Intravenous Administration of Human-Derived Mesenchymal Stem Cell-Conditioned Medium for Patients with General Malaise
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Participants
2.3. Research Participant Selection Policy
- 1.
- Patients who had not achieved satisfactory treatment effects with standard treatments provided by a general physician.
- 2.
- Patients who opted not to undergo standard drug treatments, owing to concerns about side effects, and whose physician deemed treatment with the MSC-CM to be optimal.
- Those aged 18 years or older;
- Those with normal consent capacity;
- Those who provided written informed consent;
- Those for whom the physician recognized a need for treatment.
- 1.
- Those with a history or suspicion of dementia;
- 2.
- Those who used drugs and stimulants;
- 3.
- Those who were pregnant or breastfeeding;
- 4.
- Those who were judged as unsuitable by the attending physician.
2.4. Types of MSC-CM Used for Treatment
2.5. MSC-CM Administration Method
2.6. Administration Schedule
2.7. Blood Collection
2.8. Statistical Analyses
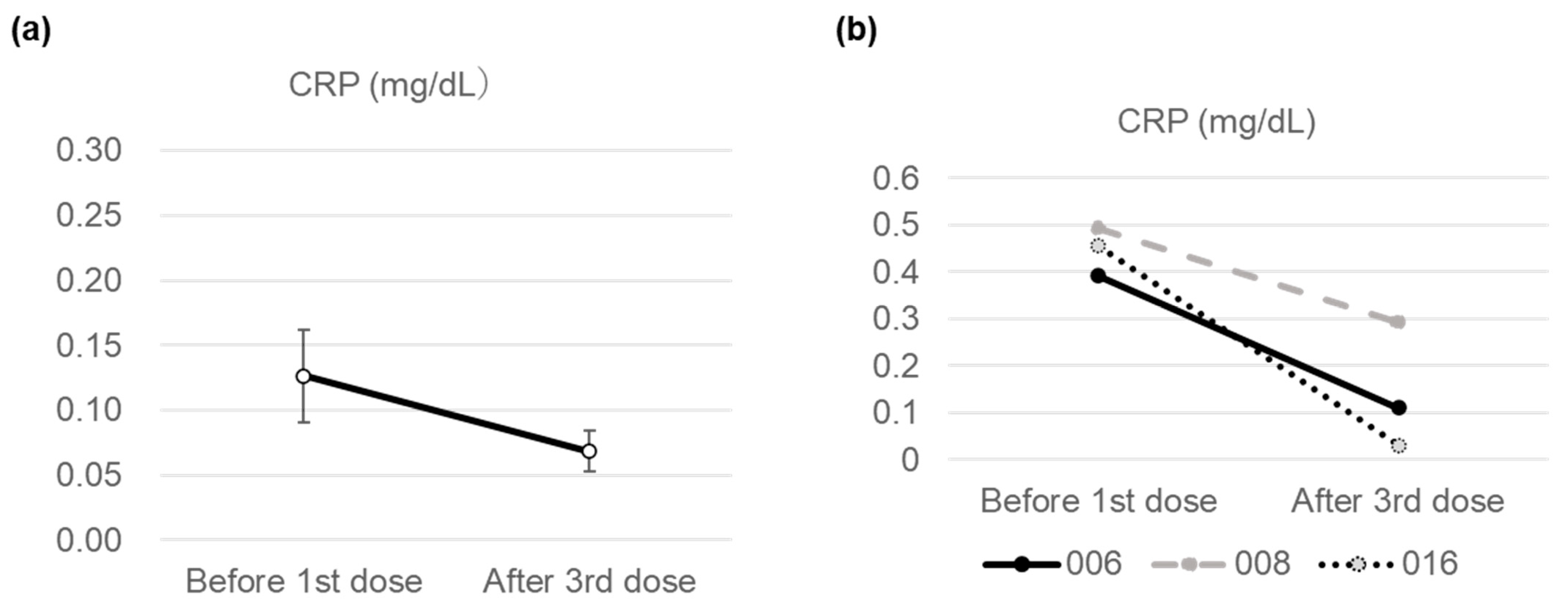
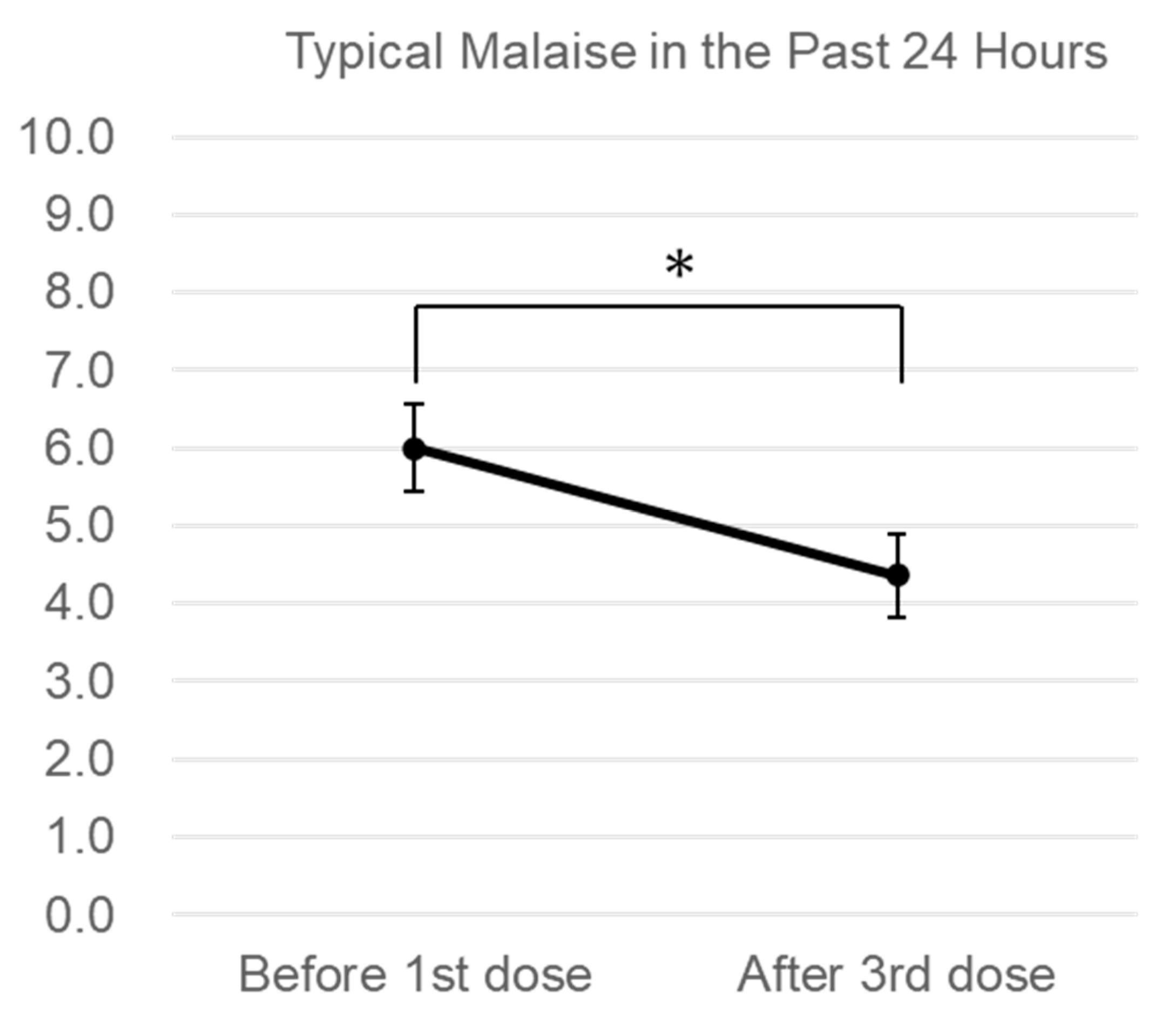
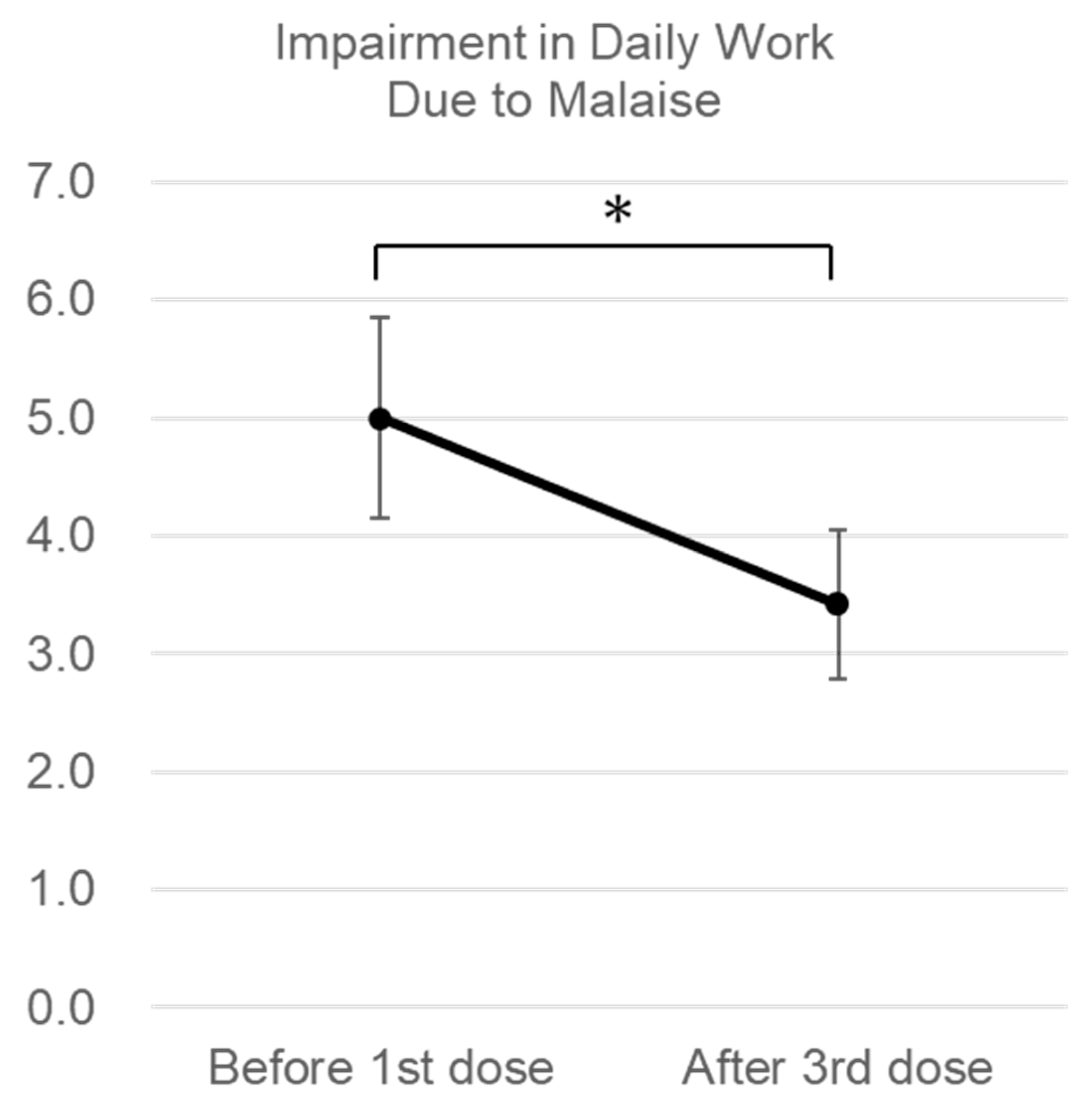
3. Results
Changes in Subjective Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD-CM | adipose tissue-derived MSC-conditioned medium |
| CRP | C-reactive protein |
| INH | inhalation |
| IV | intravenous |
| MSCs | mesenchymal stem cells |
| MSC-CM | MSC-conditioned medium |
| NRS | numerical rating scale |
| QOL | quality of life |
| STAS | serum total antioxidant status |
| UC-CM | umbilical cord-derived MSC-conditioned medium |
References
