Serum Levels of HMGB1, hS100A8/A9, and sRAGE in Patients with Knee and Hip Osteoarthritis: Inflammatory Biomarkers of Disease Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patient Characteristics
2.3. Samples
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. High-Mobility Group Box 1 (HMGB1)
4.2. Human sRAGE
4.3. Human hS100A8/9
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pettenuzzo, S.; Berardo, A.; Belluzzi, E.; Pozzuoli, A.; Ruggieri, P.; Carniel, E.L.; Fontanella, C.G. Mechanical insights into fat pads: A comparative study of infrapatellar and suprapatellar fat pads in osteoarthritis. Connect. Tissue Res. 2025, 66, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37, 3–6. [Google Scholar] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Santoni, G.; Cardinali, C.; Morelli, M.B.; Santoni, M.; Nabissi, M.; Amantini, C. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J. Neuroinflamm. 2015, 12, 21. [Google Scholar] [CrossRef]
- Rosenberg, J.H.; Rai, V.; Dilisio, M.F.; Sekundiak, T.D.; Agrawal, D.K. Increased expression of damage-associated molecular patterns (DAMPs) in osteoarthritis of human knee joint compared to hip joint. Mol. Cell. Biochem. 2017, 436, 59–69. [Google Scholar] [CrossRef]
- Bosch, M.H.v.D. Inflammation in osteoarthritis: Is it time to dampen the alarm(in) in this debilitating disease? Clin. Exp. Immunol. 2019, 19, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, C.; Lu, C.; Mo, C.; Zeng, J.; Yao, M.; Jia, B.; Liu, Z.; Yuan, P.; Xu, S. Age-related bone diseases: Role of inflammaging. J. Autoimmun. 2024, 143, 103169. [Google Scholar] [CrossRef]
- Millerand, M.; Berenbaum, F.; Jacques, C. Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 48–56. [Google Scholar]
- Taniguchi, N.; Kawakami, Y.; Maruyama, I.; Lotz, M. HMGB Proteins and Arthritis. Hum. Cell 2018, 31, 1–9. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Roth, J. Mechanisms of disease: A ‘DAMP’ view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. 2007, 3, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Mainardi, A.; Majumder, N.; Dönges, L.; Kumar, B.; Occhetta, P.; Martin, I.; Egloff, C.; Ghosh, S.; Bandyopadhyay, A.; et al. Chondrocyte Hypertrophy in Osteoarthritis: Mechanistic Studies and Models for the Identification of New Therapeutic Strategies. Cells 2022, 11, 4034. [Google Scholar] [CrossRef]
- Palumbo, A.; Atzeni, F.; Murdaca, G.; Gangemi, S. The Role of Alarmins in Osteoarthritis Pathogenesis: HMGB1, S100B and IL-33. Int. J. Mol. Sci. 2023, 24, 12143. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Hong, B.; Huang, G. Mechanism of high expression of high mobility group protein 1 in a rat model of knee osteoarthritis. J. South. Med. Univ. 2021, 41, 1142–1149. [Google Scholar] [CrossRef]
