Development of a Novel Ultrasound-Guided Needle Cricothyroidotomy Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Size and Power Analysis
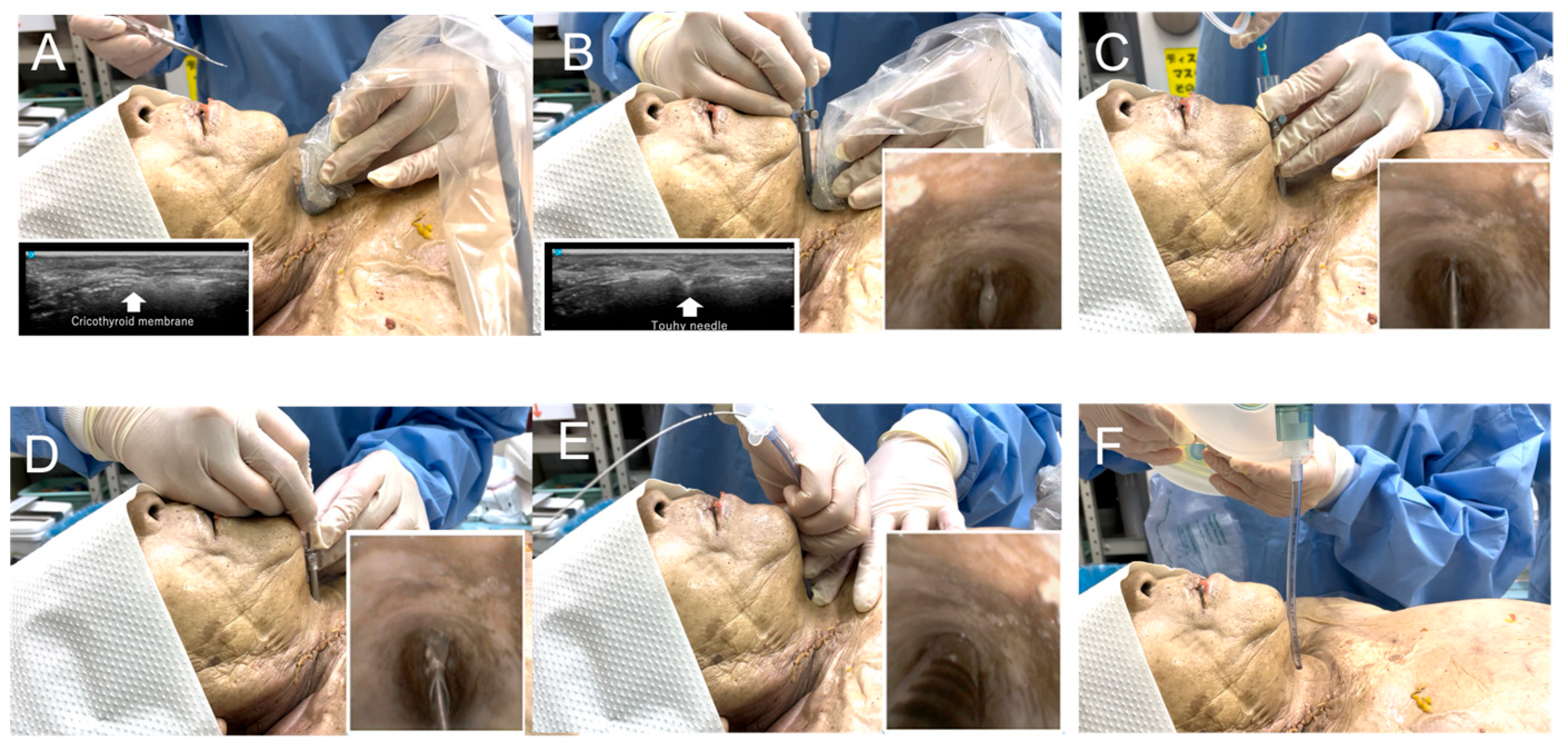
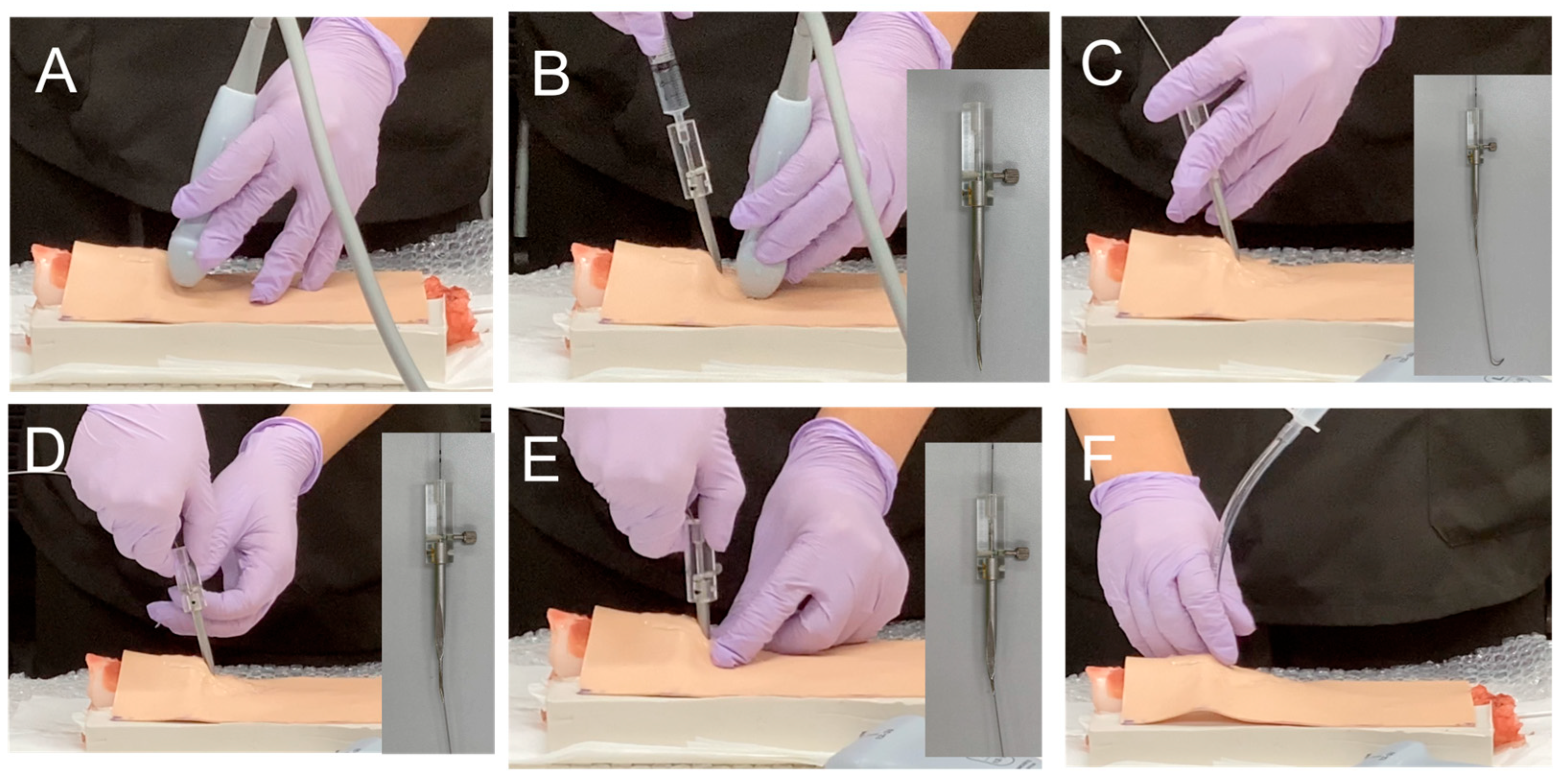
2.3. Development of an Ultrasound-Guided Cricothyroidotomy Kit
2.4. Study Preparation: Video Lecture and Hands-On Training for Cricothyroidotomy
2.5. Preparation of Porcine Ex Vivo Larynges
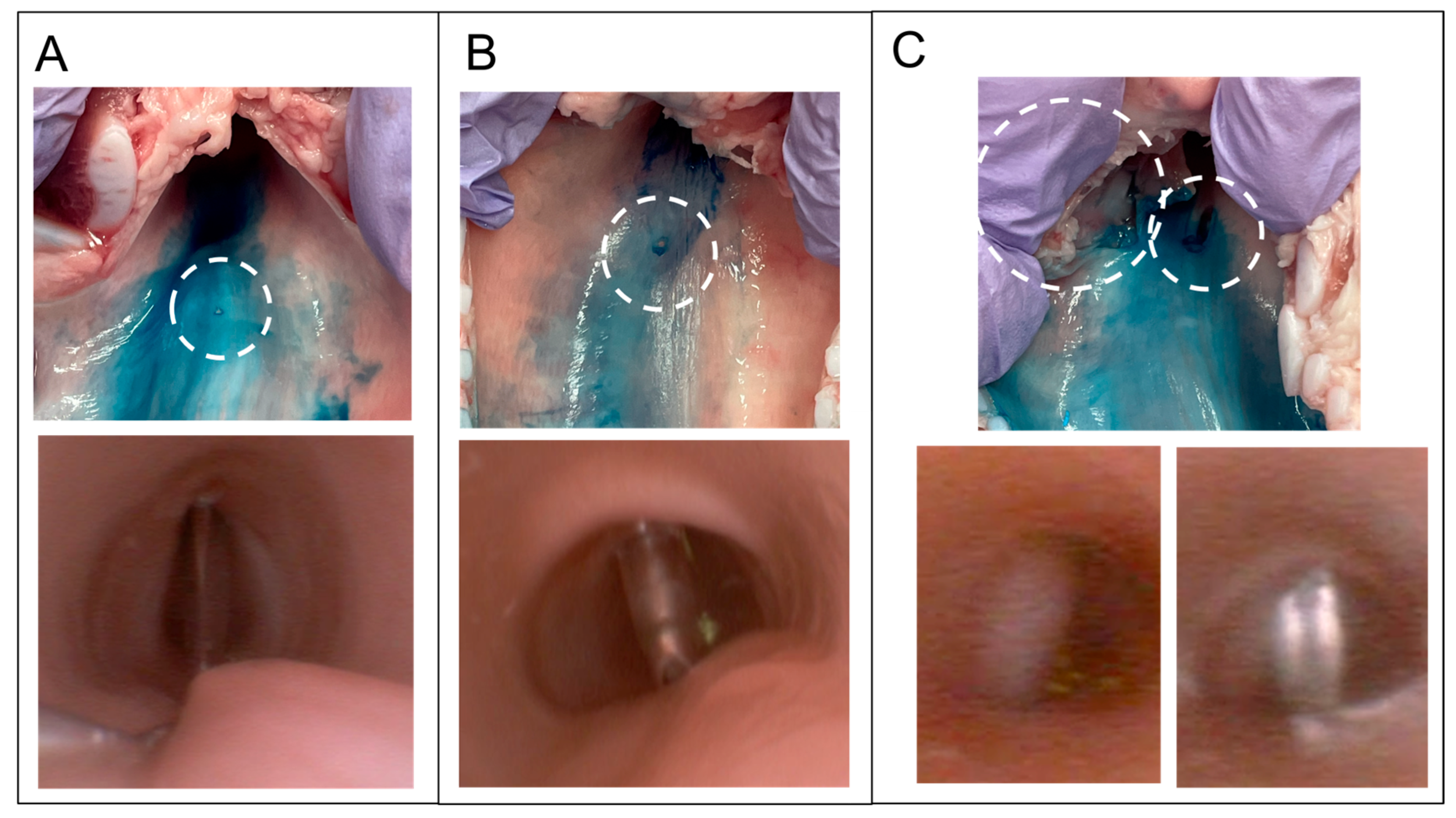
2.6. Ultrasound-Guided Cricothyroidotomy
2.7. Statistical Analysis
3. Results
Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, T.M.; Woodall, N.; Harper, J.; Benger, J.; Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: Intensive care and emergency departments. Br. J. Anaesth. 2011, 106, 632–642. [Google Scholar] [CrossRef] [PubMed]
- You-Ten, K.E.; Siddiqui, N.; Teoh, W.H.; Kristensen, M.S. Point-of-care ultrasound (POCUS) of the upper airway. Can. J. Anaesth. 2018, 65, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.S.; Teoh, W.H.; Rudolph, S.S. Ultrasonographic identification of the cricothyroid membrane: Best evidence, techniques, and clinical impact. Br. J. Anaesth. 2016, 117 (Suppl. 1), i39–i48. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Nakazawa, H.; Tokumine, J.; Yorozu, T. Real-time vs. static ultrasound-guided needle cricothyroidotomy: A randomized crossover simulation trial. Sci. Rep. 2025, 15, 8112. [Google Scholar] [CrossRef] [PubMed]
- Katayama, A.; Watanabe, K.; Tokumine, J.; Lefor, A.K.; Nakazawa, H.; Jimbo, I.; Yorozu, T. Cricothyroidotomy needle length is associated with posterior tracheal wall injury: A randomized crossover simulation study (CONSORT). Medicine 2020, 99, e19331. [Google Scholar] [CrossRef] [PubMed]
