Integrating and Simplifying Evidence to Optimize Cardiorenal Guideline-Directed Therapies
Abstract
1. Introduction
2. Catching Chronic Kidney Disease Early: Why It Matters
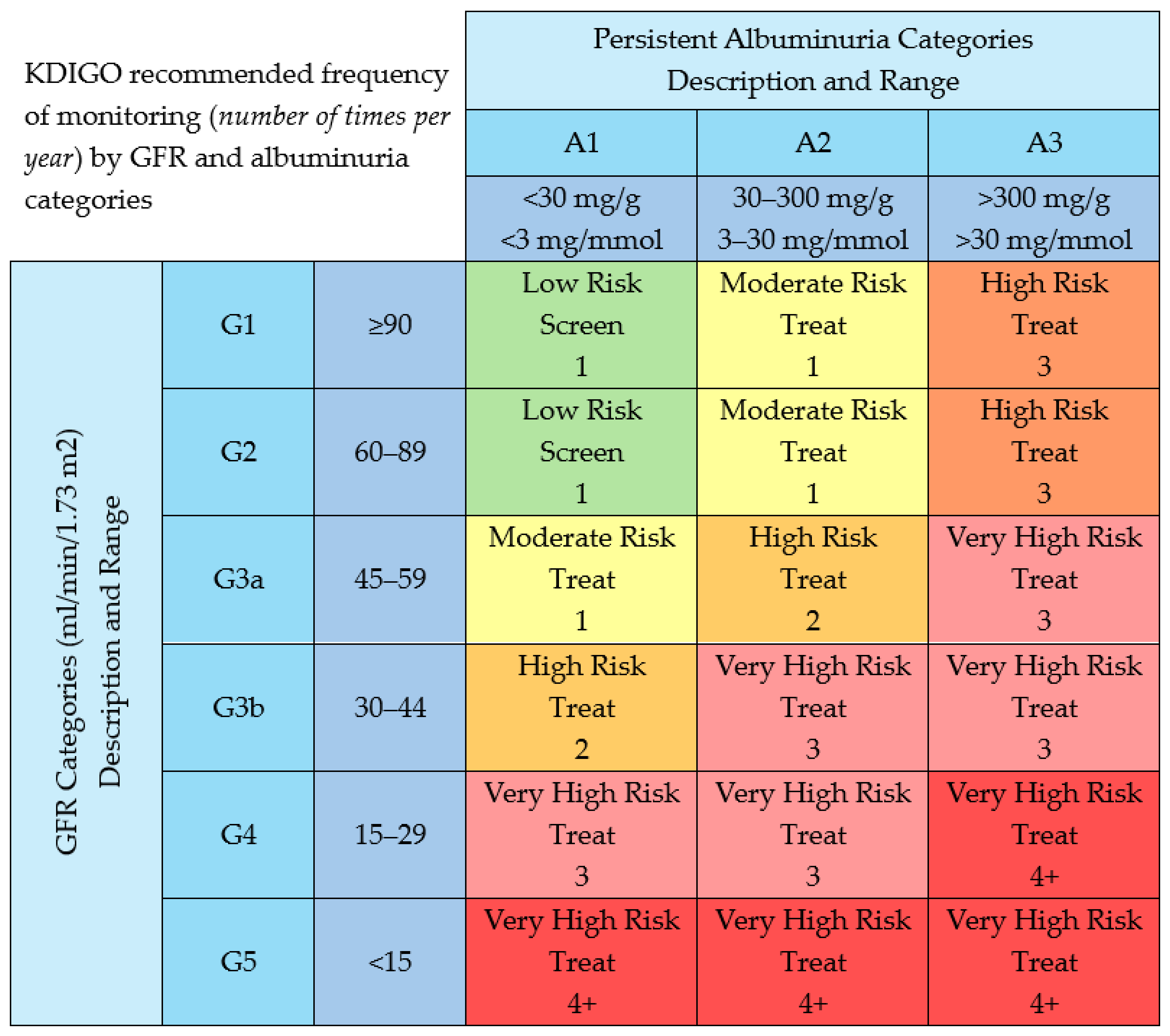
3. GFR and UACR: The Dynamic Duo for Detecting, Staging and Tracking CKD
3.1. GFR
3.2. Albumin-to-Creatinine Ratio (uACR)
4. Renal Troubles: Sorting out Acute Kidney Injury (AKI) from Worsening Renal Function (WRF)
5. The GDMT Adoption Dilemma in HF with CKD
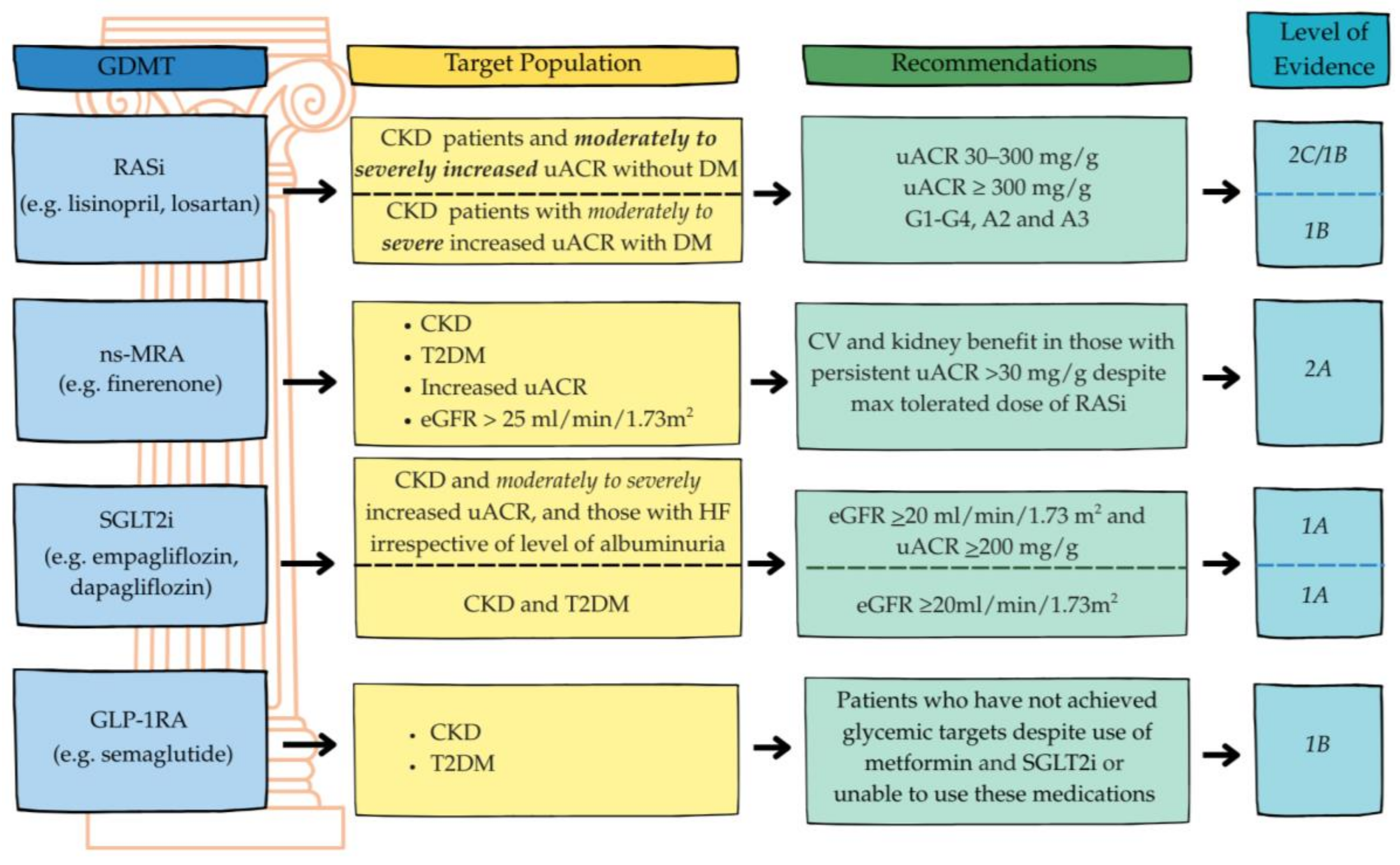
6. Therapies Delivering CV Gains in CKD
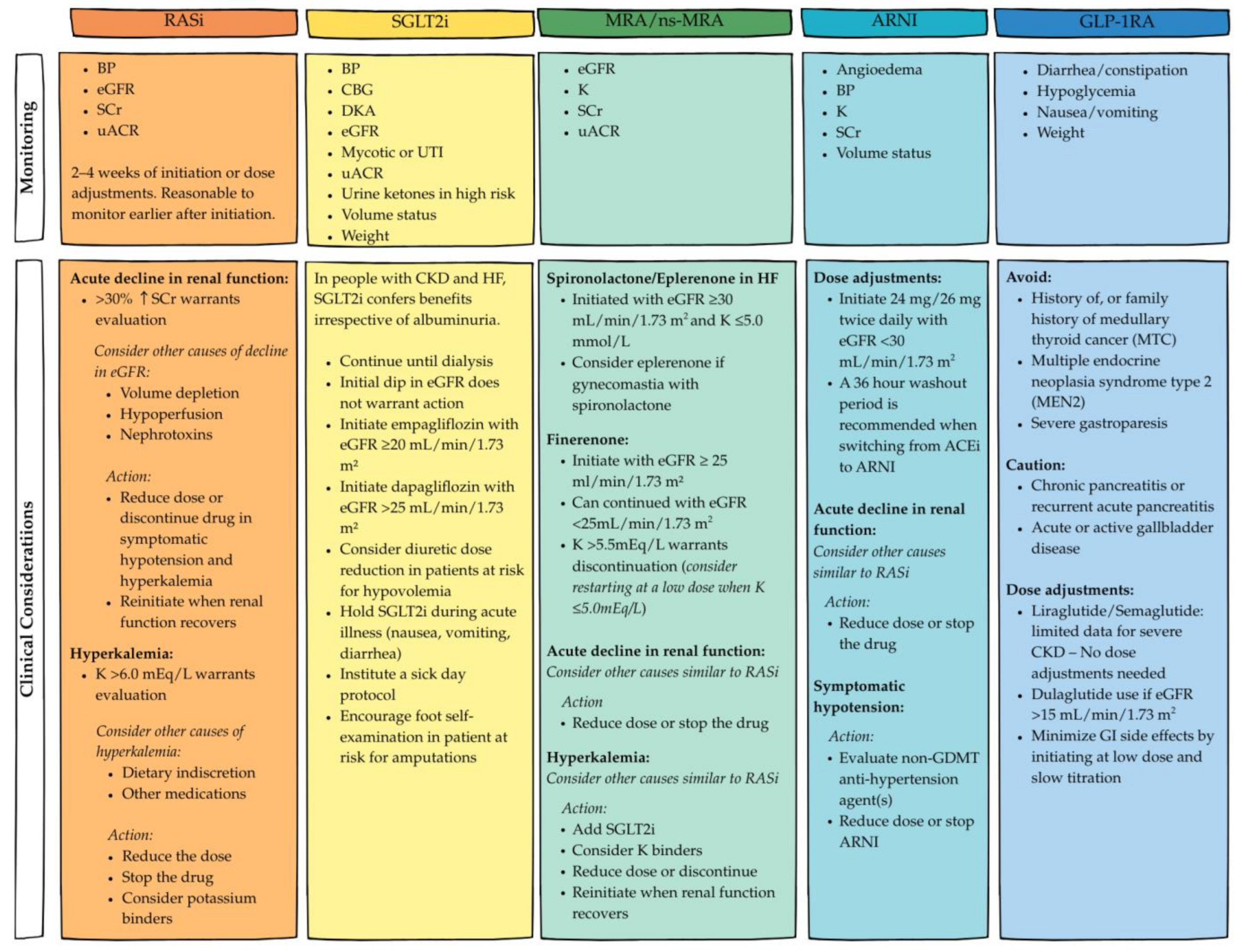
6.1. RAS Inhibitors: Angiotensin-Converting Enzyme Inhibitors (ACEis), Angiotensin II Receptor Blockers (ARBs)
6.2. Mineralocorticoid Receptor Antagonists (MRAs)
6.3. Angiotensin Receptor–Neprilysin Inhibitor (ARNI)
6.4. Sodium–Glucose Cotransporter-2 Inhibitors (SGLT2is)
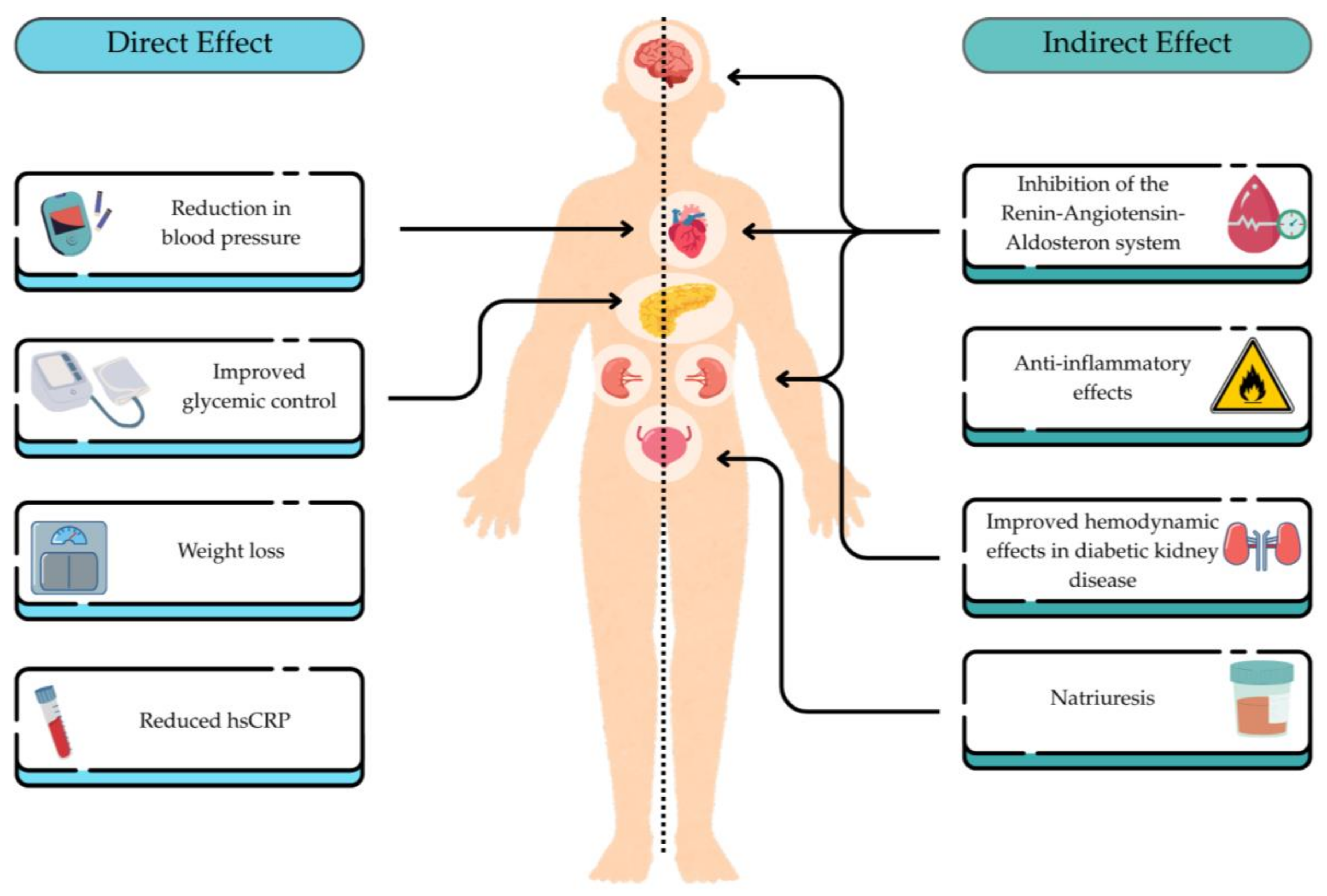
6.5. Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs)
7. From Evidence to Impact: Challenges and Opportunities of Implementing Best Practices in CKD Management
7.1. Nonpharmacological Management of CKD and HF
7.2. Pharmacological Management of CKD and HF
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEi | Angiotensin-converting enzyme inhibitors |
| ADHF | Acute decompensated heart failure |
| AKI | Acute kidney injury |
| ARB | Angiotensin II receptor blockers |
| ARNI | Angiotensin receptor-neprilysin inhibitor |
| CKD | Chronic kidney disease |
| CV | Cardiovascular |
| CVOTs | CV outcome trials |
| DM | Diabetes |
| eGFRcr | Estimated GFR based on serum creatinine |
| eGFRcr-cys | Cystatin C–based eGFR |
| ESKD | End-stage kidney disease |
| GDMT | Guideline directed medical therapy |
| GFR | GFR glomerular filtration rate |
| GLP-1RA | Glucagon-like peptide-1 receptor agonists |
| HFH | Heart failure hospitalization |
| HFmrEF | Heart failure with mildly reduced ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| HTN | Hypertension |
| KRT | Kidney replacement therapy |
| MACE | Major adverse CV events |
| RASi | Renin-angiotensin system inhibitors |
| RAAS | Renin-angiotensin-aldosterone system |
| SCr | Serum creatinine |
| SGLT2i | Sodium–glucose cotransporter 2 inhibitors |
| S/V | Sacubitril/valsartan |
| T2DM | Type 2 diabetes mellitus |
| uACR | Urine albumin-to-creatinine ratio |
| WRF | Worsening renal function |
References
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic Kidney Disease and the Global Public Health Agenda: An International Consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A Single Number for Advocacy and Communication—Worldwide More than 850 Million Individuals Have Kidney Diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Chan, G.C.-K.; Chen, J.; Chen, H.-C.; Cheng, Y.-L.; Fan, S.L.-S.; He, J.C.; Hu, W.; Lim, W.-H.; Pei, Y.; et al. Tackling Dialysis Burden around the World: A Global Challenge. Kidney Dis. 2021, 7, 167–175. [Google Scholar] [CrossRef]
