Visual Function in Alzheimer’s Disease: Current Understanding and Potential Mechanisms Behind Visual Impairment
Abstract
1. Introduction
2. Visual Information Processing
3. Visual Acuity
4. Contrast Sensitivity
5. Color Vision
6. Visual Field
7. Ocular Motility
8. Visual Perception and Stereopsis
9. Limitations and Future Directions
10. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Beta-amyloid peptides |
| AD | Alzheimer’s disease |
| AMD | Age-related macular degeneration |
| aROC | Area under the receiver operator characteristic |
| APOE | Apolipoprotein E |
| APP | Amyloid precursor protein |
| CNS | Central nervous system |
| CS | Contrast sensitivity |
| CSF | Cerebrospinal fluid |
| DPT | Digital Perception Test |
| EMA | European Medicines Agency |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| FDA | Food and Drug Administration |
| FDT | Frequency Doubling Technology |
| FH+ | Familiar history positive |
| 18F-FDG-PET | Fluorine-18 fluorodeoxyglucose positron emission tomography |
| LGN | Lateral geniculate nucleus |
| MCI | Mild cognitive impairment |
| MMSE | Mini-Mental State Examination |
| M pathway | Magnocellular pathway |
| MoCA | Montreal Cognitive Assessment |
| MRI | Magnetic Resonance Imaging |
| MT1 | Melatonin receptor 1a |
| NFT | Neurofibrillary tangles |
| OCT | Optical Coherence Tomography |
| P pathway | Parvocellular pathway |
| PET | Positron emission tomography |
| PIB-PET | Pittsburgh compound B positron emission tomography |
| PSEN 1 | Presenilin 1 |
| PSEN 2 | Presenilin 2 |
| RCG | Retinal ganglion cells |
| RNFL | Retinal nerve fiber layer |
| ROS | Reactive oxygen species |
| V1 | Primary visual cortex |
| V2 | Secondary visual cortex |
| VA | Visual acuity |
| VF | Visual field |
References
- Gale, S.A.; Acar, D.; Daffner, K.R. Dementia. Am. J. Med. 2018, 131, 1161–1169. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Soria López, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wang, M.; Sharp, S.J.; Au Yeung, S.L.; Luo, S.; Jang, H.; Jiesisibieke, Z.L.; Shi, Q.; Chen, Z.; Brage, S. Incidence of Dementia and Alzheimer’s Disease, Genetic Susceptibility, and Grip Strength Among Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad224. [Google Scholar] [CrossRef]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 107. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Picanco, L.C.; Ozela, P.F.; de Fátima de Brito Brito, M.; Pinheiro, A.A.; Padilha, E.C.; Braga, F.S.; de Paula da Silva, C.H.T.; Dos Santos, C.B.R.; Rosa, J.M.C.; da Silva Hage-Melim, L.I. Alzheimer’s Disease: A Review from the Pathophysiology to Diagnosis, New Perspectives for Pharmacological Treatment. Curr. Med. Chem. 2018, 25, 3141–3159. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Silva, M.V.F.; Loures, C.M.G.; Alves, L.C.V.; De Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hong, F.; Yang, S. Amyloidosis in Alzheimer’s Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules 2022, 27, 1210. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- El-Haddad, G.; Alavi, A.; Mavi, A.; Bural, G.; Zhuang, H. Normal variants in [18F]-fluorodeoxyglucose PET imaging. Radiol. Clin. N. Am. 2004, 42, 1063–1081. [Google Scholar] [CrossRef]