- Athanassoglou, V.; Hughes-Jones, H.; Hadjipavlou, G.; Teoh, W.H.; Kristensen, M.S.; Vanner, R. Depth to the airway lumen at the level of the cricothyroid membrane measured by ultrasound. Acta Anaesthesiol. Scand. 2020, 64, 48–52. [Google Scholar] [CrossRef]
- Develi, S.; Yalcin, B.; Yazar, F. Topographical anatomy of cricothyroid membrane and its relation with invasive airway access. Clin. Anat. 2016, 29, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Nagase, M.; Nagase, T.; Tokumine, J.; Saito, K.; Sunami, E.; Shiokawa, Y.; Matsumura, G. Formalin-free soft embalming of human cadavers using N-vinyl-2-pyrrolidone: Perspectives for cadaver surgical training and medical device development. Anat. Sci. Int. 2022, 97, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Pazderník, M. Cricothyrotomy Using QuickTrach [Video]. Prague ICU. Available online: https://www.pragueicu.com/video-courses/cricothyrotomy-using-quicktrach (accessed on 18 May 2025).
- Difficult Airway Society. Front of Neck Access (FONA) Training. Available online: https://database.das.uk.com/content/fona_training (accessed on 18 May 2025).
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Frerk, C.; Mitchell, V.S.; McNarry, A.F.; Mendonca, C.; Bhagrath, R.; Patel, A.; O’Sullivan, E.P.; Woodall, N.M.; Ahmad, I.; Difficult Airway Society Intubation Guidelines Working Group. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br. J. Anaesth. 2015, 115, 827–848. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Tong, Q.J.; Beh, Z.Y.; Quek, K.H.; Ang, B.H. A bench study comparing between scalpel-bougie technique and cannula-to-Melker technique in emergency cricothyroidotomy in a porcine model. Korean J. Anesthesiol. 2018, 71, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Andresen, Å.E.L.; Kramer-Johansen, J.; Kristiansen, T. Percutaneous vs surgical emergency cricothyroidotomy: An experimental randomized crossover study on an animal-larynx model. Acta Anaesthesiol. Scand. 2019, 63, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Yeow, C.; Greaney, L.; Foy, C.; King, W.; Patel, B. Evaluation of a novel cricothyroidotomy introducer in a simulated obese porcine model: A randomised crossover comparison with scalpel cricothyroidotomy. Anaesthesia 2018, 73, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Brännström, A.; Gellerfors, M.; Gustavsson, J.; Günther, M. Comparison of emergency surgical cricothyroidotomy and percutaneous cricothyroidotomy by experienced airway providers in an obese, in vivo porcine hemorrhage airway model. Mil. Med. Res. 2022, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Law, J.A.; Duggan, L.V.; Asselin, M.; Baker, P.; Crosby, E.; Downey, A.; Hung, O.R.; Kovacs, G.; Lemay, F.; Noppens, R.; et al. Canadian Airway Focus Group updated consensus-based recommendations for management of the difficult airway: Part 2. Planning and implementing safe management of the patient with an anticipated difficult airway. Can. J. Anaesth. 2021, 68, 1405–1436. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.S.; Teoh, W.H.; Rudolph, S.S.; Hesselfeldt, R.; Børglum, J.; Tvede, M.F. A randomised cross-over comparison of the transverse and longitudinal techniques for ultrasound-guided identification of the cricothyroid membrane in morbidly obese subjects. Anaesthesia 2016, 71, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.E.; Sweeney, T.W.; Ferre, R.M.; Strout, T.D. Bedside sonography by emergency physicians for the rapid identification of landmarks relevant to cricothyrotomy. Am. J. Emerg. Med. 2008, 26, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Sakai, W.; Nawa, Y.; Junichi, O. Obstructive shock due to tracheal perforation following long-term placement of a tracheostomy tube in a pediatric patient: A case report. JA Clin. Rep. 2022, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Vanner, R.; Crawley, S. Cric-Guide—A review of an innovative scalpel designed for adult surgical cricothyroidotomy. Trends Anaesth. Crit. Care 2023, 53, 101307. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Sharma, A.; Muddassir, K. Broken scalpel blade during emergent cricothyroidotomy: An unexpected complication in a critical situation. Cureus 2020, 12, e8868. [Google Scholar] [CrossRef] [PubMed]


| US-G | Pal-C | PAL-SI | |
|---|---|---|---|
| Accuracy of CTM identification | ○ US guidance | △ Palpation | △ Palpation |
| Size of the incision | ○ Small | ○ Small | △ Moderate |
| Reliability of airway access | ○ High | △ Low | ◎ High |
| Technical complexity | × Multi-step | ○ Simple | △ moderate |
| Risk of posterior wall injury | ○ Low | △ High | ○ Low |
| Risk of bleeding | ○ Low | ○ Low | △ High |