- Rao, N.; Brotons-Munto, F.; Moura, A.F.; Kocks, J.W.H.; Zhao, M.; Chadban, S.; Guiang, H.; Priest, S.; Brown, S. Holistic Impact of CKD: A Clinical, Economic, and Environmental Analysis by IMPACT CKD. Kidney Int. Rep. 2025. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Adamczak, M.; de Oliveira, R.B.; Massy, Z.A.; Sarafidis, P.; Agarwal, R.; Mark, P.B.; Kotanko, P.; Ferro, C.J.; et al. Cardiovascular Complications in Chronic Kidney Disease: A Review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc. Res. 2023, 119, 2017–2032. [Google Scholar] [CrossRef]
- Vanholder, R.; Annemans, L.; Bello, A.K.; Bikbov, B.; Gallego, D.; Gansevoort, R.T.; Lameire, N.; Luyckx, V.A.; Noruisiene, E.; Oostrom, T.; et al. Fighting the Unbearable Lightness of Neglecting Kidney Health: The Decade of the Kidney. Clin. Kidney J. 2021, 14, 1719–1730. [Google Scholar] [CrossRef]
- KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease—Kidney International. Available online: https://www.kidney-international.org/article/S0085-2538(23)00766-4/fulltext (accessed on 12 June 2025).
- Kushner, P.; Khunti, K.; Cebrián, A.; Deed, G. Early Identification and Management of Chronic Kidney Disease: A Narrative Review of the Crucial Role of Primary Care Practitioners. Adv. Ther. 2024, 41, 3757–3770. [Google Scholar] [CrossRef] [PubMed]
- Neale, E.P.; Middleton, J.; Lambert, K. Barriers and Enablers to Detection and Management of Chronic Kidney Disease in Primary Healthcare: A Systematic Review. BMC Nephrol. 2020, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Moriyama, T.; Schneider, M.P.; Virgitti, J.B.; Nicola, L.D.; Arnold, M.; Barone, S.; Peach, E.; Wittbrodt, E.; Chen, H.; et al. Prevalence of Undiagnosed Stage 3 Chronic Kidney Disease in France, Germany, Italy, Japan and the USA: Results from the Multinational Observational REVEAL-CKD Study. BMJ Open 2023, 13, e067386. [Google Scholar] [CrossRef]
- Tangri, N.; De Nicola, L. Findings and Implications of the REVEAL-CKD Study Investigating the Global Prevalence of Undiagnosed Stage G3 Chronic Kidney Disease. EMJ 2022, 60–65. [Google Scholar] [CrossRef]
- Tangri, N.; Peach, E.J.; Franzén, S.; Barone, S.; Kushner, P.R. Patient Management and Clinical Outcomes Associated with a Recorded Diagnosis of Stage 3 Chronic Kidney Disease: The REVEAL-CKD Study. Adv. Ther. 2023, 40, 2869–2885. [Google Scholar] [CrossRef]
- Boele-Schutte, E.; Gansevoort, R.T. Measured GFR: Not a Gold, but a Gold-Plated Standard. Nephrol. Dial. Transplant. 2017, 32, ii180–ii184. [Google Scholar] [CrossRef]
- Rowe, C.; Sitch, A.J.; Barratt, J.; Brettell, E.A.; Cockwell, P.; Dalton, R.N.; Deeks, J.J.; Eaglestone, G.; Pellatt-Higgins, T.; Kalra, P.A.; et al. Biological Variation of Measured and Estimated Glomerular Filtration Rate in Patients with Chronic Kidney Disease. Kidney Int. 2019, 96, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Shahid, I.; Anker, S.D.; Fonarow, G.C.; Fudim, M.; Hall, M.E.; Hernandez, A.; Morris, A.A.; Shafi, T.; Weir, M.R.; et al. Albuminuria and Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 81, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Abbate, M.; Zoja, C.; Remuzzi, G. How Does Proteinuria Cause Progressive Renal Damage? J. Am. Soc. Nephrol. 2006, 17, 2974–2984. [Google Scholar] [CrossRef]