- Romaus-Sanjurjo, D.; Regueiro, U.; López-López, M.; Vázquez-Vázquez, L.; Ouro, A.; Lema, I.; Sobrino, T. Alzheimer’s Disease Seen through the Eye: Ocular Alterations and Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 2486. [Google Scholar] [CrossRef]
- Dubois, B.; von Arnim, C.A.F.; Burnie, N.; Bozeat, S.; Cummings, J. Biomarkers in Alzheimer’s disease: Role in early and differential diagnosis and recognition of atypical variants. Alzheimer’s Res. Ther. 2023, 15, 175. [Google Scholar] [CrossRef]
- Arroyo-Pacheco, N.; Sarmiento-Blanco, S.; Vergara-Cadavid, G.; Castro-Leones, M.; Contreras-Puentes, N. Monoclonal therapy with Lecanemab in the treatment of mild Alzheimer’s disease: A systematic review and meta-analysis. Ageing Res. Rev. 2025, 104, 102620. [Google Scholar] [CrossRef]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef]
- Cohen, S.; van Dyck, C.H.; Gee, M.; Doherty, T.; Kanekiyo, M.; Dhadda, S.; Li, D.; Hersch, S.; Irizarry, M.; Kramer, L.D. Lecanemab Clarity AD: Quality-of-life Results from a Randomized, Double-Blind Phase 3 Trial in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2023, 10, 771–777. [Google Scholar] [CrossRef]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Mahaman, Y.A.R.; Embaye, K.S.; Huang, F.; Li, L.; Zhu, F.; Wang, J.Z.; Liu, R.; Feng, J.; Wang, X. Biomarkers used in Alzheimer’s disease diagnosis, treatment, and prevention. Ageing Res. Rev. 2022, 74, 101544. [Google Scholar] [CrossRef]
- Fereshetian, S.; Agranat, J.S.; Siegel, N.; Ness, S.; Stein, T.D.; Subramanian, M.L. Protein and Imaging Biomarkers in the Eye for Early Detection of Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2021, 5, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Marwick, B.; Yu, H.; Fadavi, H.; Tavakoli, M. Ophthalmic Biomarkers for Alzheimer’s Disease: A Review. Front. Aging Neurosci. 2021, 13, 720167. [Google Scholar] [CrossRef]
- Singh, A.; Verma, S. Use of ocular biomarkers as a potential tool for early diagnosis of Alzheimer’s disease. Indian J. Ophthalmol. 2020, 68, 555–561. [Google Scholar] [CrossRef]
- Losada, J.; Zarranz, J.J. Neurooftalmología. In Neurología, 5th ed.; Zarranz, J.J., Ed.; Elsevier: Barcelona, Spain, 2013; pp. 71–91. [Google Scholar]
- González, F. El sistema visual. In Fisiología Humana, 3rd ed.; Femenía, R., Sánchez, C., Eds.; McGraw Hill: Madrid, Spain, 2005; pp. 200–216. [Google Scholar]
- Yoonessi, A.; Yoonessi, A. Functional Assessment of Magno, Parvo and Konio-Cellular Pathways; Current State and Future Clinical Applications. J. Ophthalmic Vis. Res. 2011, 6, 119–126. [Google Scholar] [PubMed]
- Kim, N.G.; Lee, H.W. Stereoscopic Depth Perception and Visuospatial Dysfunction in Alzheimer’s Disease. Healthcare 2021, 9, 157. [Google Scholar] [CrossRef]
- Fernández, M.; Zarranz, J.J. Demencias. In Neurología, 5th ed.; Zarranz, J.J., Ed.; Elsevier: Barcelona, Spain, 2013; pp. 609–635. [Google Scholar]
- La Morgia, C.; Di Vito, L.; Carelli, V.; Carbonelli, M. Patterns of Retinal Ganglion Cell Damage in Neurodegenerative Disorders: Parvocellular vs Magnocellular Degeneration in Optical Coherence Tomography Studies. Front. Neurol. 2017, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yang, X.; Yang, H.; Sun, Z. Decreased coherence in the model of the dorsal visual pathway associated with Alzheimer’s disease. Sci. Rep. 2023, 13, 3495. [Google Scholar] [CrossRef]
