The Impact of Early Mobilization on the Incidence of Intensive Care Unit-Acquired Weakness in Patients with Sepsis in the Critical Care—The Shinshu Multicenter Prospective Cohort Study (EROSCCS Study)
Abstract
1. Introduction
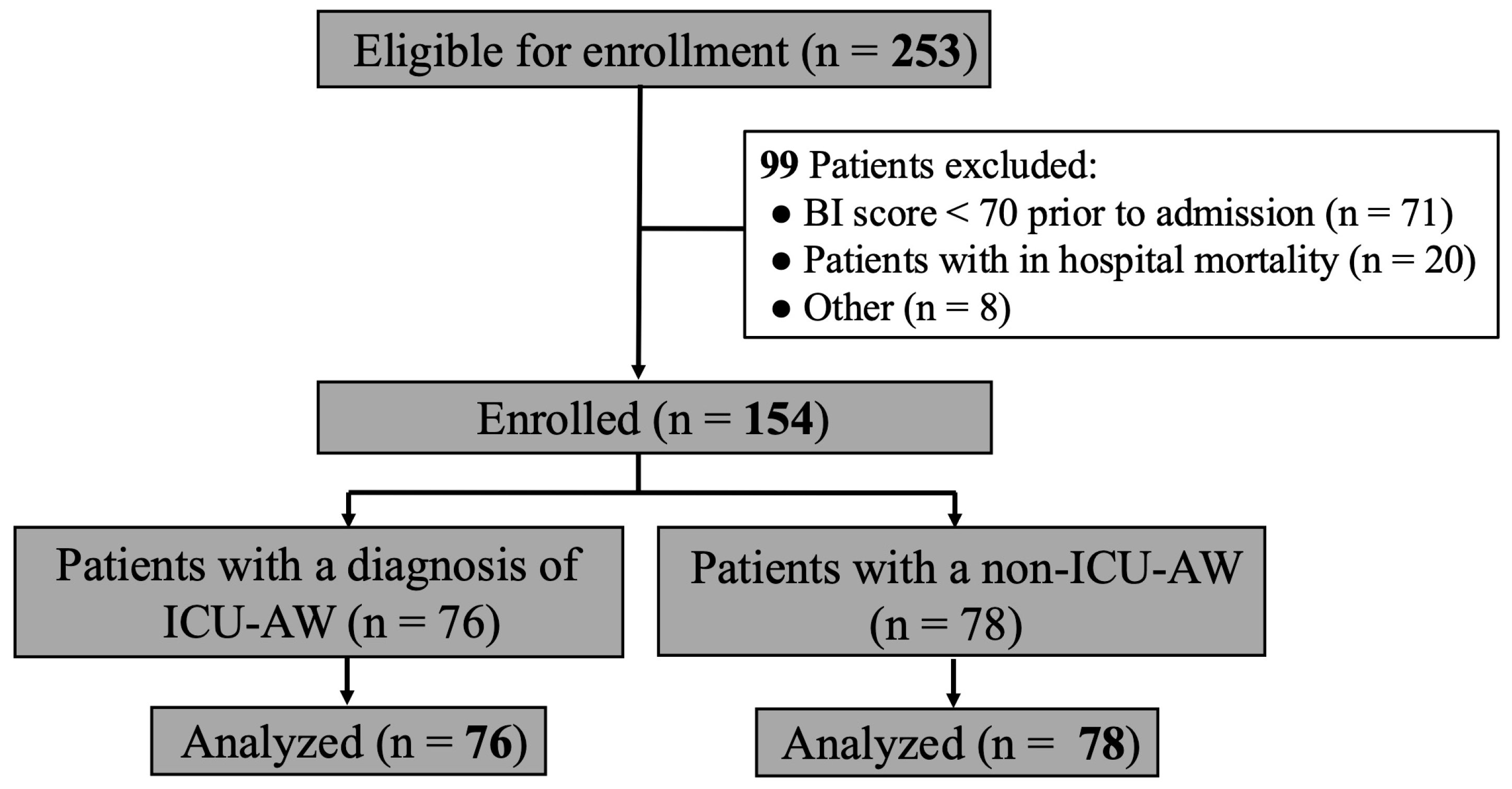
2. Materials and Methods
2.1. Study Population
2.2. Ethical Approval
2.3. Methods
2.4. Patient Data Collection During Hospitalization
2.5. Medical Research Council (MRC) Score Measurement and ICU-AW Definitions
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Study Patients
3.2. Outcome Measurement
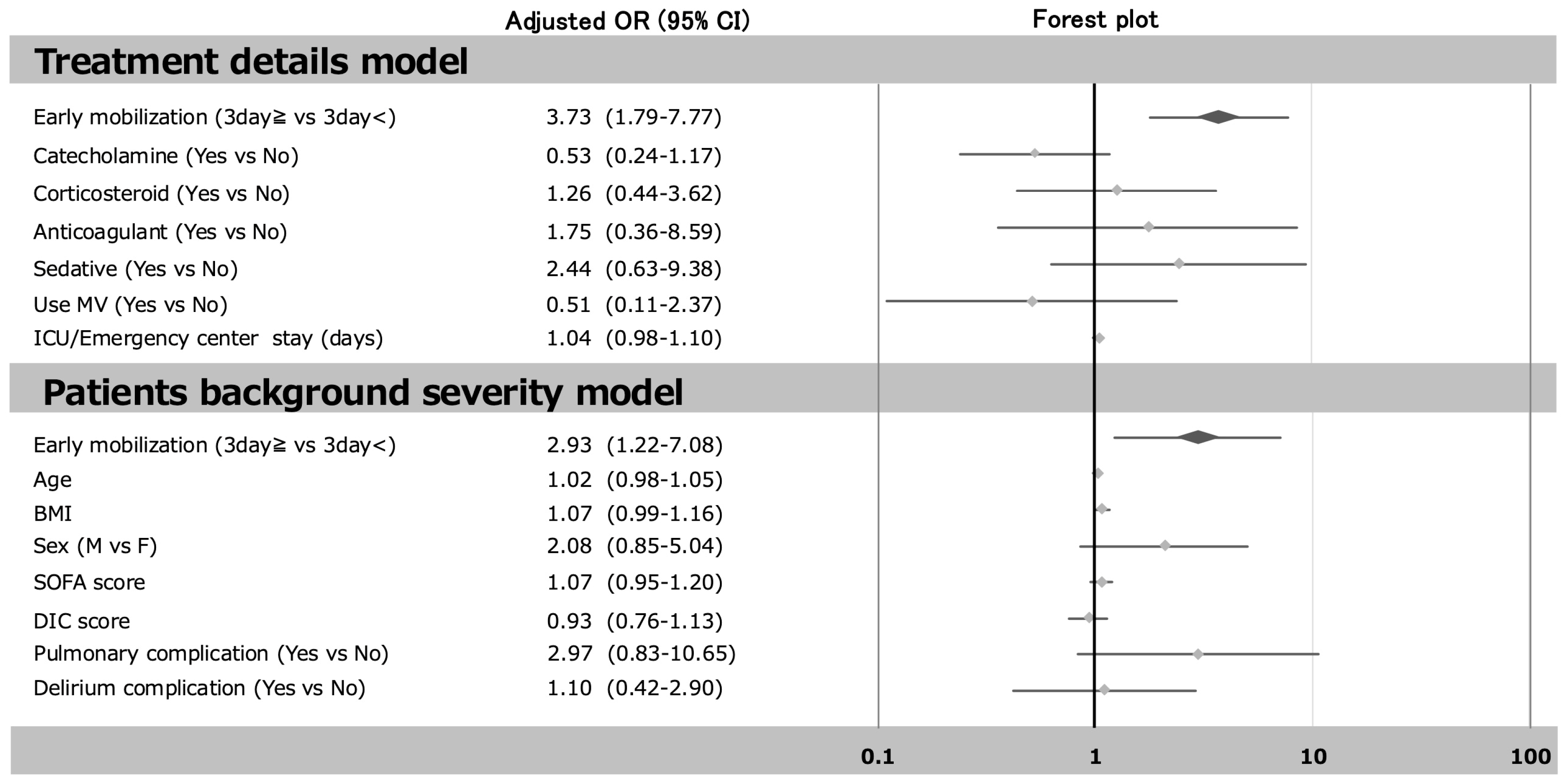
3.3. The Relationship Between Early Mobilization and the Incidence of ICU-AW
4. Discussion
4.1. Key Findings and Strengths
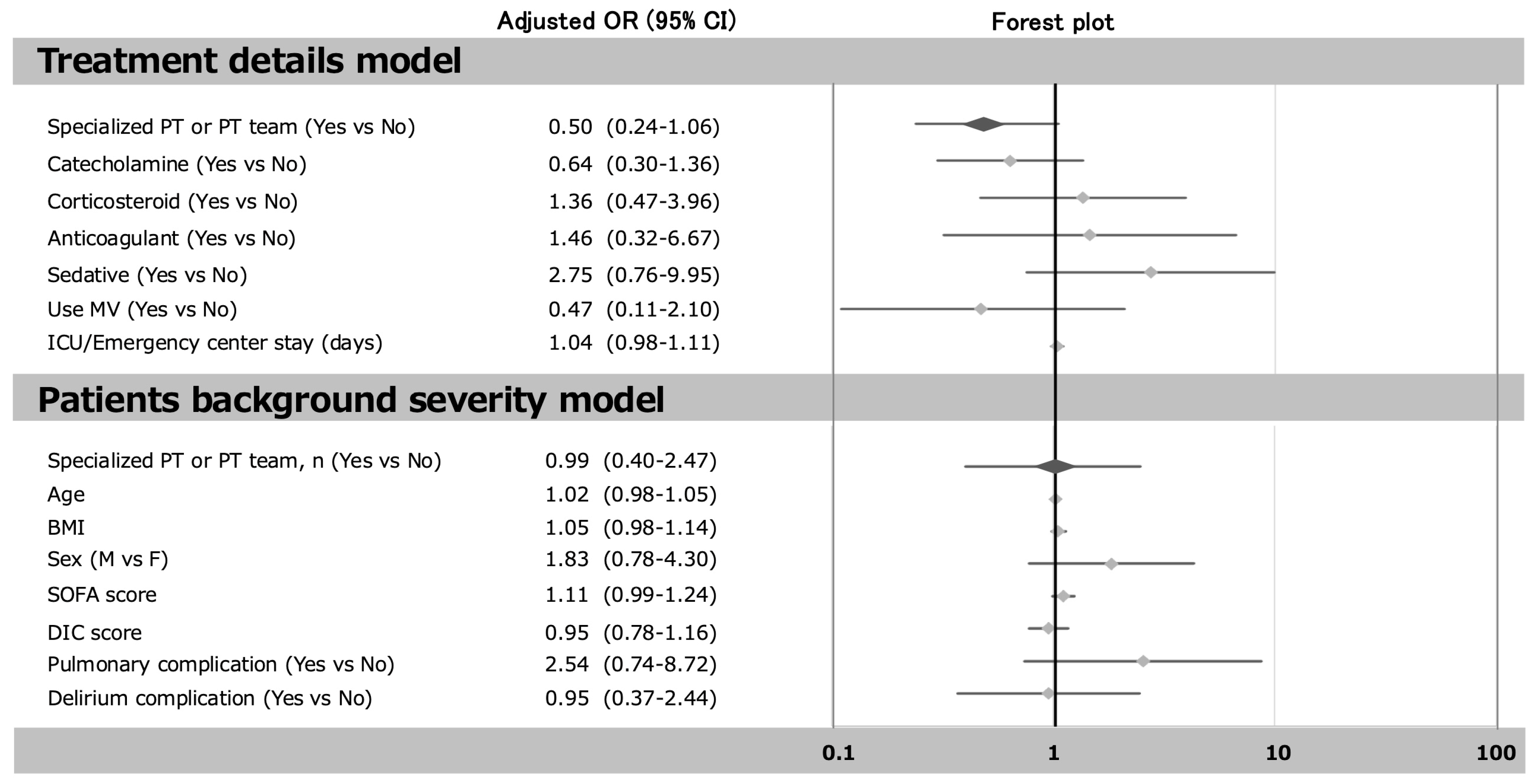
4.2. Early Mobilization and the Role of a Dedicated Team or Physiotherapist
4.3. ICU-AW and Acute Kidney Injury (AKI)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PICS | Post-Intensive Care Syndrome |
| ICU-AW | Intensive Care Unit-Acquired Weakness |
| ICU | Intensive Care Unit |
| ADL | Activities of Daily Living |
| SIRS | Systemic Inflammatory Response Syndrome |
| BI | Barthel Index |
| EMS | Electrical Muscle Stimulation |
| BMI | Body Mass Index |
| SOFA | Sequential Organ Failure Assessment |
| DIC | Disseminated Intravascular Coagulation |
| PLT | Procalcitonin |
| MV | Mechanical Ventilation |
| MRC | Medical Research Council |
| SPPB | Short Physical Performance Battery |
| AKI | Acute Kidney Injury |
References
- Stevens, R.D.; Dowdy, D.W.; Michaels, R.K.; Mendez-Tellez, P.A.; Pronovost, P.J.; Needham, D.M. Neuromuscular dysfunction acquired in critical illness: A systematic review. Intensive Care Med. 2007, 33, 1876–1891. [Google Scholar] [CrossRef] [PubMed]