- Looker, H.C.; Mauer, M.; Saulnier, P.-J.; Harder, J.L.; Nair, V.; Boustany-Kari, C.M.; Guarnieri, P.; Hill, J.; Esplin, C.A.; Kretzler, M.; et al. Changes in Albuminuria But Not GFR Are Associated with Early Changes in Kidney Structure in Type 2 Diabetes. J. Am. Soc. Nephrol. 2019, 30, 1049–1059. [Google Scholar] [CrossRef]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of Estimated Glomerular Filtration Rate and Albuminuria with All-Cause and Cardiovascular Mortality: A Collaborative Meta-Analysis of General Population Cohorts. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef]
- Verma, A.; Schmidt, I.M.; Claudel, S.; Palsson, R.; Waikar, S.S.; Srivastava, A. Association of Albuminuria With Chronic Kidney Disease Progression in Persons With Chronic Kidney Disease and Normoalbuminuria. Ann. Intern Med. 2024, 177, 467–475. [Google Scholar] [CrossRef]
- Siddiqi, F.S.; Advani, A. Endothelial-Podocyte Crosstalk: The Missing Link Between Endothelial Dysfunction and Albuminuria in Diabetes. Diabetes 2013, 62, 3647–3655. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Ali, M.A.; Wang, A.Y.-M.; Jha, V. Acute Kidney Injury in Acute Heart Failure—When to Worry and When Not to Worry? Nephrol. Dial. Transplant. 2024, 40, 10–18. [Google Scholar] [CrossRef]
- Lala, A.; Coca, S.; Feinman, J.; Hamo, C.E.; Fiuzat, M.; Abraham, W.T.; O’Connor, C.; Lindenfeld, J.; Januzzi, J.; Cavagna, I.; et al. Standardized Definitions of Changes in Kidney Function in Trials of Heart Failure: JACC Expert Panel From the HF-ARC. J. Am. Coll. Cardiol. 2025, 85, 766–781. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Solomon, S.D.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; Shah, S.J.; Lindholm, D.; et al. Dapagliflozin in Heart Failure with Preserved and Mildly Reduced Ejection Fraction: Rationale and Design of the DELIVER Trial. Eur. J. Heart Fail. 2021, 23, 1217–1225. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
- Lightfoot, C.J.; Wilkinson, T.J.; Smith, A.C. Non-Pharmacological Management of Chronic Kidney Disease. Medicine 2023, 51, 170–175. [Google Scholar] [CrossRef]
- Mc Causland, F.R.; Claggett, B.L.; Vaduganathan, M.; Desai, A.; Jhund, P.; Vardeny, O.; Fang, J.C.; de Boer, R.A.; Docherty, K.F.; Hernandez, A.F.; et al. Decline in Estimated Glomerular Filtration Rate After Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction. JAMA Cardiol. 2024, 9, 144–152. [Google Scholar] [CrossRef]
- Efficacy and Safety of Sotagliflozin in Patients with Type 2 Diabetes and Severe Renal Impairment—Cherney—2021—Diabetes, Obesity and Metabolism—Wiley Online Library. Available online: https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.14513 (accessed on 12 June 2025).
- Packer, M.; Butler, J.; Zeller, C.; Pocock, S.J.; Brueckmann, M.; Ferreira, J.P.; Filippatos, G.; Usman, M.S.; Zannad, F.; Anker, S.D. Blinded Withdrawal of Long-Term Randomized Treatment With Empagliflozin or Placebo in Patients With Heart Failure. Circulation 2023, 148, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Fonarow, G.C.; Greene, S.J.; DeVore, A.D.; Kavati, A.; Sikirica, S.; Albert, N.M.; Duffy, C.I.; Hill, C.L.; Patterson, J.H.; et al. Contemporary Treatment Patterns and Clinical Outcomes of Comorbid Diabetes Mellitus and HFrEF: The CHAMP-HF Registry. JACC Heart Fail. 2020, 8, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Coats, A.J.; Filippatos, G.; Ruschitzka, F.; Ferrari, R.; Piepoli, M.F.; Delgado Jimenez, J.F.; Metra, M.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-Year Follow-up Outcomes and Differences across Regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef]