- Rizzo, M.; Anderson, S.W.; Dawson, J.; Nawrot, M. Vision and cognition in Alzheimer’s disease. Neuropsychologia 2000, 38, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Polo, V.; Rodrigo, M.J.; Garcia-Martin, E.; Otin, S.; Larrosa, J.M.; Fuertes, M.I.; Bambo, M.P.; Pablo, L.E.; Satue, M. Visual dysfunction and its correlation with retinal changes in patients with Alzheimer’s disease. Eye 2017, 31, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Salobrar-García, E.; De Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Rojas, P.; Vazirani, R.; Amarante, C.; Yubero, R.; Gil, P.; Pinazo-Durán, M.D.; et al. Changes in visual function and retinal structure in the progression of Alzheimer’s disease. PLoS ONE 2019, 14, e0220535. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Hurtado, L.; López-Cuenca, I.; de Hoz, R.; Salas, M.; Sánchez-Puebla, L.; Ramírez-Toraño, F.; Matamoros, J.A.; Fernández-Albarral, J.A.; Rojas, P.; Alfonsín, S.; et al. Alzheimer’s disease: A continuum with visual involvements. Front. Psychol. 2023, 14, 1124830. [Google Scholar] [CrossRef]
- Nolan, J.M.; Loskutova, E.; Howard, A.N.; Moran, R.; Mulcahy, R.; Stack, J.; Bolger, M.; Dennison, J.; Akuffo, K.O.; Owens, N.; et al. Macular pigment, visual function, and macular disease among subjects with Alzheimer’s disease: An exploratory study. J. Alzheimer’s Dis. 2014, 42, 1191–1202. [Google Scholar] [CrossRef]
- Cormack, F.K.; Tovee, M.; Ballard, C. Contrast sensitivity and visual acuity in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2000, 15, 614–620. [Google Scholar] [CrossRef]
- Dhapola, R.; Sharma, P.; Kumari, S.; Vellingiri, B.; Medhi, B.; HariKrishnaReddy, D. Exploring Retinal Neurodegeneration in Alzheimer’s Disease: A Molecular and Cellular Perspective. Neurotox. Res. 2025, 43, 22. [Google Scholar] [CrossRef]
- Ramírez, A.I.; de Hoz, R.; Salobrar-García, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef]
- Rodríguez-Arellano, J.J.; Parpura, V.; Zorec, R.; Verkhratsky, A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 2016, 323, 170–182. [Google Scholar] [CrossRef]
- Hutton, J.T.; Morris, J.L.; Elias, J.W.; Poston, J.N. Contrast sensitivity dysfunction in Alzheimer’s disease. Neurology 1993, 43, 2328–2330. [Google Scholar] [CrossRef]
- Schlotterer, G.; Moscovitch, M.; Crapper-McLachlan, D. Visual processing deficits as assessed by spatial frequency contrast sensitivity and backward masking in normal ageing and Alzheimer’s disease. Brain 1984, 107, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Neargarder, S.A.; Stone, E.R.; Cronin-Golomb, A.; Oross, S. The Impact of acuity on performance of four clinical measures of contrast sensitivity in Alzheimer’s disease. J. Gerontol. B Psychol. Sci. Soc. Sci. 2003, 58, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.; Bandelow, S.; Hogervorst, E. Visual impairment in Alzheimer’s disease: A critical review. J. Alzheimer’s Dis. 2010, 21, 15–34. [Google Scholar] [CrossRef]
- He, X.F.; Liu, Y.T.; Peng, C.; Zhang, F.; Zhuang, S.; Zhang, J.S. Optical coherence tomography assessed retinal nerve fiber layer thickness in patients with Alzheimer’s disease: A meta-analysis. Int. J. Ophthalmol. 2012, 5, 401–405. [Google Scholar]