- Aulin, C.; Lassacher, T.; Palmblad, K.; Erlandsson Harris, H. Early Stage Blockade of the Alarmin HMGB1 Reduces Cartilage Destruction in Experimental OA. Osteoarthr. Cartil. 2020, 28, 698–707. [Google Scholar] [CrossRef]
- Li, Z.C.; Cheng, G.Q.; Hu, K.Z.; Li, M.Q.; Zang, W.P.; Dong, Y.Q.; Wang, W.L.; Liu, Z.D. Correlation of Synovial Fluid HMGB-1 Levels with Radiographic Severity of Knee Osteoarthritis. Clin. Investig. Med. 2011, 34, E298. [Google Scholar] [CrossRef]
- He, C.; Chen, C.; Jiang, X.; Hui, L.; Li-Xin, W.; Tao, X. The role of AGEs in pathogenesis of cartilage destruction in osteoarthritis. Bone Joint. Res. 2022, 11, 292–300. [Google Scholar] [CrossRef]
- MMengis, T.; Bernhard, L.; Nüesch, A.; Heggli, I.; Herger, N.; Devan, J.; Marcus, R.; Laux, C.J.; Brunner, F.; Farshad, M.; et al. Expression of toll-like receptors in cartilage endplates cells: A role of toll-like receptor 2 in pro-inflammatory and -catabolic gene expression. Cells 2024, 13, 1402. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Tayyib, N.A.; Ramaiah, P.; Alshahrani, S.H.; Margiana, R.; Almalki, S.G.; Kareem, A.K.; Zabibah, R.S.; Shbeer, A.M.; Ali, S.H.J.; Mustafa, Y.F. Soluble receptor for advanced glycation end products (sRAGE) is associated with obesity rates: A systematic review and meta-analysis of cross-sectional study. BMC Endocr. Disord. 2023, 23, 275. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. The Development of Millard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef] [PubMed]
- Erusalimsky, J.D. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. 2021, 42, 101958. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, N.; Ghorbani, M.; Shariatifar, H.; Farasat, A. In silico assessment of human Calprotectin subunits (S100A8/A9) in presence of sodium and calcium ions using Molecular Dynamics simulation approach. PLoS ONE 2019, 14, e0224095. [Google Scholar] [CrossRef]
- Berg, W.v.D. Osteoarthritis Year 2010 in Review: Pathomechanisms. Osteoarthr. Cartil. 2011, 19, 338–341. [Google Scholar] [CrossRef]
- Kang, K.Y.; Woo, J.W.; Park, S.H. S100A8/A9 as a biomarker for synovial inflammation and joint damage in patients with rheumatoid arthritis. Korean J. Intern. Med. 2014, 29, 12–19. [Google Scholar] [CrossRef] [PubMed]
- van Kooten, N.J.T.; Blom, A.B.; van Manen, I.J.T.; Theeuwes, W.F.; Roth, J.; Gorris, M.A.J.; Walgreen, B.; Sloetjes, A.W.; Helsen, M.M.; Vitters, E.L.; et al. S100A8/A9 drives monocytes towards M2-like macrophage differentiation and associates with M2-like macrophages in osteoarthritic synovium. Rheumatology 2025, 64, 332–343. [Google Scholar] [CrossRef] [PubMed]
