Surgery for Complex vs. Simple Native Left-Sided Endocarditis: Insights from an Extended Follow-Up on Survival, Recurrent Infection, and Valve Durability
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Study Endpoints
2.4. Definitions
2.5. Surgical Management
2.6. Statistical Analysis
3. Results
3.1. Patient Cohort and Disease Classification
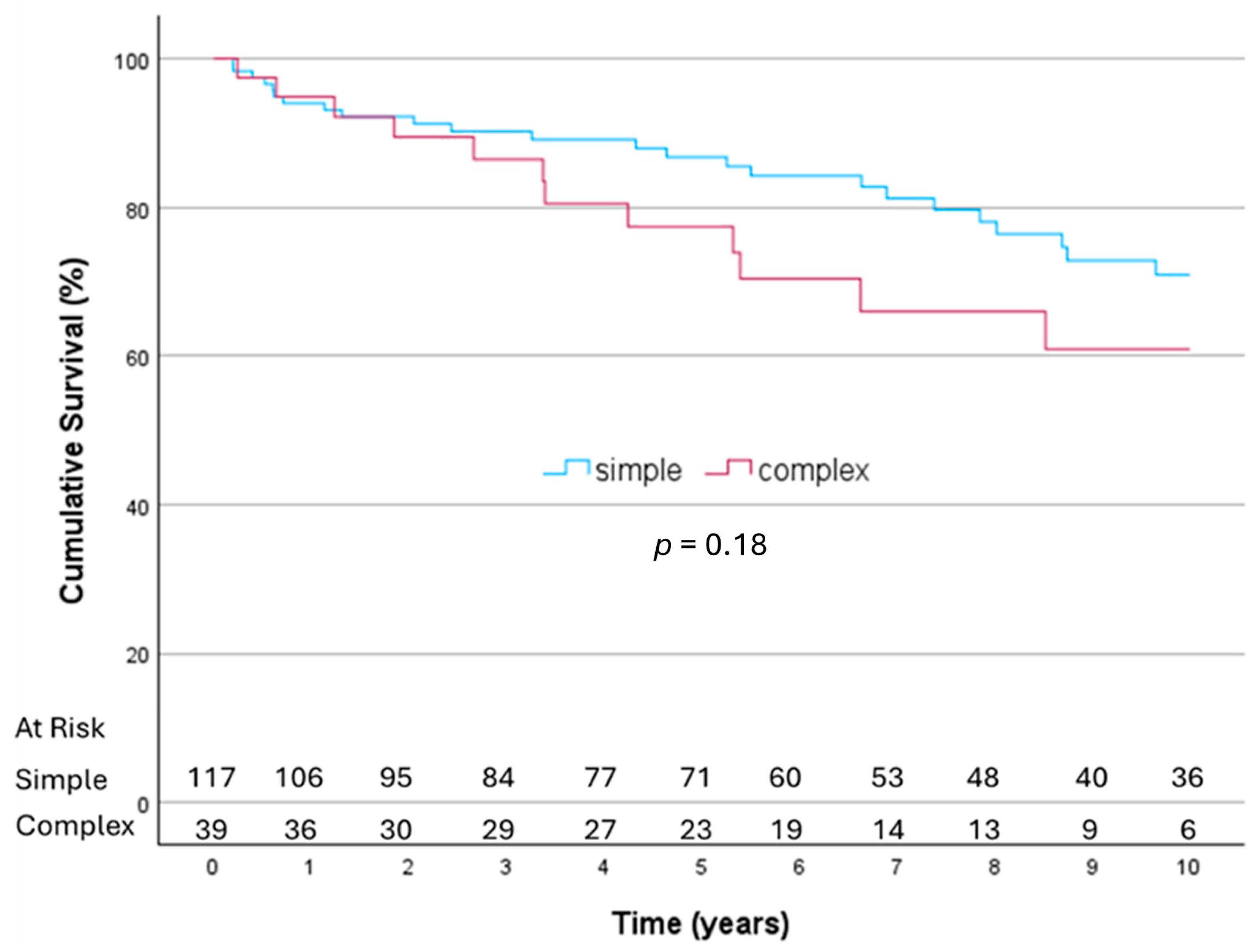
3.2. Early and Long-Term Survival
3.3. Re-Endocarditis
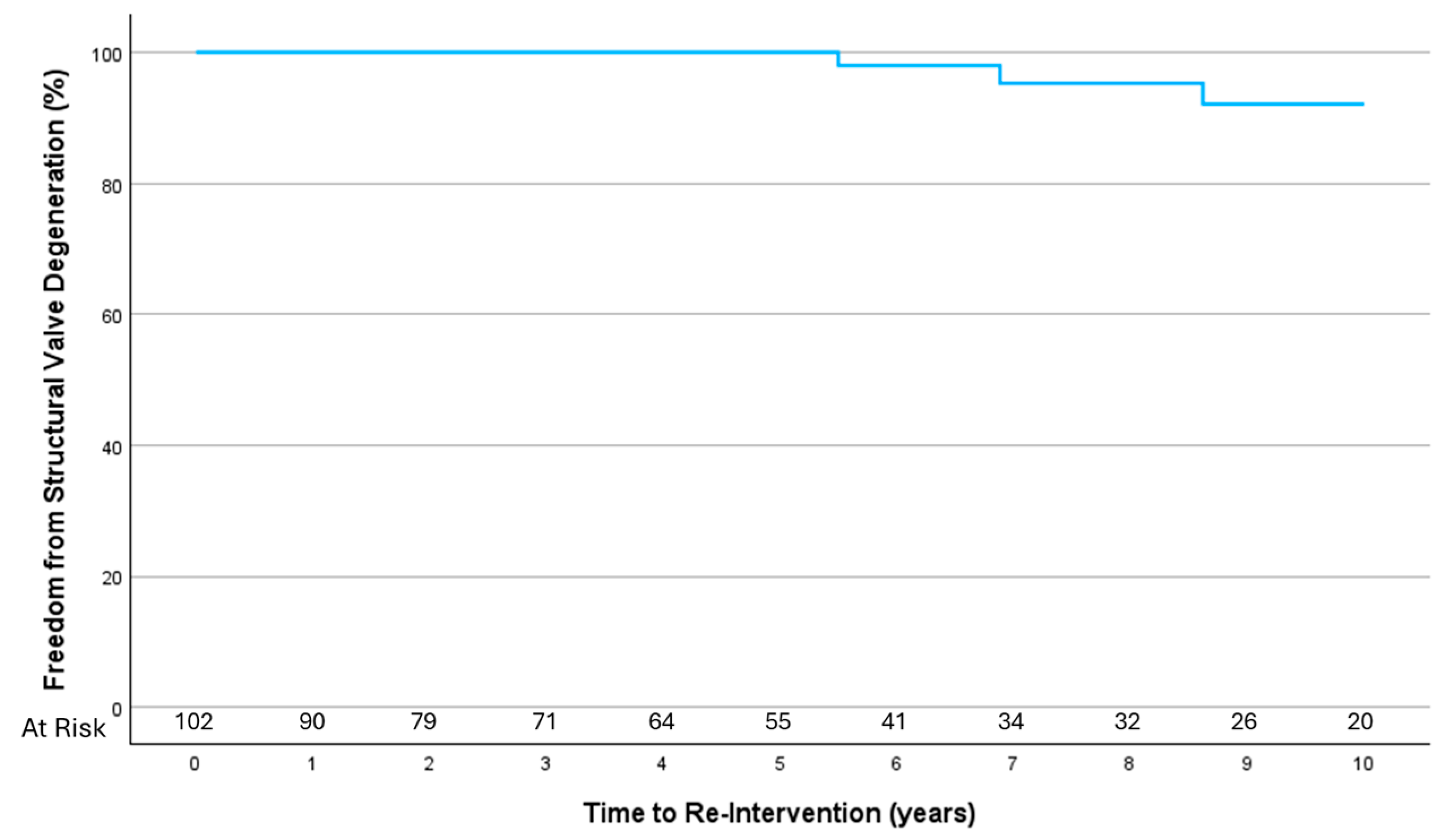
3.4. Structural Valve Degeneration
3.5. Mitral Valve Repair
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BAV | Bicuspid aortic valve |
| CABG | Coronary arteries bypass surgery |
| CHF | Congestive heart failure |
| CONS | Coagulase-negative staphylococcus aureus |
| COPD | Chronic obstructive pulmonary disease |
| DM | Diabetes mellitus |
| EF | Ejection fraction |
| HACEK group | Hemophilus, Actinobacillus, Cardiobacterium, Eikenella, Kingella |
| HTN | Hypertension |
| ICU | Intensive care unit |
| IE | Infective endocarditis |
| IVDU | Intravenous drug user |
| LAA | Left atrial appendage |
| MV | Mitral valve |
| PVD | Peripheral vascular disease |
| RHD | Rheumatic heart disease |
| SVD | Structural valve degeneration |
| VIV | Valve in valve |
| TF | Trans-femoral |
| TV | Tricuspid valve |
Appendix A