- Natelson, B.H.; Brunjes, D.L.; Mancini, D. Chronic fatigue syndrome and cardiovascular disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Ba, J.; Chen, Y.; Liu, D. Fatigue in adults with type 2 diabetes: A systematic review and meta-analysis. West J. Nurs. Res. 2021, 43, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, K.; Kapoor, D. Fatigue in cirrhosis. J. Clin. Exp. Hepatol. 2022, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Polikandrioti, M.; Kalafatakis, F.; Koutelekos, I.; Kokoularis, D. Fatigue in heart failure outpatients: Levels, associated factors, and the impact on quality of life. Arch. Med. Sci. Athérosclér Dis. 2019, 4, e103–e112. [Google Scholar] [CrossRef]
- Zeraatkar, D.; Ling, M.; Kirsh, S.; Jassal, T.; Pitre, T.; Chakraborty, S.; Turner, T.; Turkstra, L.; Mclntyre, R.S.; Izcovich, A.; et al. Interventions for the management of post- COVID-19 condition (long COVID): Protocol for a living systematic review and network meta-analysis. BMJ Open 2025, 15, e086407. [Google Scholar] [CrossRef]
- Witham, M.D.; Adams, F.; McSwiggan, S.; Kennedy, G.; Kabir, G.; Belch, J.J.; Khan, F. Effect of intermittent vitamin D3 on vascular function and symptoms in chronic fatigue syndrome—A randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 287–294. [Google Scholar] [CrossRef]
- Yamato, M.; Tamura, Y.; Eguchi, A.; Kume, S.; Miyashige, Y.; Nakano, M.; Watanabe, Y.; Kataoka, Y. Brain interleukin-1β and the intrinsic receptor antagonist control peripheral toll-like receptor 3-mediated suppression of spontaneous activity in rats. PLoS ONE 2014, 9, e90950. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef]
- Kennedy, G.; Spence, V.A.; McLaren, M.; Hill, A.; Underwood, C.; Belch, J.J. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic. Biol. Med. 2005, 39, 584–589. [Google Scholar] [CrossRef]
- Daniel, S.; Hou, R.; Galea, I.; Bulters, D. C-reactive protein and fatigue after subarachnoid haemorrhage. Brain Behav. Immun. Health 2025, 48, 101046. [Google Scholar] [CrossRef]