- van Lent, P.L.E.M.; Blom, A.B.; Schelbergen, R.F.P.; Slöetjes, A.; Lafeber, F.P.J.G.; Lems, W.F.; Cats, H.; Vogl, T.; Roth, J.; Berg, W.B.v.D. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012, 64, 1466–1476. [Google Scholar] [CrossRef]
- Carrión, M.; Juarranz, Y.; Martínez, C.; González-Álvaro, I.; Pablos, J.L.; Gutiérrez-Cañas, I.; Gomariz, R.P. IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatology 2013, 52, 2177–2186. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Huang, W. Identification of S100A8 as a common diagnostic biomarkers and exploring potential pathogenesis for osteoarthritis and metabolic syndrome. Front. Immunol. 2023, 14, 1185275. [Google Scholar] [CrossRef]
- Xie, J.W.; Wang, Y.; Xiao, K.; Xu, H.; Luo, Z.Y.; Li, L.; Pei, F.X.; Kraus, V.B.; Huang, Z.Y. Alpha defensin-1 attenuates surgically induced osteoarthritis in association with promoting M1 to M2 macrophage polarization. Osteoarthr. Cartil. 2021, 29, 1048–1059. [Google Scholar] [CrossRef]
- Ma, C.A.; Leung, Y.Y. Exploring the Link between Uric Acid and Osteoarthritis. Front. Med. 2017, 4, 225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chan, Y.T.; Yung, P.S.H.; Tuan, R.S.; Jiang, Y. Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis. Front. Cell Dev. Biol. 2021, 8, 607764. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Haque, Q.S.; Tabrez, S.; Choi, I.; Ahmad, S. Biochemical and immunological parameters as indicators of osteoarthritis subjects: Role of OH-collagen in auto-antibodies generation. EXCLI J. 2015, 14, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Zappia, J.; Sanchez, C.; Florin, A.; Dubuc, J.E.; Henrotin, Y. The Damage-Associated Molecular Patterns (DAMPs) as Potential Targets to Treat Osteoarthritis: Perspectives from a Review of the Literature. Front. Med. 2021, 7, 607186. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef]
- Salonen, E.M. A rapid and sensitive solid-phase enzyme immunoassay for C-reactive protein. J. Immunol. Methods 1982, 48, 45–50. [Google Scholar] [CrossRef]
- Correia, L.C.; Lima, J.C.; Gerstenblith, G.; Magalhães, L.P.; Moreira, A.; Barbosa, O.; Dumet, J.; Passos, L.C.; D’Oliveira, J.A.; Esteves, J.P. Correlation between turbidimetric and nephelometric methods of measuring C-reactive protein in patients with unstable angina or non-ST elevation acute myocardial infarction. Arq. Bras. Cardiol. 2003, 81, 129–133. [Google Scholar] [CrossRef]
- Terada, C.; Yoshida, A.; Nasu, Y.; Mori, S.; Tomono, Y.; Tanaka, M.; Takahashi, H.K.; Nishibori, M.; Ozaki, T.; Nishida, K. Gene expression and localization of high-mobility group box chromosomal protein-1 (HMGB-1) in human osteoarthritic cartilage. Acta Med. Okayama 2011, 65, 369–377. [Google Scholar] [CrossRef]