- Bánfi-Bacsárdi, F.; Pilecky, D.; Vámos, M.; Majoros, Z.; Török, G.M.; Borsányi, T.D.; Dékány, M.; Solymossi, B.; Andréka, P.; Duray, G.Z.; et al. The Effect of Kidney Function on Guideline-directed Medical Therapy Implementation and Prognosis in Heart Failure with Reduced Ejection Fraction. Clin. Cardiol. 2024, 47, e24244. [Google Scholar] [CrossRef] [PubMed]
- Ostrominski, J.W.; Arnold, S.V.; Butler, J.; Fonarow, G.C.; Hirsch, J.S.; Palli, S.R.; Donato, B.M.K.; Parrinello, C.M.; O’Connell, T.; Collins, E.B.; et al. Prevalence and Overlap of Cardiac, Renal, and Metabolic Conditions in US Adults, 1999-2020. JAMA Cardiol. 2023, 8, 1050–1060. [Google Scholar] [CrossRef]
- Hou, F.F.; Zhang, X.; Zhang, G.H.; Xie, D.; Chen, P.Y.; Zhang, W.R.; Jiang, J.P.; Liang, M.; Wang, G.B.; Liu, Z.R.; et al. Efficacy and Safety of Benazepril for Advanced Chronic Renal Insufficiency. N. Engl. J. Med. 2006, 354, 131–140. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D.; The Collaborative Study Group. The Effect of Angiotensin-Converting-Enzyme Inhibition on Diabetic Nephropathy. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, G.; Chiurchiu, C.; Ruggenenti, P. Proteinuria Predicting Outcome in Renal Disease: Nondiabetic Nephropathies (REIN). Kidney Int. Suppl. 2004, S90–S96. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L. Heart Failure With Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Kamath, N.S.; El Kossi, M.; El Nahas, A.M. The Impact of Stopping Inhibitors of the Renin–Angiotensin System in Patients with Advanced Chronic Kidney Disease. Nephrol. Dial. Transplant. 2010, 25, 3977–3982. [Google Scholar] [CrossRef]
- Onuigbo, M.A.C.; Onuigbo, N.T.C. Late-Onset Renal Failure from Angiotensin Blockade (LORFFAB) in 100 CKD Patients. Int. Urol. Nephrol. 2008, 40, 233–239. [Google Scholar] [CrossRef]
- Qiao, Y.; Shin, J.-I.; Chen, T.K.; Inker, L.A.; Coresh, J.; Alexander, G.C.; Jackson, J.W.; Chang, A.R.; Grams, M.E. Association Between Renin-Angiotensin System Blockade Discontinuation and All-Cause Mortality Among Persons with Low Estimated Glomerular Filtration Rate. JAMA Intern. Med. 2020, 180, 718–726. [Google Scholar] [CrossRef]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.F.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P. STOP ACEi Trial Investigators Renin-Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef]
- Jaffe, I.Z.; Mendelsohn, M.E. Angiotensin II and Aldosterone Regulate Gene Transcription via Functional Mineralocortocoid Receptors in Human Coronary Artery Smooth Muscle Cells. Circ. Res. 2005, 96, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Buffolo, F.; Tetti, M.; Mulatero, P.; Monticone, S. Aldosterone as a Mediator of Cardiovascular Damage. Hypertension 2022, 79, 1899–1911. [Google Scholar] [CrossRef]