- Shankar, V.; Wilhelmy, J.; Curtis, E.J.; Michael, B.; Cervantes, L.; Mallajosyula, V.; Davis, R.W.; Snyder, M.; Younis, S.; Robinson, W.H.; et al. Oxidative stress is a shared characteristic of ME/CFS and Long COVID. Proc. Natl. Acad. Sci. USA 2025, 122, e2426564122. [Google Scholar] [CrossRef]
- Castillo-Melendez, M.; Yawno, T.; Jenkin, G.; Miller, S.L. Stem cell therapy to protect and repair the developing brain: A review of mechanisms of action of cord blood and amnion epithelial derived cells. Front. Neurosci. 2013, 7, 194. [Google Scholar] [CrossRef]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Ben-Hur, T.; Offen, D.; Abramsky, O.; Melamed, E.; Karussis, D. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: Results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Fričová, D.; Korchak, J.A.; Zubair, A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. NPJ Regen. Med. 2020, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, B.; Panahi, M.; Saraygord-Afshari, N.; Taheri, N.; Bilici, M.; Jafari, D.; Alizadeh, E. The secretome of mesenchymal stem cells and oxidative stress: Challenges and opportunities in cell-free regenerative medicine. Mol. Biol. Rep. 2021, 48, 5607–5619. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, Y.; Hori, M.; Mizuno, T.; Harada-Shiba, M. Humoral factors secreted from adipose tissue-derived mesenchymal stem cells ameliorate atherosclerosis in Ldlr−/− mice. Cardiovasc. Res. 2019, 115, 1041–1051. [Google Scholar] [CrossRef]
- Uchiyama, A.; Motegi, S.I.; Sekiguchi, A.; Fujiwara, C.; Perera, B.; Ogino, S.; Yokoyama, Y.; Ishikawa, O. Mesenchymal stem cells-derived MFG-E8 accelerates diabetic cutaneous wound healing. J. Dermatol. Sci. 2017, 86, 187–197. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, N.; Hong, H.; Qi, J.; Zhang, S.; Wang, J. Mesenchymal stem cells: Therapeutic mechanisms for stroke. Int. J. Mol. Sci. 2022, 23, 2550. [Google Scholar] [CrossRef]
