerebral palsy: Reliability, validity, and sensitivity of the Generic Core Scales and Cerebral Palsy Module. Dev. Med. Child Neurol. 2006, 48, 442–449. [Google Scholar] [CrossRef]
- Christine Resch, C.; Van Kruijsbergen, M.; Ketelaar, M.; Hurks, P.; Adair, B.; Imms, C.; De Kloet, A.; Piskur, B.; Van Heugten, C. Assessing participation of children with acquired brain injury and cerebral palsy: A systematic review of measurement properties. Dev. Med. Child Neurol. 2020, 62, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Chiarello, L.A.; King, G.A.; Novak, I.; Stoner, T.; Fiss, A.L. Participation-based therapy for children with physical disabilities. Disabil. Rehabil. 2012, 34, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Allonsius, F.; de Kloet, A.; Bedell, G.; Van Markus-Doornbosch, F.; Rosema, S.; Meesters, J.; Vliet Vlieland, T.; Van der Holst, M. Participation restrictions in youth with ABI in pediatric rehabilitation. Int. J. Environ. Res. Public Health 2021, 18, 1625. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, O.; Peterson, M.D.; Balemans, A.C.J.; Hurvitz, E.A. Exercise and physical activity recommendations for people with cerebral palsy. Dev. Med. Child Neurol. 2016, 58, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, M.A.T.; Backx, F.J.G.; Takken, T.; Wittink, H.; Benner, J.; Mollema, J.; De Groot, J.F. Factors associated with physical activity in children and adolescents with a physical disability: A systematic review. Dev. Med. Child Neurol. 2015, 57, 137–148. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Be aged <18 years at study entry. | Do not have a completed and signed informed consent form. |
| Have a medical diagnosis of acquired brain injury in the subacute or chronic stage. | Have medical comorbidities that contraindicate safe exercise (e.g., cardiac or respiratory instability, and uncontrolled seizures). |
| Be able to understand simple instructions from the program. | Failure to cooperate during the preliminary tests of the program. |
| Items | Description of Items |
|---|---|
| Name of the intervention for the experimental/comparison group | Experimental group: sport-based exercise program and usual care sessions. Control group: usual care sessions. |
| Rationale | |
| Materials used in the intervention | Experimental group: The program is centered around different sports disciplines, which are adapted to participants’ functional condition, as well as different generic equipment, such as balls, chairs, tables, and platforms, among others. |
| Intervention procedures | Experimental group: The content of this program is designed around a sport-based framework incorporating motor patterns and task demands from various sports disciplines. It uses a circuit format to promote functional movement, progressive task complexity, and social interaction in small groups tailored by age and functional ability. Group composition will consider motor proficiency, cognitive, and behavioral profiles to balance skill levels and promote peer support. Strategies such as individualized task modification, use of visual and verbal cues, and structured routines will support engagement. Control group: Usual care encompasses a range of therapies (e.g., occupational therapy, physiotherapy, psychological support) provided by the rehabilitation services within the center. Typical frequency is 2–3 sessions per week, mainly individual or small group-based, and does not routinely include sport-based or structured group exercise. Following the post-intervention assessment, participants from this control group can participate in the same program sessions upon request. |
| Provider | Experimental group: The program will be delivered by a professional with expertise in neurological disorders and adapted physical exercise. Control group: Usual care for this group will be provided by professionals from the rehabilitation center. |
| Mode of intervention delivery | Experimental group: Presential, group-based sessions (from two to four participants). Control group: Presential, individual sessions. |
| Setting of intervention | Screening, interventions, and assessments will be conducted at the pediatric hospital, located in Madrid, Spain. |
| Dosage | Experimental group: Participants from this group will receive a 3-month exercise program which consists of sixteen sessions, 1 h session/day for 2 days/week. Control group: This group will continue with their usual care as usual 2 days/week, over 3 months, and then will be reassessed. |
| Tailoring | The session’s content will be continuously tailored and adapted by a research team to meet the individual needs and functional levels of each participant, thereby ensuring precise execution and security. |
| Modifications | Not applicable. |
| Fidelity assessment | The team responsible for delivering and managing the intervention will conduct a phone follow-up every 2 weeks, as well as other strategies to monitor the adherence and quality of the intervention (session attendance, fidelity checklists). Additionally, this professional will track the progress of participants from the control group during these follow-up calls. |
| Study Period | |||
|---|---|---|---|
| Allocation | Baseline | Completion | |
| ENROLLMENT: | |||
| Eligibility screen |  | ||
| Informed Consent |  | ||
| INTERVENTIONS: | |||
| Sports-based exercise + Usual Care |  | ||
| Usual care |  | ||
| ASSESSMENTS: | |||
| Sociodemographic, anthropometric, and comorbidity data |  | ||
| Health-related quality of life (PedsQL) |  |  | |
| Physical Activity (GPAQ) |  |  | |
| Participation (CASP) |  |  | |
| Motor functioning (BOT-2) |  |  | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Suárez, A.; Pérez-Rodríguez, M.; Castrillo, A.; Pérez-Tejero, J. Sport-Based Exercise in Pediatric Acquired Brain Injury: Protocol for a Randomized Controlled Trial. J. Clin. Med. 2025, 14, 5970. https://doi.org/10.3390/jcm14175970
Gutiérrez-Suárez A, Pérez-Rodríguez M, Castrillo A, Pérez-Tejero J. Sport-Based Exercise in Pediatric Acquired Brain Injury: Protocol for a Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(17):5970. https://doi.org/10.3390/jcm14175970
Chicago/Turabian StyleGutiérrez-Suárez, Andrea, Marta Pérez-Rodríguez, Agurtzane Castrillo, and Javier Pérez-Tejero. 2025. "Sport-Based Exercise in Pediatric Acquired Brain Injury: Protocol for a Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 17: 5970. https://doi.org/10.3390/jcm14175970
APA StyleGutiérrez-Suárez, A., Pérez-Rodríguez, M., Castrillo, A., & Pérez-Tejero, J. (2025). Sport-Based Exercise in Pediatric Acquired Brain Injury: Protocol for a Randomized Controlled Trial. Journal of Clinical Medicine, 14(17), 5970. https://doi.org/10.3390/jcm14175970