- Nanas, S.; Kritikos, K.; Angelopoulos, E.; Siafaka, A.; Tsikriki, S.; Poriazi, M.; Zervakis, D.; Routsi, C.; Roussos, C. Predisposing factors for critical illness polyneuro myopathy in a multidisciplinary intensive care unit. Acta Neurol Scand. 2008, 118, 175–181. [Google Scholar] [CrossRef]
- Jaber, S.; Petrof, B.J.; Jung, B.; Chanques, G.; Berthet, J.-P.; Rabuel, C.; Bouyabrine, H.; Courouble, P.; Koechlin-Ramonatxo, C.; Sebbane, M.; et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am. J. Respir. Crit. Care Med. 2011, 183, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, P.; Schaeffer, A.F.; Leitao, C.B.; Rech, T.H.; Nedel, W.L. Association between neuromuscular blocking agents and the devel opment of intensive care unit-acquired weakness (ICU-AW): A systematic review with meta-analysis and trial sequential analysis. Anaesth. Crit. Care Pain. Med. 2023, 42, 101202. [Google Scholar]
- Herridge, M.S.; Cheung, A.M.; Tansey, C.M.; Matte-Martyn, A.; Diaz-Granados, N.; Al-Saidi, F.; Cooper, A.B.; Guest, C.B.; Mazer, C.D.; Mehta, S.; et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003, 348, 683–693. [Google Scholar] [CrossRef]
- Papazian, L.; Forel, J.M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.M.; Perez, D.; Seghboyan, J.M.; et al. Neuromuscular blockers in early acute respiretory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Amaya-Villar, R.; García-Garmendía, J.L.; Madrazo-Osuna, J.; Ortiz-Leyba, C. Effect of critical illness poly neuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients. Crit. Care Med. 2005, 33, 349–354. [Google Scholar] [CrossRef]
- De Jonghe, B.; Bastuji-Garin, S.; Durand, M.-C.; Malissin, I.; Rodrigues, P.; Cerf, C.; Outin, H.; Sharshar, T. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit. Care Med. 2007, 35, 2007–2015. [Google Scholar] [CrossRef]