- Neves, B.R.O.; de Freitas, S.; Borelli, P.; Rogero, M.M.; Fock, R.A. Delphinidin-3-O-glucoside in vitro suppresses NF-κB and changes the secretome of mesenchymal stem cells affecting macrophage activation. Nutrition 2023, 105, 111853. [Google Scholar] [CrossRef]
- Saleem, R.; Mohamed-Ahmed, S.; Elnour, R.; Berggreen, E.; Mustafa, K.; Al-Sharabi, N. Conditioned Medium from Bone Marrow Mesenchymal Stem Cells Restored Oxidative Stress-Related Impaired Osteogenic Differentiation. Int. J. Mol. Sci. 2021, 22, 13458. [Google Scholar] [CrossRef]
- Pezzotta, A.; Bovio, A.; Imberti, B.; Locatelli, M.; Corna, D.; Cerullo, D.; Gastoldi, S.; Benigni, A.; Remuzzi, G.; Morigi, M.; et al. Mesenchymal stromal cell secretome reduces lung injury and thrombo-inflammation induced by SARS-CoV-2 spike protein. Stem Cell Res. Ther. 2025, 16, 324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sano, M.; Tanabe, A.; Urushihata, N.; Liu, X.-L. Effect of human adipose-derived mesenchymal stem cell conditioned medium on musculoskeletal pain. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Inami, N. Safety assessment of multiple systemic administration of human mesenchymal stem cell-conditioned medium for various chronic diseases. PLoS ONE 2025, 20, e0322497. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Kumari, M. Age modification of the relationship between C-reactive protein and fatigue: Findings from Understanding Society (UKHLS). Psychol. Med. 2018, 48, 1341–1349. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.G.; Lee, D.S.; Son, C.G. Oxidative stress is a convincing contributor to idiopathic chronic fatigue. Sci. Rep. 2018, 8, 12890. [Google Scholar] [CrossRef]
- Kumada, M.; Kihara, S.; Sumitsuji, S.; Kawamoto, T.; Matsumoto, S.; Ouchi, N.; Arita, Y.; Okamoto, Y.; Shimomura, I.; Hiraoka, H.; et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 85–89. [Google Scholar] [CrossRef]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef]