| Key characteristics | Accurate CTM identification Less invasive Multiple cumbersome steps | Simple procedure Small incision High risk for wall injury | High reliability of airway access High invasive Risk for wall injury and bleeding |
| Measurements | US-G | Pal-C | Pal-SI | p Value |
|---|---|---|---|---|
| Success rate % (n) | 100 (22) | 100 (22) | 95 (21) | 1.0 |
| Procedure time m (1st, 3rd) (s) | 80 (63, 115) | 22 (16, 27) | 51 (44, 74) | <0.001 |
| Tracheal wall injury rate % (n) | 18 (4) | 31 (7) | 23 (5) | 0.68 |
| Severe wall injury rate % (n) | 0 (0) | 5 (1) | 14 (3) | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, H.; Nakazawa, H.; Tokumine, J.; Nagase, M.; Saito, K.; Yorozu, T.; Moriyama, K. Development of a Novel Ultrasound-Guided Needle Cricothyroidotomy Device. J. Clin. Med. 2025, 14, 5871. https://doi.org/10.3390/jcm14165871
Watanabe H, Nakazawa H, Tokumine J, Nagase M, Saito K, Yorozu T, Moriyama K. Development of a Novel Ultrasound-Guided Needle Cricothyroidotomy Device. Journal of Clinical Medicine. 2025; 14(16):5871. https://doi.org/10.3390/jcm14165871
Chicago/Turabian StyleWatanabe, Hidenobu, Harumasa Nakazawa, Joho Tokumine, Miki Nagase, Koichiro Saito, Tomoko Yorozu, and Kiyoshi Moriyama. 2025. "Development of a Novel Ultrasound-Guided Needle Cricothyroidotomy Device" Journal of Clinical Medicine 14, no. 16: 5871. https://doi.org/10.3390/jcm14165871
APA StyleWatanabe, H., Nakazawa, H., Tokumine, J., Nagase, M., Saito, K., Yorozu, T., & Moriyama, K. (2025). Development of a Novel Ultrasound-Guided Needle Cricothyroidotomy Device. Journal of Clinical Medicine, 14(16), 5871. https://doi.org/10.3390/jcm14165871