- Risacher, S.L.; WuDunn, D.; Tallman, E.F.; West, J.D.; Gao, S.; Farlow, M.R.; Brosch, J.R.; Apostolova, L.G.; Saykin, A.J. Visual contrast sensitivity is associated with the presence of cerebral amyloid and tau deposition. Brain Commun. 2020, 2, fcaa019. [Google Scholar] [CrossRef] [PubMed]
- Pache, M.; Smeets, C.H.W.; Fontana Gasio, P.; Savaskan, E.; Flammer, J.; Wirz-Justice, A.; Kaiser, H.J. Colour vision deficiencies in Alzheimer’s disease. Age Ageing. 2003, 32, 422–426. [Google Scholar] [CrossRef]
- Bassi, C.J.; Solomon, K.; Young, D. Vision in aging and dementia. Optom. Vis. Sci. 1993, 70, 809–813. [Google Scholar] [CrossRef]
- Wijk, H.; Sivik, L. Some aspects of colour perception among patients with Alzheimer’s disease. Scand. J. Caring Sci. 1995, 9, 3–9. [Google Scholar] [CrossRef]
- Salobrar-Garcia, E.; De Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Yubero, R.; Gil, P.; Triviño, A.; Ramirez, J. Ophthalmologic Psychophysical Tests Support OCT Findings in Mild Alzheimer’s Disease. J. Ophthalmol. 2015, 2015, 736949. [Google Scholar] [CrossRef]
- Vidal, K.S.M.; Decleva, D.; Barboni, M.T.S.; Nagy, B.V.; De Menezes, P.A.H.; Aher, A.; Coutinho, A.M.; Squarzoni, P.; Faria, D.P.; Duran, F.L.S.; et al. The Association Between Acquired Color Deficiency and PET Imaging of Neurodegeneration in Mild Cognitive Impairment and Alzheimer Disease. Investig. Ophthalmol. Vis. Sci. 2022, 63, 20. [Google Scholar] [CrossRef]
- Brewer, A.A.; Barton, B. Visual cortex in aging and Alzheimer’s disease: Changes in visual field maps and population receptive fields. Front. Psychol. 2014, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Wirz-Justice, A.; Olivieri, G.; Pache, M.; Kräuchi, K.; Brydon, L.; Jockers, R.; Müller-Spahn, F.; Meyer, P. Distribution of Melatonin MT1 Receptor Immunoreactivity in Human Retina. J. Histochem. Cytochem. 2002, 50, 519–526. [Google Scholar] [CrossRef]
- Banc, A.; Kedar, S. Interpretation of the Visual Field in Neuro-ophthalmic Disorders. Curr. Neurol. Neurosci. Rep. 2024, 24, 67–81. [Google Scholar] [CrossRef]
- Pelak, V.S.; Hills, W. Vision in Alzheimer’s disease: A focus on the anterior afferent pathway. Neurodegener. Dis. Manag. 2018, 8, 49–67. [Google Scholar] [CrossRef]
- Trick, G.L.; Trick, L.R.; Morris, P.; Wolf, M. Visual field loss in senile dementia of the Alzheimer’s type. Neurology 1995, 45, 68–74. [Google Scholar] [CrossRef]
- Steffes, R.; Thralow, J. Visual Field Limitation in the Patient with Dementia of the Alzheimer’s Type. J. Am. Geriatr. Soc. 1987, 35, 198–204. [Google Scholar] [CrossRef]
- Valenti, D.A. Alzheimer’s Disease: Screening Biomarkers Using Frequency Doubling Technology Visual Field. ISRN Neurol. 2013, 2013, 989583. [Google Scholar] [CrossRef]
- Aykan, U.; Akdemir, M.O.; Yildirim, O.; Varlibas, F. Screening for Patients with Mild Alzheimer Disease Using Frequency Doubling Technology Perimetry. Neuroophthalmology 2013, 37, 239–246. [Google Scholar] [CrossRef]
- Cesareo, M.; Martucci, A.; Ciuffoletti, E.; Mancino, R.; Cerulli, A.; Sorge, R.P.; Martorana, A.; Sancesario, G.; Nucci, C. Association between Alzheimer’s Disease and Glaucoma: A Study Based on Heidelberg Retinal Tomography and Frequency Doubling Technology Perimetry. Front. Neurosci. 2015, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Visual Field Defects in Alzheimer’s Disease Patients May Reflect Differential Pathology in the Primary Visual Cortex. Optom. Vis. Sci. 1996, 73, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhang, W.; Jiao, B.; Yang, Q.; Zhang, X.; Chen, R.; Wang, X.; Xiao, X.; Zhu, Y.; Liao, W.; et al. Correlation between retinal structure and brain multimodal magnetic resonance imaging in patients with Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1088829. [Google Scholar] [CrossRef]