- Sharshar, T.; Bastuji-Garin, S.; Stevens, R.D.; Durand, M.-C.; Malissin, I.; Rodriguez, P.; Cerf, C.; Outin, H.; De Jonghe, B. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit. Care Med. 2009, 37, 3047–3053. [Google Scholar] [CrossRef]
- La Via, L.; Sangiorgio, G.; Stefani, S.; Marino, A.; Nunnari, G.; Cocuzza, S.; La Mantia, I.; Cacopardo, B.; Stracquadanio, S.; Spampinato, S.; et al. The Global Burden of Sepsis and Septic Shock. Epidemiologic 2024, 5, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.; Figueirêdo, B.B.; Vasconcelos, A.; de Andrade, É.M.; Dornelas de Andrade, A.; Reinaux, C. Is transcutaneous electrical muscle stimulation an alternative for preventing acquired muscle weakness in the pediatric intensive care unit? A scoping review. Pediatr. Pulmonol. 2019, 54, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Unoki, T.; Hayashida, K.; Kawai, Y.; Taito, S.; Ando, M.; Iida, Y.; Kasai, F.; Kawasaki, T.; Kozu, R.; Kondo, Y.; et al. Japanese Clinical Practice Guidelines for Rehabilitation in Critically Ill Patients 2023 (J-ReCIP 2023). J. Intensive Care 2023, 11, 47. [Google Scholar] [CrossRef]
- Hodgson, C.L.; Schaller, S.J.; Nydahl, P.; Timenetsky, K.T.; Needham, D.M. Ten strategies to optimize early mobilization and rehabilitation in intensive care. Crit. Care 2021, 25, 324. [Google Scholar] [CrossRef]
- Sakai, Y.; Yamamoto, S.; Karasawa, T.; Sato, M.; Nitta, K.; Okada, M.; Takeshige, K.; Ikegami, S.; Imamura, H.; Horiuchi, H. Effects of early rehabilitation in sepsis patients by a specialized physical therapist in an emergency center on the return to activities of daily living independence: A retrospective cohort study. PLoS ONE 2022, 17, e0266348. [Google Scholar] [CrossRef]