- Jiang, L.; Rong, J.; Wang, Y.; Hu, F.; Bao, C.; Li, X.; Zhao, Y. The Relationship Between Body Mass Index and Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Jt. Bone Spine 2011, 78, 150–155. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, W.; Wang, Y.; Rong, J.; Bao, C.; Liu, Y.; Zhao, Y.; Wang, C. Body Mass Index and Susceptibility to Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Jt. Bone Spine 2012, 79, 291–297. [Google Scholar] [CrossRef]
- Ke, X.; Jin, G.; Yang, Y.; Cao, X.; Fang, R.; Feng, X.; Lei, B. Synovial Fluid HMGB-1 Levels Are Associated with Osteoarthritis Severity. Clin. Lab. 2015, 61, 809–818. [Google Scholar] [CrossRef]
- Shao, B.; Xu, Y.; Jia, M.; Li, C.-X.; Gong, Z.-C. Association of HMGB1 Levels in Synovial Fluid with the Severity of Temporomandibular Joint Osteoarthritis. BMC Musculoskelet. Disord. 2023, 24, 183. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Bingxia, M.; Lingli, D. The Role of HMGB1 in Rheumatic Diseases. Front. Immunol. 2022, 13, 815257. [Google Scholar] [CrossRef]
- Wenzhao, L.; Jiangdong, N.; Deye, S.; Muliang, D.; Junjie, W.; Xianzhe, H.; Mingming, Y.; Jun, H. Dual Regulatory Roles of HMGB1 in Inflammatory Reaction of Chondrocyte Cells and Mice. Cell Cycle 2019, 18, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Liu, Y.; Han, Y.; Wang, J. Expression and Significance of High-Mobility Group Protein B1 (HMGB1) and the Receptor for Advanced Glycation End-Product (RAGE) in Knee Osteoarthritis. Med. Sci. Monit. 2016, 22, 2105–2112. [Google Scholar] [CrossRef]
- Chayanupatkul, M.; Honsawek, S. Soluble receptor for advanced glycation end products (sRAGE) in plasma and synovial fluid is inversely associated with disease severity of knee osteoarthritis. Clin. Biochem. 2010, 43, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Schelbergen, R.F.P.; de Munter, W.; Bosch, M.H.J.v.D.; Lafeber, F.P.J.G.; Sloetjes, A.; Vogl, T.; Roth, J.; Berg, W.B.v.D.; van der Kraan, P.M.; Blom, A.B.; et al. Alarmins S100A8/S100A9 Aggravate Osteophyte Formation in Experimental Osteoarthritis and Predict Osteophyte Progression in Early Human Symptomatic Osteoarthritis. Ann. Rheum. Dis. 2016, 75, 218–225. [Google Scholar] [CrossRef]
- Blom, A.B.; Bosch, M.H.v.D.; Davidson, E.N.B.; Roth, J.; Vogl, T.; van de Loo, F.A.; Koenders, M.; van der Kraan, P.M.; Geven, E.J.; van Lent, P.L. The alarmins S100A8 and S100A9 mediate acute pain in experimental synovitis. Arthritis Res. Ther. 2020, 22, 199. [Google Scholar] [CrossRef]
- Mahler, E.; Zweers, M.; Van Lent, P.; Blom, A.; Van Den Hoogen, F.; Van Den Berg, W.; Roth, J.; Vogl, T.; Bijlsma, J.W.; van den Ende, C.H.; et al. Association between serum levels of the proinflammatory protein S100A8/A9 and clinical and structural characteristics of patients with established knee, hip, and hand osteoarthritis. Scand. J. Rheumatol. 2015, 44, 56–60. [Google Scholar] [CrossRef]