- Vardeny, O.; Wu, D.H.; Desai, A.; Rossignol, P.; Zannad, F.; Pitt, B.; Solomon, S.D.; RALES Investigators. Influence of Baseline and Worsening Renal Function on Efficacy of Spironolactone in Patients with Severe Heart Failure: Insights from RALES (Randomized Aldactone Evaluation Study). J. Am. Coll. Cardiol. 2012, 60, 2082–2089. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Abreu, P.; McMurray, J.J.V.; van Veldhuisen, D.J.; Swedberg, K.; Pocock, S.J.; Vincent, J.; Lins, K.; Rossignol, P.; Pitt, B.; et al. Renal Function Stratified Dose Comparisons of Eplerenone versus Placebo in the EMPHASIS-HF Trial. Eur. J. Heart Fail. 2019, 21, 345–351. [Google Scholar] [CrossRef]
- Cooper, L.B.; Lippmann, S.J.; Greiner, M.A.; Sharma, A.; Kelly, J.P.; Fonarow, G.C.; Yancy, C.W.; Heidenreich, P.A.; Hernandez, A.F. Use of Mineralocorticoid Receptor Antagonists in Patients with Heart Failure and Comorbid Diabetes Mellitus or Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6, e006540. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Pitt, B.; McMurray, J.J.V.; Pocock, S.J.; Solomon, S.D.; Pfeffer, M.A.; Zannad, F.; Rossignol, P. Steroidal MRA Across the Spectrum of Renal Function: A Pooled Analysis of RCTs. JACC Heart Fail. 2022, 10, 842–850. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Cherney, D.Z.I.; Desai, A.S.; Testani, J.M.; Verma, S.; Chinnakondepalli, K.; Dolling, D.; Patel, S.; Dahl, M.; Eudicone, J.M.; et al. Sodium Zirconium Cyclosilicate for Management of Hyperkalemia During Spironolactone Optimization in Patients with Heart Failure. J. Am. Coll. Cardiol. 2025, 85, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Zannad, F.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Jamal, W.; Steubl, D.; Schueler, E.; et al. Interplay of Mineralocorticoid Receptor Antagonists and Empagliflozin in Heart Failure: EMPEROR-Reduced. J. Am. Coll. Cardiol. 2021, 77, 1397–1407. [Google Scholar] [CrossRef]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and Kidney Outcomes with Finerenone in Patients with Type 2 Diabetes and Chronic Kidney Disease: The FIDELITY Pooled Analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Mc Causland, F.R.; Lefkowitz, M.P.; Claggett, B.; Packer, M.; Senni, M.; Gori, M.; Jhund, P.S.; McGrath, M.M.; Rouleau, J.L.; Shi, V.; et al. Angiotensin-Neprilysin Inhibition and Renal Outcomes Across the Spectrum of Ejection Fraction in Heart Failure. Eur. J. Heart Fail. 2022, 24, 1591–1598. [Google Scholar] [CrossRef]
- Mc Causland, F.R.; Lefkowitz, M.P.; Claggett, B.; Anavekar, N.S.; Senni, M.; Gori, M.; Jhund, P.S.; McGrath, M.M.; Packer, M.; Shi, V.; et al. Angiotensin-Neprilysin Inhibition and Renal Outcomes in Heart Failure With Preserved Ejection Fraction. Circulation 2020, 142, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R. Ending the Fear Depriving Patients with HFpEF and CKD of Lifesaving Therapies. JACC Heart Fail. 2025, 13, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes. JAMA Cardiol. 2021, 6, 1–11. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Herrington, W.G.; Savarese, G.; Haynes, R.; Marx, N.; Mellbin, L.; Lund, L.H.; Dendale, P.; Seferovic, P.; Rosano, G.; Staplin, N.; et al. Cardiac, Renal, and Metabolic Effects of Sodium-Glucose Co-Transporter 2 Inhibitors: A Position Paper from the European Society of Cardiology Ad-Hoc Task Force on Sodium-Glucose Co-Transporter 2 Inhibitors. Eur. J. Heart Fail. 2021, 23, 1260–1275. [Google Scholar] [CrossRef]