- Sakai, Y.; Yamamoto, S.; Karasawa, T.; Sato, M.; Nitta, K.; Okada, M.; Ikegami, S.; Imamura, H.; Horiuchi, H. Early Rehabilitation Provided by Specialized Physical Therapist in an Emergency Center Reduces Pulmonary Complications in Patients with Sepsis: A Retrospective Cohort Study. Int. J. Phys. Med. Rehabil. 2020, 8, 550. [Google Scholar]
- Anekwe, D.E.; Biswas, S.; Bussières, A.; Spahija, J. Early rehabilitation reduces the likelihood of developing intensive care unit-acquired weakness: A systematic review and meta-analysis. Physiotherapy 2020, 107, 1–10. [Google Scholar] [CrossRef]
- Sidiras, G.; Patsaki, I.; Karatzanos, E.; Dakoutrou, M.; Kouvarakos, A.; Mitsiou, G.; Routsi, C.; Stranjalis, G.; Nanas, S.; Gerovasili, V. Long term follow-up of quality of life and functional ability in patients with ICU acquired Weakness—A post hoc analysis. J. Crit. Care 2019, 53, 223–230. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 2008, 36, 296–327. [Google Scholar] [CrossRef]
- Lundin-Olsson, L.; Nyberg, L.; Gustafson, Y.; Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 12, 61–65. [Google Scholar]
- Collin, C.; Wade, D.T.; Davies, S.; Horne, V. The Barthel ADL Index: A reliability study. Int. Disabil. Stud. 1988, 10, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Fossat, G.; Baudin, F.; Courtes, L.; Bobet, S.; Dupont, A.; Bretagnol, A.; Benzekri-Lefèvre, D.; Kamel, T.; Muller, G.; Bercault, N.; et al. Effect of In-Bed Leg Cycling and Electrical Stimulation of the Quadriceps on Global Muscle Strength in Critically Ill Adults: A Randomized Clinical Trial. JAMA 2018, 320, 368–378. [Google Scholar] [CrossRef]