- Sekar, A.; Panouillères, M.T.N.; Kaski, D. Detecting Abnormal Eye Movements in Patients with Neurodegenerative Diseases—Current Insights. Eye Brain 2024, 16, 3–16. [Google Scholar] [CrossRef]
- Qi, J.; Lian, T.; Guo, P.; He, M.; Li, J.; Li, J.; Luo, D.; Zhang, Y.; Huang, Y.; Liu, G.; et al. Abnormal eye movements: Relationship with clinical symptoms and predictive value for Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1471698. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Cui, L.; Du, Y.; Liu, X.; Wang, C.; Zhao, J.; Qiao, H.; Li, Z.; Dong, M. Analysis of eye movement features in patients with Alzheimer’s disease based on intelligent eye movement analysis and evaluation system. J. Alzheimer’s Dis. 2024, 102, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.V.Z.A.; Pais, M.V.; Bellan, A.F.R.; Tahira, A.C.; Dos Santos, B.; Sant’Ana, L.C.F.G.; Radanovic, M.; Forlenza, O.V. Impact of Cognitive Demand on Eye Movement Pattern in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 85–95. [Google Scholar] [CrossRef]
- Javaid, F.Z.; Brenton, J.; Guo, L.; Cordeiro, M.F. Visual and ocular manifestations of Alzheimer’s disease and their use as biomarkers for diagnosis and progression. Front. Neurol. 2016, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.A.; Granada, J.; Fernández, G. Memory-driven eye movements prospectively predict dementia in people at risk of Alzheimer’s disease. Alzheimer’s Dement. 2022, 14, e12386. [Google Scholar] [CrossRef]
- Hannonen, S.; Andberg, S.; Kärkkäinen, V.; Rusanen, M.; Lehtola, J.M.; Saari, T.; Korhonen, V.; Hokkanen, L.; Hallikainen, M.; Hänninen, T.; et al. Shortening of Saccades as a Possible Easy-to-Use Biomarker to Detect Risk of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 88, 609–618. [Google Scholar] [CrossRef]
- Ma, X.T.; Yao, L.L.; Liu, S.W.; Weng, X.F.; Bao, R.Y.; Yang, Y.F.; Li, Y.F.; Sun, Y.Y.; Xu, D.; Jia, Z.Y.; et al. The link between eye movements and cognitive function in mild to moderate Alzheimer’s disease. Exp. Brain Res. 2025, 243, 39. [Google Scholar] [CrossRef]
- Opwonya, J.; Doan, D.N.T.; Kim, S.G.; Kim, J.I.; Ku, B.; Kim, S.; Park, S.; Kim, J.U. Saccadic Eye Movement in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2022, 32, 193–227. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, H.; Siéroff, É. Visual Perceptual Disorders in Alzheimer’s Disease. Geriatr. Psychol. Neuropsychiatr. Vieil. 2019, 17, 307–316. [Google Scholar]
- Lee, C.N.; Ko, D.; Suh, Y.W.; Park, K.W. Cognitive functions and stereopsis in patients with Parkinson’s disease and Alzheimer’s disease using 3-dimensional television: A case controlled trial. PLoS ONE 2015, 10, e1023229. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.P.; Murphy, D.F.; Noad, R.F. The Utility of Visual and Spatial Perception Tests in Alzheimer’s Disease: A Systematic Review. Dement. Geriatr. Cogn. Disord. 