- Ruan, G.; Xu, J.; Wang, K.; Zheng, S.; Wu, J.; Ren, J.; Bian, F.; Chang, B.; Zhu, Z.; Han, W.; et al. Associations between serum S100A8/S100A9 and knee symptoms, joint structures and cartilage enzymes in patients with knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Poste, G. Bring on the biomarkers. Nature 2011, 469, 156–157. [Google Scholar] [CrossRef]
- Mischak, H.; Vlahou, A.; Ioannidis, J.P.A. Technical aspects and inter-laboratory variability in native peptide profiling: The CE-MS experience. Clin. Biochem. 2013, 46, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef]
- Lee, J.W.; Devanarayan, V.; Barrett, Y.C.; Weiner, R.; Allinson, J.; Fountain, S.; Keller, S.; Weinryb, I.; Green, M.; Duan, L.; et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm. Res. 2006, 23, 312–328. [Google Scholar] [CrossRef]
- Plebani, M. Errors in clinical laboratories or errors in laboratory medicine? Clin. Chem. Lab. Med. 2006, 44, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Alad, M.; Yousef, F.; Epure, L.M.; Lui, A.; Grant, M.P.; Merle, G.; Eliopoulos, N.; Barralet, J.; Antoniou, J.; Mwale, F. Unraveling Osteoarthritis: Mechanistic Insights and Emerging Therapies Targeting Pain and Inflammation. Biomolecules 2025, 15, 874. [Google Scholar] [CrossRef]
| Hip OA (M ± SD) | KOA (M ± SD) | p-Value | |
|---|---|---|---|
| n | 49 | 45 | - |
| Female/Male (n) | 27/22 | 26/19 | 0.60 |
| Age (range) | 51–75 | 51–74 | - |
| Age (years) | 63.00 ± 6.04 | 64.91 ± 5.74 | 0.73 |
| VAS pain | 4.93 ± 1.79 | 5.50 ± 2.37 | 0.07 |
| WOMAC pain | 9.84 ± 3.18 | 9.02 ± 4.68 | 0.41 |
| WOMAC stiffness | 4.08 ± 1.63 | 3.28 ± 2.00 | 0.10 |
| WOMAC function | 33.06 ± 11.95 | 31.40 ± 13.80 | 0.68 |
| Hip OA (M ± SD) | KOA (M ± SD) | p-Value | |
|---|---|---|---|
| ESR (mm/h) | 17.87 ± 9.84 | 19.25 ± 15.20 | 0.84 |
| CRP (mg/L) | 2.73 ± 1.93 | 7.10 ± 19.75 | 0.70 |
| Haematocrit (L/L) | 0.41 ± 0.04 | 0.42 ± 0.04 | 0.79 |
| Platelets × 109/L | 245.52 ± 61.61 | 260.95 ± 58.58 | 0.14 |
| Leukocytes × 109/L | 7.06 ± 2.48 | 7.21 ± 2.44 | 0.81 |
| Albumin (g/L) | 43.38 ± 4.31 | 48.89 ± 9.30 | 0.72 |
| Alpha 1 globulin (g/L) | 2.92 ± 0.43 | 5.45 ± 0.74 | <0.001 * |
| Alpha 2 globulin (g/L) | 4.35 ± 1.16 | 9.15 ± 1.88 | <0.001 * |
| Beta globulin (g/L) | 8.11 ± 1.05 | 11.66 ± 1.79 | <0.001 * |
| Gamma globulin (g/L) | 7.56 ± 1.91 | 13.41 ± 5.51 | <0.001 * |
| A/G ratio | 1.51 ± 0.21 | 1.42 ± 0.24 | 0.10 |
| Hip OA (M ± SD) | KOA (M ± SD) | p-Value | |
|---|---|---|---|
| sRAGE (ng/mL) | 566.76 ± 22.89 | 499.97 ± 18.71 | 0.84 |