- Nave, A.H.; Rackoll, T.; Grittner, U.; Bläsing, H.; Gorsler, A.; Nabavi, D.G.; Audebert, H.J.; Klostermann, F.; Müller-Werdan, U.; Steinhagen-Thiessen, E.; et al. Physical Fitness Training in Patients with Subacute Stroke (PHYS-STROKE): Multicentre, randomised controlled, endpoint blinded trial. BMJ 2019, 366, l5101. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery as sessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Chaves, A.M.; Torres, S.J.; Palacios, L.; Alvarado, J.I.; Stozitzky, M.V.; Santacruz, H.C.A. Prospective ultrasonographic evaluation of femoral and vastus intermedius muscles as predictors of ICU-acquired weakness in critically ill patients. J. Ultrasound 2025, 28, 447–454. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Tyml, K.; Swarbreck, S.; Pape, C.; Secor, D.; Koropatnick, J.; Feng, Q.; Veldhuizen, R.A.W.; Gill, S.E. Voluntary running exercise protects against sepsis-induced early inflammatory and pro-coagulant responses in aged mice. Crit. Care 2017, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Verceles, A.C.; Wells, C.L.; Sorkin, J.D.; Terrin, M.L.; Beans, J.; Jenkins, T.; Goldberg, A.P. A multimodal rehabilitation program for patients with ICU acquired weakness improves ventilator weaning and discharge home. J. Crit. Care 2018, 47, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Dubb, R.; Nydahl, P.; Hermes, C.; Schwabbauer, N.; Toonstra, A.; Parker, A.M.; Kaltwasser, A.; Needham, D.M. Barriers and Strategies for Early Mobilization of Patients in Intensive Care Units. Ann. Am. Thorac. Soc. 2016, 13, 724–730. [Google Scholar] [CrossRef]
- Teixeira, J.P.; Mayer, K.P.; Griffin, B.R.; George, N.; Jenkins, N.; Pal, C.A.; González-Seguel, F.; Neyra, J.A. Intensive Care Unit-Acquired Weakness in Patients with Acute Kidney Injury: A Contemporary Review. Am. J. Kidney Dis. 2023, 81, 336–351. [Google Scholar] [CrossRef]