2023, 52, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lemos, R.; Figueiredo, P.; Santana, I.; Simões, M.R.; Castelo-Branco, M. Temporal integration of 3D coherent motion cues defining visual objects of unknown orientation is impaired in amnestic mil cognitive impairment and Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 28, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Rami, L.; Serradell, M.; Bosch, B.; Villar, A.; Molinuevo, J. Perception Digital Test (PDT) for the assessment of incipient visual disorder in initial Alzheimer’s disease. Neurología 2007, 22, 342–347. [Google Scholar]
- Giannakopoulos, P.; Gold, G.; Duc, M.; Michel, J.P.; Hof, P.R.; Bouras, C. Neuroanatomic correlates of visual agnosia in Alzheimer’s disease A clinicopathologic study. Neurology 1999, 52, 71–77. [Google Scholar] [CrossRef]
- Josephs, K.A.; Josephs, K.A. Prosopagnosia: Face blindness and its association with neurological disorders. Brain Commun. 2024, 6, fcae002. [Google Scholar] [CrossRef]
- Kalló, G.; Emri, M.; Varga, Z.; Ujhelyi, B.; Tozsér, J.; Csutak, A.; Csősz, É. Changes in the chemical barrier composition of tears in Alzheimer’s disease reveal potential tear diagnostic biomarkers. PLoS ONE 2016, 11, e0158000. [Google Scholar] [CrossRef]
- Gharbiya, M.; Visioli, G.; Trebbastoni, A.; Albanese, G.M.; Colardo, M.; D’Antonio, F.; Segatto, M.; Lambiase, A. Beta-Amyloid Peptide in Tears: An Early Diagnostic Marker of Alzheimer’s Disease Correlated with Choroidal Thickness. Int. J. Mol. Sci. 2023, 24, 2590. [Google Scholar] [CrossRef]
- Gijs, M.; Ramakers, I.H.G.B.; Visser, P.J.; Verhey, F.R.J.; van de Waarenburg, M.P.H.; Schalkwijk, C.G.; Nuijts, R.M.M.A.; Webers, C.A.B. Association of tear fluid amyloid and tau levels with disease severity and neurodegeneration. Sci. Rep. 2021, 11, 22675. [Google Scholar] [CrossRef]
- Kenny, A.; Jiménez-Mateos, E.M.; Zea-Sevilla, M.A.; Rábano, A.; Gili-Manzanaro, P.; Prehn, J.H.M.; Henshall, D.C.; Ávila, J.; Engel, T.; Hernández, F. Proteins and microRNAs are differentially expressed in tear fluid from patients with Alzheimer’s disease. Sci. Rep. 2019, 9, 15437. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, V.; Hannonen, S.; Rusanen, M.; Lehtola, J.M.; Saari, T.; Uusitalo, H.; Leinonen, V.; Thiede, B.; Kaarniranta, K.; Koivisto, A.M.; et al. Tear fluid reflects the altered protein expressions of Alzheimer’s disease patients in proteins involved in protein repair and clearance system or the regulation of cytoskeleton. J. Alzheimer’s Dis. 2024, 18. [Google Scholar] [CrossRef] [PubMed]
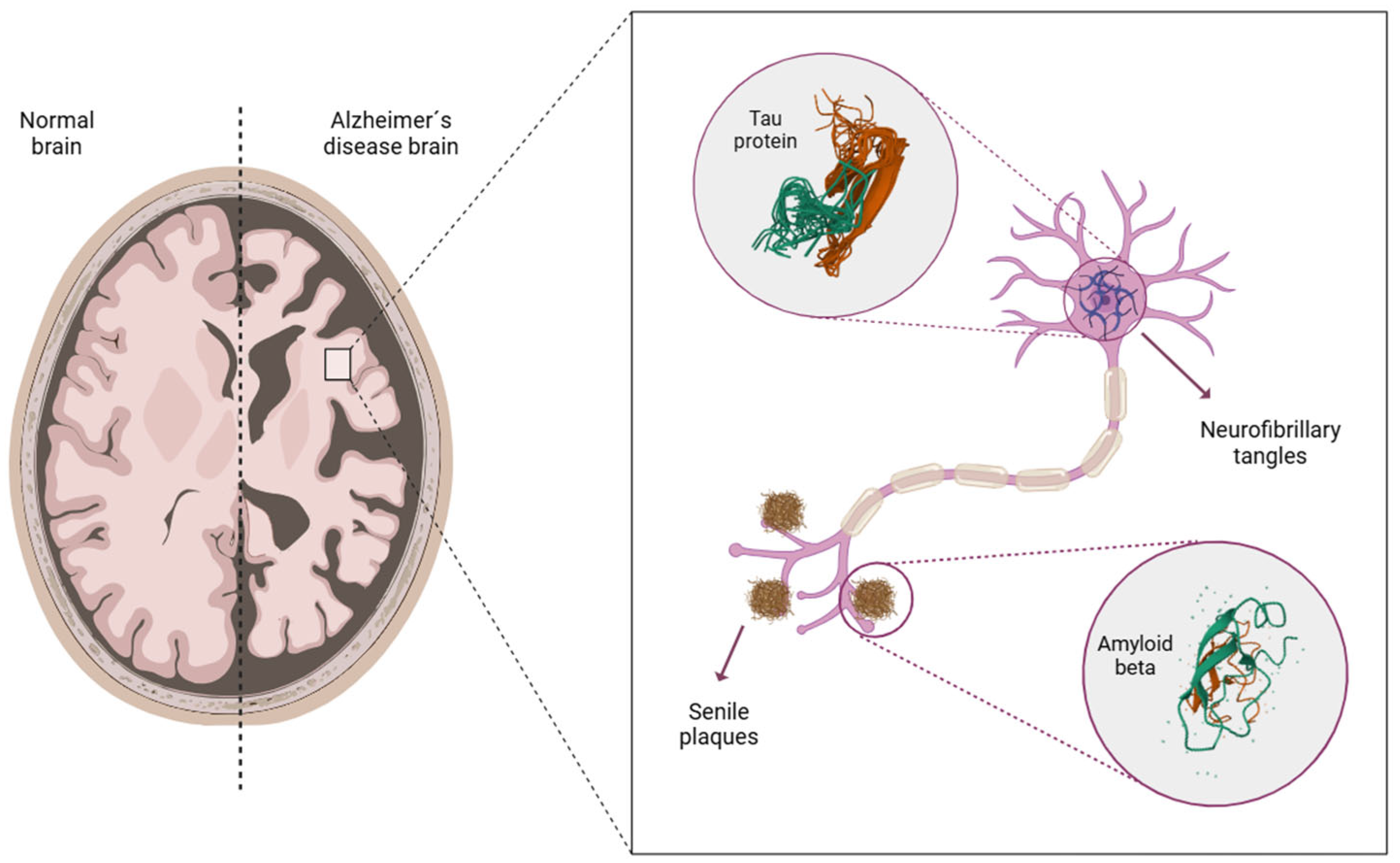
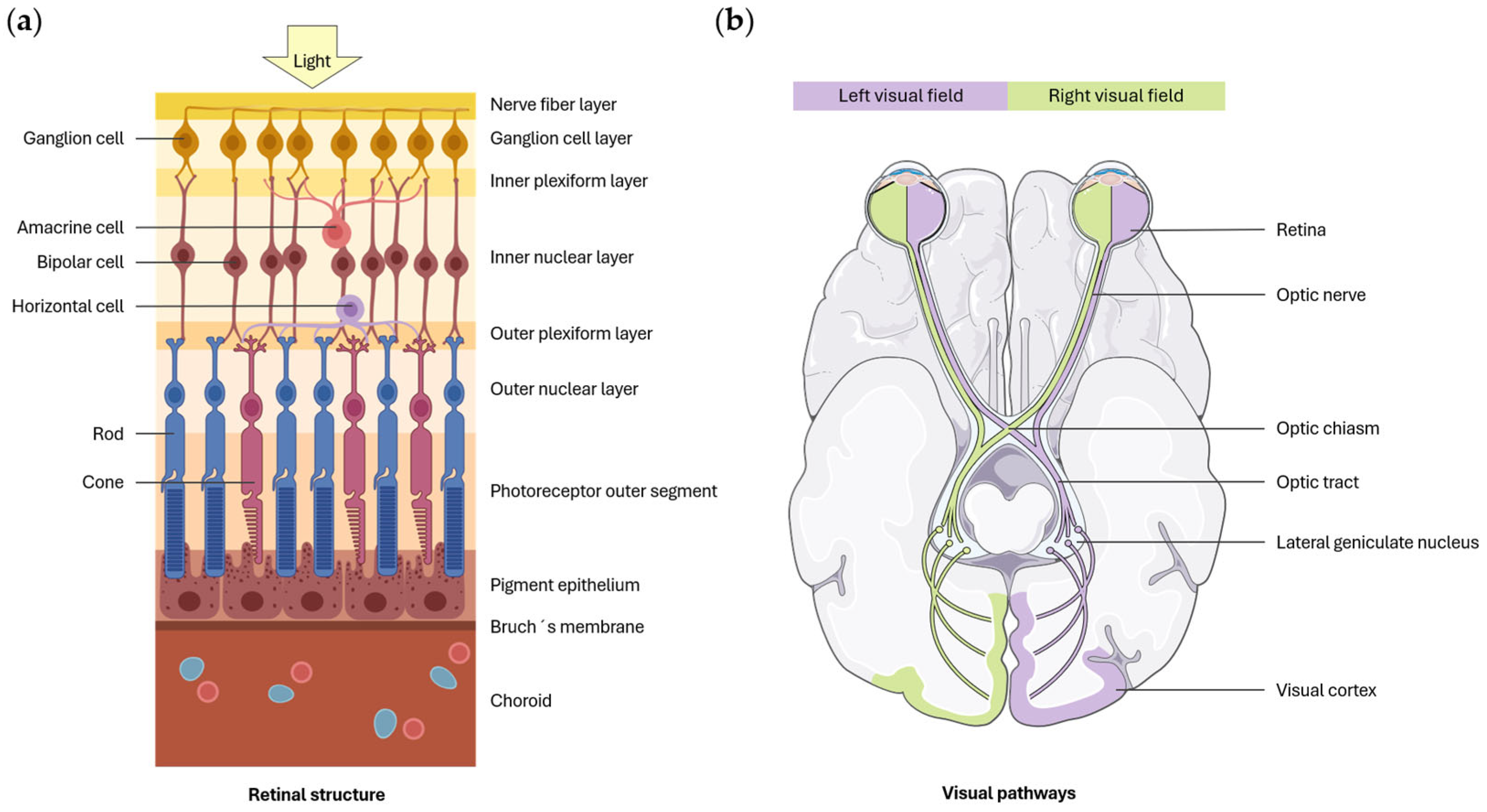
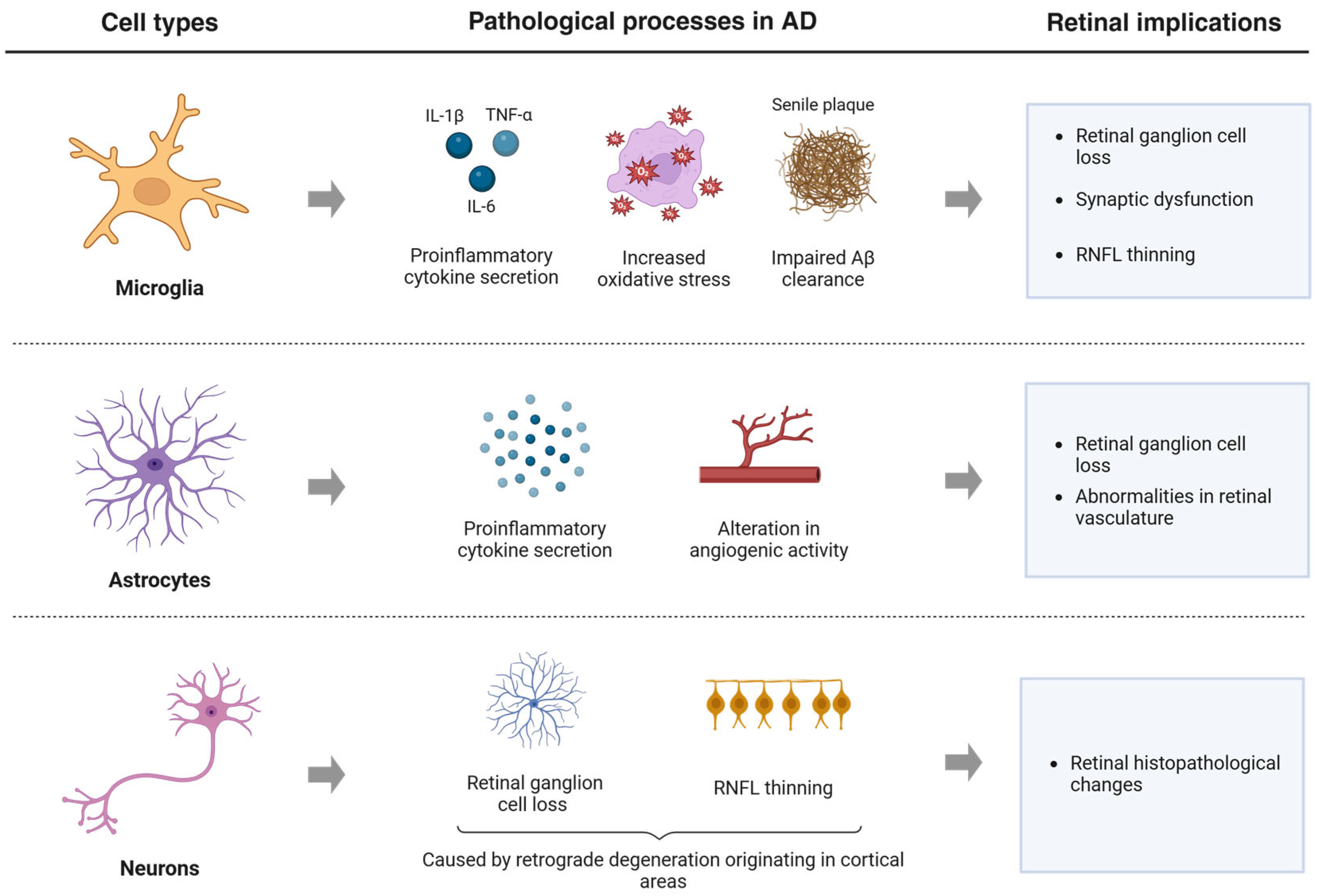

| Study | Sample Size | Test | Main Findings |
|---|---|---|---|
| Rizzo et al. [34] | 22 controls 43 mild AD | Sloan letters | No significant differences between groups. |
| Polo et al. [35] | 24 controls 24 mild-moderate AD | LogMAR ETDRS | No significant differences between groups. Correlation between VA and macular and RNFL thickness. No correlation between VA and MMSE. |
| Salobrar-García et al. [36] | 40 controls 39 mild AD 21 moderate AD | Snellen | Significant decrease in VA in patients with mild and moderate AD compared to controls. High predictive value of the VA test. Correlation between VA and MMSE. |