| No | Surgery Date | Age | Simple/ Complex | Surgery | Re-do Procedure | Etiology | Date | Time Elapsed |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 April 2005 | 46 | Simple | AVR + MVR bioprostheses | Re-do MVR bioprosthesis + TV annuloplasty | SVD | 21 February 2012 | 7 years |
| 2 | 25 April 2006 | 43 | Simple | MV repair: resection of P2, part of P1, sliding plasty, annuloplasty | MVR mechanical | Early failure susp. Re-endocarditis | 4 May 2006 | 9 days |
| 3 | 31 May 2006 | 55 | Simple | AVR Mechanical | Re-AVR mechanical | Paravalvular leak | 11 June 2006 | 11 days |
| 4 | 28 May 2006 | 48 | Simple | MVR Mechanical + concomitant TV repair | re-do AVR mechanical + implantation of epicardial electrode | Late aortic regurgitation | 22 July 2021 | 15 years |
| 5 | 29 November 2006 | 77 | Simple | AVR + MVR bioprostheses | VIV mitral | SVD | 1 January 2022 | 16 years |
| 6 | 7 March 2007 | 44 | Simple | AVR bioprosthesis | Re-do CABG | IHD | 15 February 2015 | 8 years |
| 7 | 26 March 2008 | 66 | Simple | AVR bioprosthesis + CABG | TF VIV Evolut R 23 | SVD | 19 February 2020 | 12 years |
| 8 | 9 July 2009 | 56 | Simple | MVR bioprosthesis + CABG+ closure of LAA | TF mitral VIV Sapien 3 26 + PCI to RCA | SVD | 2 April 2020 | 11 years |
| 9 | 23 May 2012 | 55 | Complex | Aortic root replacement freestyle, MV repair: patch reconstruction of aorto-mitral continuity and anterior leaflet | Re-root replacement with biological composite graft | Re-endocarditis | 16 January 2024 | 12 years |