| Variables | ICU-AW (n = 76) | Non-ICU-AW (n = 78) | p-Value |
|---|---|---|---|
| Age (years) | 76.8 ± 12.9 | 74.5 ± 11.6 | 0.25 |
| Men/women, n (%) | 44 (58)/32 (42) | 54 (69)/24 (31) | 0.144 |
| BMI (kg/m2) | 21.4 (19.4, 23.8) | 21.8 (19.9, 24.8) | 0.255 |
| Severity score | |||
| SOFA score | 7 (5, 12) | 6 (4, 9) | 0.074 |
| DIC score | 2 (1, 4.5) | 2 (1, 4) | 1.000 |
| PCT (ng/mL) | 31.0 (2.27, 55.2) | 22.3 (2.8, 97.9) | 0.721 |
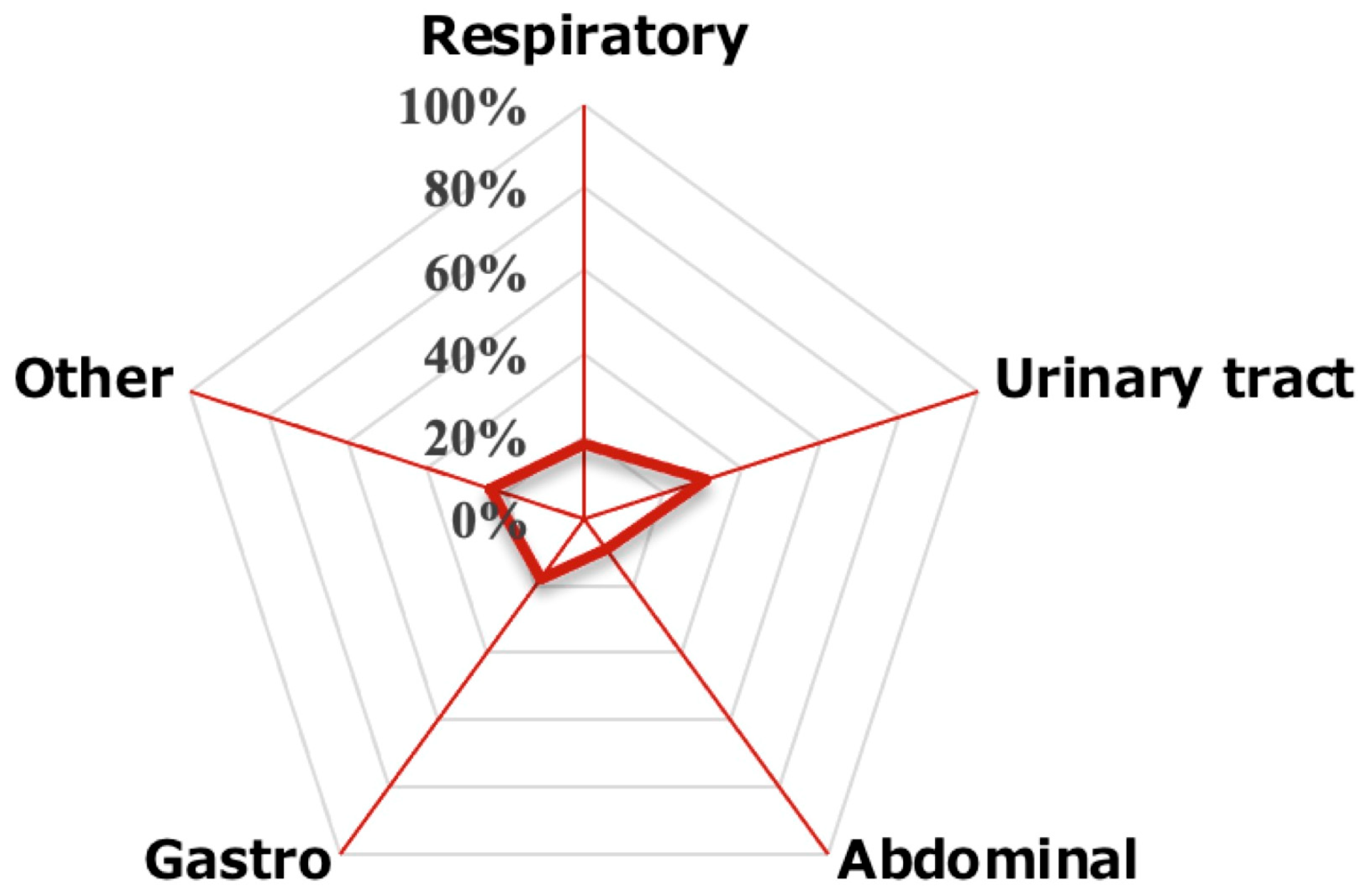
| Primary category of infection | |||
| Respiratory, n (%) | 14 (18) | 12 (15) | |
| Urinary tract, n (%) | 23 (31) | 31 (40) | |
| Abdominal, n (%) | 7 (9) | 10 (13) | 0.22 |
| Gastroenteritis, n (%) | 15 (18) | 16 (21) | |
| Others, n (%) | 17 (24) | 9 (11) | |
| Treatment details | |||
| Catecholamine, n (%) | 47 (62) | 54 (69) | 0.334 |
| Corticosteroid, n (%) | 14 (18) | 11 (14) | 0.468 |
| Anticoagulant, n (%) | 6 (8) | 4 (5) | 0.016 |
| Sedative, n (%) | 20 (26) | 15 (19) | 0.145 |
| Use MV, n (%) | 14 (18) | 12 (15) | 0.615 |
| Start of mobilization (day) | 4 (3, 4) | 2 (1, 4) | p < 0.001 |
| Specialized PT or PT team, n (%) | 43 (57) | 51 (65) | 0.263 |
| ICU/Emergency center stay (days) | 5 (2, 9) | 4 (2.3, 6) | 0.168 |
| Baseline ADL | |||
| Barthel index baseline (points) | 0 (0, 20) | 10 (0, 35) | 0.015 |
| Variables | ICU-AW (n = 76) | Non-ICU-AW (n = 78) | p-Value |
|---|---|---|---|
| Outcome | |||
| Barthel index discharge (points) | 65 (17.5, 87.5) | 90 (80, 100) | p < 0.001 |
| SPPB total (points) | 6.5 (2, 11.5) | 10 (5, 12) | 0.042 |
| Delirium complication, n (%) | 19 (25) | 20 (26) | 0.864 |