| Elvira-Hurtado et al. [37] | 53 controls 13 FH+ 23 MCI 25 mild AD 21 moderate AD | Snellen | Significant decrease in VA in patients with MCI, mild AD and moderate AD compared to controls. No significant differences between FH+ group and controls. High predictive value of the AV test. Correlation between VA and MMSE. |
| Nolan et al. [38] | 33 controls 36 moderate AD | LogMAR ETDRS | Significant decrease in VA in patients with moderate AD compared to controls |
| Study | Sample Size | Test | Main Findings |
|---|---|---|---|
| Polo et al. [35] | 24 controls 24 mild-moderate AD | Pelli-Robson CSV-1000E | Significant reduction in CS in AD patients with Pelli-Robson test. Significant reduction in CS in AD patients at lower spatial frequencies (3, 6, 12 cpd) with CSV-1000E test. Correlation between CS and macular and RNFL thickness. No correlation between CS and MMSE. |
| Salobrar-García et al. [36] | 40 controls 39 mild AD 21 moderate AD | CSV-1000E | Significant decrease in CS in patients with mild and moderate AD at all the spatial frequencies tested (3, 6, 12, 18 cpd). High predictive value of CS test, especially at high spatial frequencies. Correlation between CS and MMSE. |
| Elvira-Hurtado et al. [37] | 53 controls 13 FH+ 23 MCI 25 mild AD 21 moderate AD | CSV-1000E | Significant differences in higher spatial frequencies (6, 12, 18 cpd) of CS between the controls and AD groups. Differences between MCI and controls only at 12 and 18 cpd. High predictive value of CS test, especially at high spatial frequencies. Correlation between CS and MMSE. |
| Nolan et al. [38] | 33 controls 36 moderate AD | LogMAR ETDRS | Significant decrease in CS in patients with moderate AD at all spatial frequencies. |
| Hutton et al. [43] | 6 controls 6 mild-moderate AD | Computerized contrast sensitivity system | Significant decrease in CS in patients with mild-moderate AD at high spatial frequencies. |
| Schlotterer et al. [44] | 11 young controls 11 elderly controls 10 AD | - | No significant differences between the elderly controls and AD patients. |
| Study | Sample Size | Test | Main Findings |
|---|---|---|---|
| Polo et al. [35] | 24 controls 24 mild-moderate AD | Color Vision Recorder (Farnsworth and L’Anthony) | Significant impairment of color vision in patients with AD. Correlation between color vision and RNFL and macular parameters in isolated sectors. No correlation between color vision and MMSE. |
| Salobrar-García et al. [36] | 40 controls 39 mild AD 21 moderate AD | Roth 28-Hue | Higher number of total nonspecific, tritan axis and deutan axis errors in patients with mild and moderate AD. High predictive value of color vision test. Correlation between color vision test errors and MMSE. |
| Elvira-Hurtado et al. [37] | 53 controls 13 FH+ 23 MCI 25 mild AD 21 moderate AD | Farnsworth 28-Hue | Higher number of total nonspecific, tritan axis and deutan axis errors in AD patients. No significant differences between FH+ group and controls. High predictive value of the color vision test. Correlation between color vision test errors and MMSE. |
| Pache et al. [49] | 25 controls 26 mild-severe AD | Ishihara PV-16 | Higher number of total nonspecific errors in AD patients. No correlation between color vision and severity of the disease. |
| Bassi et al. [50] | 10 young controls 11 age-matched controls 10 probable AD 10 other dementias | L’Anthony D-15 | No significant differences between patients with AD and age-matched controls. |