- Kraus, B.J.; Weir, M.R.; Bakris, G.L.; Mattheus, M.; Cherney, D.Z.I.; Sattar, N.; Heerspink, H.J.L.; Ritter, I.; von Eynatten, M.; Zinman, B.; et al. Characterization and Implications of the Initial Estimated Glomerular Filtration Rate “dip” upon Sodium-Glucose Cotransporter-2 Inhibition with Empagliflozin in the EMPA-REG OUTCOME Trial. Kidney Int. 2021, 99, 750–762. [Google Scholar] [CrossRef]
- Zinman, B.; Nauck, M.A.; Bosch-Traberg, H.; Frimer-Larsen, H.; Ørsted, D.D.; Buse, J.B.; LEADER Publication Committee on behalf of the LEADER Trial Investigators. Liraglutide and Glycaemic Outcomes in the LEADER Trial. Diabetes Ther. 2018, 9, 2383–2392. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Rossing, P.; Baeres, F.M.M.; Bakris, G.; Bosch-Traberg, H.; Gislum, M.; Gough, S.C.L.; Idorn, T.; Lawson, J.; Mahaffey, K.W.; Mann, J.F.E.; et al. The Rationale, Design and Baseline Data of FLOW, a Kidney Outcomes Trial with Once-Weekly Semaglutide in People with Type 2 Diabetes and Chronic Kidney Disease. Nephrol. Dial. Transplant. 2023, 38, 2041–2051. [Google Scholar] [CrossRef]
- Muskiet, M.H.A.; Tonneijck, L.; Smits, M.M.; van Baar, M.J.B.; Kramer, M.H.H.; Hoorn, E.J.; Joles, J.A.; van Raalte, D.H. GLP-1 and the Kidney: From Physiology to Pharmacology and Outcomes in Diabetes. Nat. Rev. Nephrol. 2017, 13, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, H.M.; Lingvay, I.; Brown, P.M.; Deanfield, J.; Brown-Frandsen, K.; Kahn, S.E.; Plutzky, J.; Node, K.; Parkhomenko, A.; Rydén, L.; et al. Long-Term Kidney Outcomes of Semaglutide in Obesity and Cardiovascular Disease in the SELECT Trial. Nat. Med. 2024, 30, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Apperloo, E.M.; Gorriz, J.L.; Soler, M.J.; Cigarrán Guldris, S.; Cruzado, J.M.; Puchades, M.J.; López-Martínez, M.; Waanders, F.; Laverman, G.D.; van der Aart-van der Beek, A.; et al. Semaglutide in Patients with Overweight or Obesity and Chronic Kidney Disease without Diabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Nat. Med. 2025, 31, 278–285. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Kato, N.P.; Nagatomo, Y.; Kawai, F.; Kitai, T.; Mizuno, A. Fluid Restriction for Patients with Heart Failure: Current Evidence and Future Perspectives. J. Pers. Med. 2024, 14, 741. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Bansal, N.; Cavanaugh, K.L.; Chang, A.; Crowley, S.; Delgado, C.; Estrella, M.M.; Ghossein, C.; Ikizler, T.A.; Koncicki, H.; et al. KDOQI US Commentary on the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2025, 85, 135–176. [Google Scholar] [CrossRef]
- Yan, B.; Su, X.; Xu, B.; Qiao, X.; Wang, L. Effect of Diet Protein Restriction on Progression of Chronic Kidney Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0206134. [Google Scholar] [CrossRef]