| 10 | 22 August 2012 | 57 | Simple | MVR bioprosthesis + CABG | TF mitral VIV sapien 3 + PCI to SVG | SVD | 19 February 2018 | 6 years |
| 11 | 1 September 2014 | 53 | Complex | AVR mechanical | Removal of pacemaker leads | Device-related right-sided endocarditis, normal prosthetic valve | 10 November 2020 | 6 years |
| 12 | 7 September 2015 | 66 | Simple | MVR bioprosthesis + TV annuloplasty + lt.-sided cryo-ablation + Excision of LAA | Mitral VIV Sapien 3 | SVD | 18 April 2024 | 9 years |
| 13 | 18 November 2020 | 60 | Simple | MV repair: resection of posterior leaflet p2–3, annuloplasty + CABG | MVR bioprosthesis + TV annuloplasty | Early failure | 10 December 2020 | 3 weeks |
| 14 | 13 June 2022 | 47 | Simple | MVR mechanical | CABG | IHD | 4 July 2023 | 1 year |
References
- Li, M.; Kim, J.B.; Sastry, B.K.S.; Chen, M. Infective endocarditis. Lancet 2024, 404, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Marsan, N.A.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- Shavit, R.; Orvin, K.; Toledano, R.; Shaked, H.; Rubchevsky, V.; Shapira, Y.; Kornowski, R.; Aravot, D.; Sharony, R. Does Perivalvular Involvement Affect the Long-Term Surgical Outcomes of Primary Left-Sided Endocarditis? Am. J. Cardiol. 2023, 186, 135–141. [Google Scholar] [CrossRef]
- STS Adult Cardiac Surgery Database Data Specifications. Version 4.20.2. Available online: http://www.sts.org (accessed on 16 January 2025).