| Wijk et al. [51] | 12 controls 12 AD | NCS color order system | No significant differences between groups. |
| Salobrar-García et al. [52] | 28 controls 23 mild-AD | Roth 28-Hue | Higher number of tritan nonspecific errors in AD patients. High predictive value of the tritan errors. Correlation between tritan errors and MMSE. |
| Vidal et al. [53] | 18 controls 23 MCI 13 AD | Cambridge Color Test | Poorer color vision in AD patients along the protan, deutan, and tritan axes compared to controls. |
| Study | Sample Size | Test | Main Findings |
|---|---|---|---|
| Qi et al. [61] | 42 controls 63 MCI due to AD 49 dementia due to AD | EyeKnow | Abnormalities in lateral fixation, prosaccades, antisaccades, and memory saccades in AD patients. Correlation between eye movement alterations and global cognition, various cognitive domains, and daily living activities. High predictive value of the eye movement alterations. |
| Tao et al. [62] | 20 controls 23 MCI 23 AD | EyeKnow | Alterations in lateral fixation, prosaccades, and antisaccades in AD patients. No differences in smooth pursuit movements. Correlation between some oculomotor parameters and cognitive function. High predictive value of the combination of lateral fixation and antisaccades. |
| Zuben et al. [63] | 26 controls 47 MCI 18 AD | Tobii TX300 | Differences in eye movement behavior between control subjects and patients with MCI and AD. |
| Parra et al. [65] | 42 controls 65 MCI | ViewMind | Differences in fixation and saccades between groups. Oculomotor behavior analysis can effectively predict the progression to AD. |
| Hannonen et al. [66] | 37 controls 20 MCI 21 mild AD | Tobii TX300 | Differences in saccades between control subjects and patients with MCI and AD. |
| Ma et al. [67] | 34 controls 80 mil-moderate AD | EyeKnow | Abnormalities in prosaccades and antisaccades in AD patients. No differences in pursuit and fixation tasks. Prosaccades and antisaccades were correlated with global cognitive function and specific cognitive domains. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvite-Piñeiro, T.; López-López, M.; Regueiro, U.; Pías-Peleteiro, J.M.; Sobrino, T.; Lema, I. Visual Function in Alzheimer’s Disease: Current Understanding and Potential Mechanisms Behind Visual Impairment. J. Clin. Med. 2025, 14, 5963. https://doi.org/10.3390/jcm14175963
Alvite-Piñeiro T, López-López M, Regueiro U, Pías-Peleteiro JM, Sobrino T, Lema I. Visual Function in Alzheimer’s Disease: Current Understanding and Potential Mechanisms Behind Visual Impairment. Journal of Clinical Medicine. 2025; 14(17):5963. https://doi.org/10.3390/jcm14175963
Chicago/Turabian StyleAlvite-Piñeiro, Tania, Maite López-López, Uxía Regueiro, Juan Manuel Pías-Peleteiro, Tomás Sobrino, and Isabel Lema. 2025. "Visual Function in Alzheimer’s Disease: Current Understanding and Potential Mechanisms Behind Visual Impairment" Journal of Clinical Medicine 14, no. 17: 5963. https://doi.org/10.3390/jcm14175963
APA StyleAlvite-Piñeiro, T., López-López, M., Regueiro, U., Pías-Peleteiro, J. M., Sobrino, T., & Lema, I. (2025). Visual Function in Alzheimer’s Disease: Current Understanding and Potential Mechanisms Behind Visual Impairment. Journal of Clinical Medicine, 14(17), 5963. https://doi.org/10.3390/jcm14175963