- Costa, D.; Patella, G.; Provenzano, M.; Ielapi, N.; Faga, T.; Zicarelli, M.; Arturi, F.; Coppolino, G.; Bolignano, D.; De Sarro, G.; et al. Hyperkalemia in CKD: An Overview of Available Therapeutic Strategies. Front. Med. 2023, 10, 1178140. [Google Scholar] [CrossRef]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef]
- Brown, R.S. Association Between Dietary Potassium Intake and Serum Potassium in CKD. Kidney Int. Rep. 2023, 8, 1479–1480. [Google Scholar] [CrossRef]
- Naismith, D.J.; Braschi, A. An Investigation into the Bioaccessibility of Potassium in Unprocessed Fruits and Vegetables. Int. J. Food Sci. Nutr. 2008, 59, 438–450. [Google Scholar] [CrossRef]
- Parpia, A.S.; L’Abbé, M.; Goldstein, M.; Arcand, J.; Magnuson, B.; Darling, P.B. The Impact of Additives on the Phosphorus, Potassium, and Sodium Content of Commonly Consumed Meat, Poultry, and Fish Products Among Patients with Chronic Kidney Disease. J. Ren. Nutr. 2018, 28, 83–90. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, Tolerability and Efficacy of up-Titration of Guideline-Directed Medical Therapies for Acute Heart Failure (STRONG-HF): A Multinational, Open-Label, Randomised, Trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Cotter, G.; Deniau, B.; Davison, B.; Edwards, C.; Adamo, M.; Arrigo, M.; Barros, M.; Biegus, J.; Celutkiene, J.; Čerlinskaitė-Bajorė, K.; et al. Optimization of Evidence-Based Heart Failure Medications After an Acute Heart Failure Admission: A Secondary Analysis of the STRONG-HF Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 114–124. [Google Scholar] [CrossRef]
- Tomasoni, D.; Davison, B.; Adamo, M.; Pagnesi, M.; Mebazaa, A.; Edwards, C.; Arrigo, M.; Barros, M.; Biegus, J.; Čelutkienė, J.; et al. Safety Indicators in Patients Receiving High-Intensity Care After Hospital Admission for Acute Heart Failure: The STRONG-HF Trial. J. Card. Fail. 2024, 30, 525–537. [Google Scholar] [CrossRef]
- Packer, M.; McMurray, J.J.V. Rapid Evidence-based Sequencing of Foundational Drugs for Heart Failure and a Reduced Ejection Fraction. Eur. J. Heart Fail. 2021, 23, 882–894. [Google Scholar] [CrossRef]
- Agarwal, R.; Green, J.B.; Heerspink, H.J.L.; Mann, J.F.E.; McGill, J.B.; Mottl, A.K.; Rosenstock, J.; Rossing, P.; Vaduganathan, M.; Brinker, M.; et al. Finerenone with Empagliflozin in Chronic Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2025. [Google Scholar] [CrossRef]
- Chaiyakunapruk, N.; Tan, X.; Liang, Y.; Guevarra, M.; Xie, L.; Cheng, A.Y.Y. Real-World Effectiveness of Adding Newer Generation GLP-1RA to SGLT2i in Type 2 Diabetes. Cardiovasc. Diabetol. 2025, 24, 177. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Hauske, S.J.; Canziani, M.E.; Caramori, M.L.; Cherney, D.; Cronin, L.; Heerspink, H.J.L.; Hugo, C.; Nangaku, M.; Rotter, R.C.; et al. Efficacy and Safety of Aldosterone Synthase Inhibition with and without Empagliflozin for Chronic Kidney Disease: A Randomised, Controlled, Phase 2 Trial. Lancet 2024, 403, 379–390. [Google Scholar] [CrossRef]