| Plumonary complication, n (%) | 18 (24) | 5 (6) | 0.002 |
| Dischage to home, n (%) | 29 (38) | 62 (79) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, Y.; Taniuchi, K.; Karasawa, T.; Matsui, K.; Matsumoto, T.; Ikegami, S.; Imamura, H.; Horiuchi, H. The Impact of Early Mobilization on the Incidence of Intensive Care Unit-Acquired Weakness in Patients with Sepsis in the Critical Care—The Shinshu Multicenter Prospective Cohort Study (EROSCCS Study). J. Clin. Med. 2025, 14, 5904. https://doi.org/10.3390/jcm14165904
Sakai Y, Taniuchi K, Karasawa T, Matsui K, Matsumoto T, Ikegami S, Imamura H, Horiuchi H. The Impact of Early Mobilization on the Incidence of Intensive Care Unit-Acquired Weakness in Patients with Sepsis in the Critical Care—The Shinshu Multicenter Prospective Cohort Study (EROSCCS Study). Journal of Clinical Medicine. 2025; 14(16):5904. https://doi.org/10.3390/jcm14165904
Chicago/Turabian StyleSakai, Yasunari, Kohei Taniuchi, Takuma Karasawa, Ken Matsui, Takeshi Matsumoto, Shota Ikegami, Hiroshi Imamura, and Hiroshi Horiuchi. 2025. "The Impact of Early Mobilization on the Incidence of Intensive Care Unit-Acquired Weakness in Patients with Sepsis in the Critical Care—The Shinshu Multicenter Prospective Cohort Study (EROSCCS Study)" Journal of Clinical Medicine 14, no. 16: 5904. https://doi.org/10.3390/jcm14165904
APA StyleSakai, Y., Taniuchi, K., Karasawa, T., Matsui, K., Matsumoto, T., Ikegami, S., Imamura, H., & Horiuchi, H. (2025). The Impact of Early Mobilization on the Incidence of Intensive Care Unit-Acquired Weakness in Patients with Sepsis in the Critical Care—The Shinshu Multicenter Prospective Cohort Study (EROSCCS Study). Journal of Clinical Medicine, 14(16), 5904. https://doi.org/10.3390/jcm14165904