| HMGB1 (ng/mL) | 7.11 ± 1.46 | 21.72 ± 3.50 | <0.001 * |
| hS100A8/A9 (ng/mL) | 664.88 ± 38.90 | 1227.06 ± 175.34 | <0.001 * |
| Hip OA (M ± SD) | Control (M ± SD) | p-Value | |
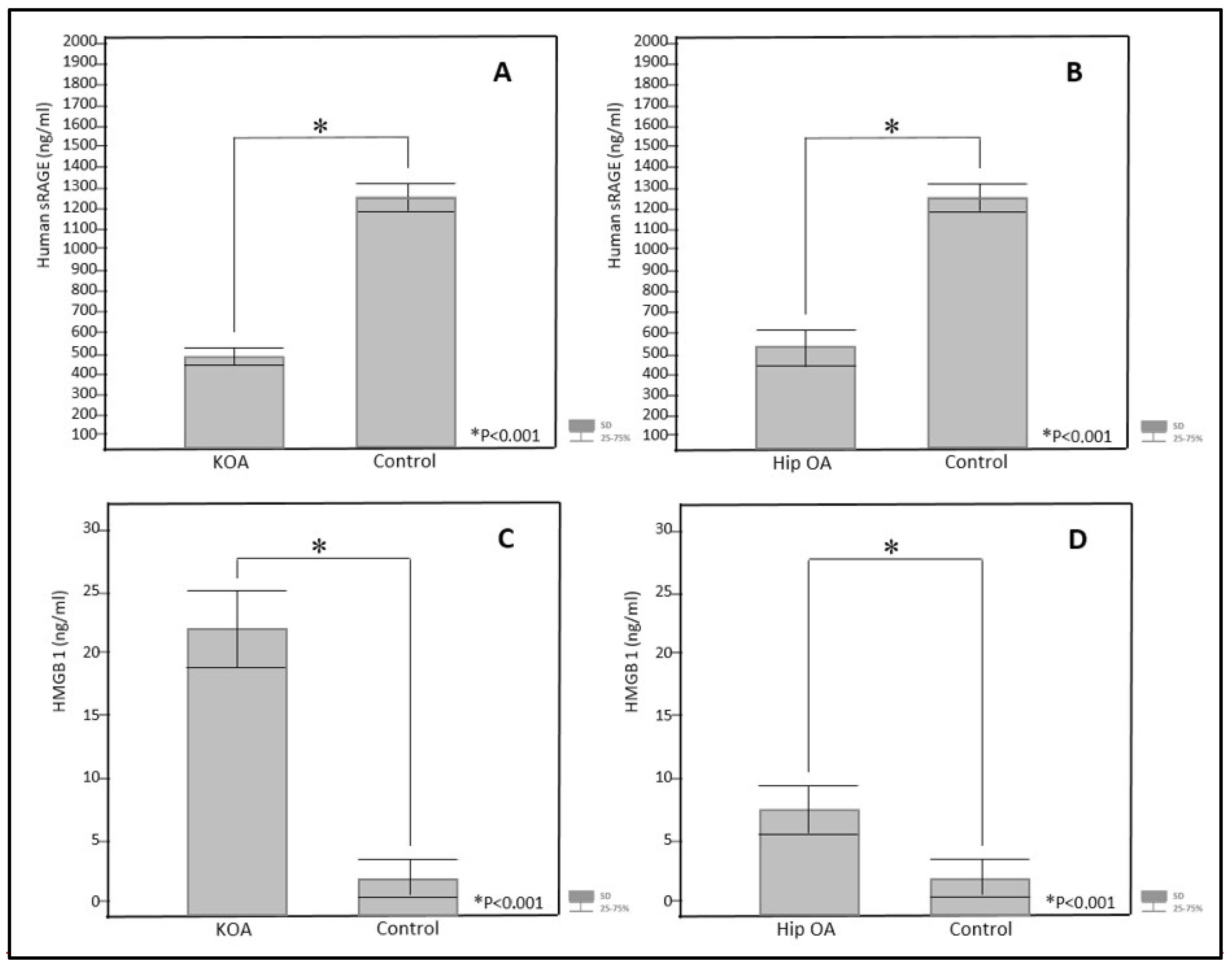
| sRAGE (ng/mL) | 566.76 ± 22.89 | 1273.87 ± 58.92 | <0.001 * |
| HMGB1 (ng/mL) | 7.11 ± 1.46 | 1.64 ± 0.80 | <0.001 * |
| hS100A8/A9 (ng/mL) | 664.88 ± 38.90 | 916.21 ± 123.13 | 0.24 |
| KOA (M ± SD) | Control (M ± SD) | p-Value | |
| sRAGE (ng/mL) | 499.97 ± 18.71 | 1273.8 ± 58.92 | <0.001 * |
| HMGB1 (ng/mL) | 21.72 ± 3.50 | 1.64 ± 0.80 | <0.001 * |
| hS100A8/A9 (ng/mL) | 1227.06 ± 175.34 | 916.21 ± 123.13 | 0.55 |
| Grade 1 n = 9 (M ± SD) | Grade 2 n = 13 (M ± SD) | Grade 3 n = 12 (M ± SD) | Grade 4 n = 11 (M ± SD) | ||
|---|---|---|---|---|---|
| HMGB1 ng/mL | HMGB1 ng/mL | HMGB1 ng/mL | HMGB1 ng/mL | p-Value | |
| KOA | 8.93 ± 1.32 | 18.64 ± 2.04 | 21.84 ± 2.68 | 35.68 ± 3.47 | <0.01 * |
| Hip OA | 5.51 ± 0.23 | 8.24 ± 1.06 | 11.46 ± 1.37 | 16.57 ± 2.71 | <0.01 * |
| hS100A8/A9 ng/mL | hS100A8/A9 ng/mL | hS100A8/A9 ng/mL | hS100A8/A9 ng/mL | p-Value | |
| KOA | 971.03 ± 124.71 | 832.8 ± 97.23 | 901.84 ± 98.69 | 1106.21 ± 134.77 | 0.86 |
| Hip OA | 498.81 ± 31.25 | 576.24 ± 87.42 | 621.21 ± 67.14 | 613.49 ± 97.87 | 0.72 |
| sRAGE ng/mL | sRAGE ng/mL | sRAGE ng/mL | sRAGE ng/mL | p-Value | |
| KOA | 369.69 ± 29.66 | 501.98 ± 39.41 | 498.84 ± 46.74 | 572.34 ± 21.39 | 0.81 |
| Hip OA | 481.81 ± 73.81 | 612.15 ± 46.28 | 591.76 ± 23.57 | 673.97 ± 31.15 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusac-Kukić, S.; Višnić, A.; Vidaković, M.R.; Bobek, D. Serum Levels of HMGB1, hS100A8/A9, and sRAGE in Patients with Knee and Hip Osteoarthritis: Inflammatory Biomarkers of Disease Activity. J. Clin. Med. 2025, 14, 5931. https://doi.org/10.3390/jcm14175931
Rusac-Kukić S, Višnić A, Vidaković MR, Bobek D. Serum Levels of HMGB1, hS100A8/A9, and sRAGE in Patients with Knee and Hip Osteoarthritis: Inflammatory Biomarkers of Disease Activity. Journal of Clinical Medicine. 2025; 14(17):5931. https://doi.org/10.3390/jcm14175931
Chicago/Turabian StyleRusac-Kukić, Sandra, Alenka Višnić, Maja Rogić Vidaković, and Dubravka Bobek. 2025. "Serum Levels of HMGB1, hS100A8/A9, and sRAGE in Patients with Knee and Hip Osteoarthritis: Inflammatory Biomarkers of Disease Activity" Journal of Clinical Medicine 14, no. 17: 5931. https://doi.org/10.3390/jcm14175931
APA StyleRusac-Kukić, S., Višnić, A., Vidaković, M. R., & Bobek, D. (2025). Serum Levels of HMGB1, hS100A8/A9, and sRAGE in Patients with Knee and Hip Osteoarthritis: Inflammatory Biomarkers of Disease Activity. Journal of Clinical Medicine, 14(17), 5931. https://doi.org/10.3390/jcm14175931