- Neuen, B.L.; Heerspink, H.J.L.; Vart, P.; Claggett, B.L.; Fletcher, R.A.; Arnott, C.; de Oliveira Costa, J.; Falster, M.O.; Pearson, S.-A.; Mahaffey, K.W.; et al. Estimated Lifetime Cardiovascular, Kidney, and Mortality Benefits of Combination Treatment with SGLT2 Inhibitors, GLP-1 Receptor Agonists, and Nonsteroidal MRA Compared With Conventional Care in Patients with Type 2 Diabetes and Albuminuria. Circulation 2024, 149, 450–462. [Google Scholar] [CrossRef]
- Neuen, B.L.; Yeung, E.K.; Rangaswami, J.; Vaduganathan, M. Combination Therapy as a New Standard of Care in Diabetic and Non-Diabetic Chronic Kidney Disease. Nephrol. Dial. Transplant. 2025, 40, i59–i69. [Google Scholar] [CrossRef]
- Rashid, A.M.; Khan, M.S.; Cherney, D.Z.I.; Mehta, A.; Rangaswami, J.; Shafi, T.; Butler, J. Rapid and Simultaneous Initiation of Guideline-Directed Kidney Therapies in Patients with CKD and Type 2 Diabetes. J. Am. Soc. Nephrol. 2025. [Google Scholar] [CrossRef]
- Murphy, D.P.; Wolfson, J.; Reule, S.; Johansen, K.L.; Ishani, A.; Drawz, P.E. Kidney Outcomes with Sodium-Glucose Cotransporter-2 Inhibitor Initiation after AKI among Veterans with Diabetic Kidney Disease. Kidney360 2024, 5, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Perrin, P.; Muller, C.; Dimitrov, Y.; Chantrel, F.; Heitz, M.; Woerly, A.; Bazin, D.; Faller, A.-L.; Krummel, T.; Hannedouche, T. Assessment of SGLT2 Inhibitors’ Safety and Discontinuation Causes in Patients with Advanced Chronic Kidney Disease: Insights from a Real-World Data Analysis. Clin. Kidney J. 2024, 17, sfae169. [Google Scholar] [CrossRef] [PubMed]

| Trial | Agent (Dose) | Median Follow-Up (yrs) | Composite Cardiovascular Endpoint | Exploratory Renal Composite Endpoint | Macroalbuminuria | Worsening eGFR | Renal Replacement Therapy |
|---|---|---|---|---|---|---|---|
| LEADER | Liraglutide 1.8 mg/d | 3.8 | 13% relative risk reduction HR: 0.87 (95% CI: 0.78–0.97) p = 0.01 | 0.78 [0.67–0.92] * | 0.74 [0.60–0.91] * | 0.89 [0.67–1.19] | 0.87 [0.61–1.24] |
| REWIND | Dulaglutide 1.5 mg/wk | 5.4 | 11% relative risk reduction HR: 0.89 (95% CI: 0.79–0.99) p = 0.026 | 0.85 [0.77–0.93] * | 0.77 [0.68–0.87] * | 0.89 [0.78–1.01] | 0.75 [0.39–1.44] |
| SUSTAIN-6 | Semaglutide 0.5/1 mg/wk | 2.1 | 26% relative risk reduction HR: 0.74 (95% CI: 0.58–0.95) p =< 0.001 | 0.64 [0.46–0.88] * | 0.54 [0.37–0.77] * | 1.28 [0.64–2.58] | 0.91 [0.40–2.07] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, H.; Puckett, C.; Lucas, Y.Q. Integrating and Simplifying Evidence to Optimize Cardiorenal Guideline-Directed Therapies. J. Clin. Med. 2025, 14, 5883. https://doi.org/10.3390/jcm14165883
Singh H, Puckett C, Lucas YQ. Integrating and Simplifying Evidence to Optimize Cardiorenal Guideline-Directed Therapies. Journal of Clinical Medicine. 2025; 14(16):5883. https://doi.org/10.3390/jcm14165883
Chicago/Turabian StyleSingh, Harleen, Carrie Puckett, and Yennie Q. Lucas. 2025. "Integrating and Simplifying Evidence to Optimize Cardiorenal Guideline-Directed Therapies" Journal of Clinical Medicine 14, no. 16: 5883. https://doi.org/10.3390/jcm14165883
APA StyleSingh, H., Puckett, C., & Lucas, Y. Q. (2025). Integrating and Simplifying Evidence to Optimize Cardiorenal Guideline-Directed Therapies. Journal of Clinical Medicine, 14(16), 5883. https://doi.org/10.3390/jcm14165883