- Kore, R.A.; Wang, X.; Ding, Z.; Griffin, R.J.; Tackett, A.J.; Mehta, J.L. MSC exosome-mediated cardioprotection in ischemic mouse heart comparative proteomics of infarct and peri-infarct areas. Mol. Cell Biochem. 2021, 476, 1691–1704. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Q.; Liu, X.; Zhang, T.; Wang, S.; Zhou, L.; Zou, L.; Fan, F.; Chi, H.; Sun, J.; et al. Exosomal microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells inhibits myocardial ischemia-reperfusion injury by reducing TLR4 and activating the PI3K/Akt signaling pathway. Int. Immunopharmacol. 2021, 101, 107592. [Google Scholar] [CrossRef]

| Improvement of Chief Complaints | Improvement in Other Symptoms | No Improvement | |
|---|---|---|---|
| 1st dose | 52.6% (10/19) | 15.8% (3/19) | 31.6% (6/19) |
| 2nd dose | 52.6% (10/19) | 21.1% (4/19) | 26.3% (5/19) |
| 3rd dose | 52.6% (10/19) | 26.3% (5/19) | 21.1% (4/19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inami, N. Intravenous Administration of Human-Derived Mesenchymal Stem Cell-Conditioned Medium for Patients with General Malaise. J. Clin. Med. 2025, 14, 5884. https://doi.org/10.3390/jcm14165884
Inami N. Intravenous Administration of Human-Derived Mesenchymal Stem Cell-Conditioned Medium for Patients with General Malaise. Journal of Clinical Medicine. 2025; 14(16):5884. https://doi.org/10.3390/jcm14165884
Chicago/Turabian StyleInami, Norihito. 2025. "Intravenous Administration of Human-Derived Mesenchymal Stem Cell-Conditioned Medium for Patients with General Malaise" Journal of Clinical Medicine 14, no. 16: 5884. https://doi.org/10.3390/jcm14165884
APA StyleInami, N. (2025). Intravenous Administration of Human-Derived Mesenchymal Stem Cell-Conditioned Medium for Patients with General Malaise. Journal of Clinical Medicine, 14(16), 5884. https://doi.org/10.3390/jcm14165884