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Said, S.M.; Abdelsattar, Z.M.; Schaff, H.V.; Greason, K.L.; Daly, R.C.; Pochettino, A.; Joyce, L.D.; Dearani, J.A. Outcomes of surgery for infective endocarditis: A single-Centre experience of 801 patients. Eur. J. Cardiothorac. Surg. 2018, 53, 435–439. [Google Scholar] [CrossRef]
- Fedoruk, L.M.; Jamieson, W.R.E.; Ling, H.; MacNab, J.S.; Germann, E.; Karim, S.S.; Lichtenstein, S.V. Predictors of recurrence and reoperation for prosthetic valve endocarditis after valve replacement surgery for native valve endocarditis. J. Thorac. Cardiovasc. Surg. 2009, 137, 326–333. [Google Scholar] [CrossRef]
- David, T.E.; Regesta, T.; Gavra, G.; Armstrong, S.; Maganti, M.D. Surgical treatment of paravalvular abscess: Long-term results. Eur. J. Cardiothorac. Surg. 2007, 31, 43–48. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kim, Y.-J.; Kim, S.-H.; Sun, B.J.; Kim, D.-H.; Yun, S.-C.; Song, J.-M.; Choo, S.J.; Chung, C.-H.; Song, J.-K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef]
- Harris, W.M.; Sinha, S.; Caputo, M.; Angelini, G.D.; Ahmed, E.M.; Rajakaruna, C.; Benedetto, U.; Vohra, H.A. Surgical outcomes and optimal approach to treatment of aortic valve endocarditis with aortic root abscess. J. Card. Surg. 2022, 37, 1917–1925. [Google Scholar] [CrossRef]
- Yang, B.; Caceres, J.; Farhat, L.; Le, T.; Brown, B.; Pierre, E.S.; Wu, X.; Kim, K.M.; Patel, H.J.; Deeb, G.M. Root abscess in the setting of infectious endocarditis: Short and long-term outcomes. JTCVS 2021, 162, 1049–1059. [Google Scholar] [CrossRef]
- Pizzino, F.; Paradossi, U.; Trimarchi, G.; Benedetti, G.; Marchi, F.; Chiappino, S.; Conti, M.; Di Bella, G.; Murzi, M.; Di Sibio, S.; et al. Clinical Features and Patient Outcomes in Infective Endocarditis with Surgical Indication: A Single-Centre Experience. J. Cardiovasc. Dev. Dis. 2024, 11, 138. [Google Scholar] [CrossRef]
- Citro, R.; Chan, K.-L.; Miglioranza, M.H.; Laroche, C.; Benvenga, R.M.; Furnaz, S.; Magne, J.; Olmos, C.; Paelinck, B.P.; Pasquet, A.; et al. EURO ENDO Investigators group. Clinical profile and outcome of recurrent infective endocarditis. Heart 2022, 108, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, M.E.; Mehaffey, J.H.; Chang, S.-C.; O’gAra, P.T.; Mack, M.J.; Goldstone, A.B.; Chikwe, J.; Gillinov, A.M.; Wu, C.; Fontana, G.P.; et al. Bioprosthetic vs mechanical aortic valve replacement in patients 40-75 years. JACC 2025, 85, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, M.; Anttila, V.; Rautava, P.; Gunn, J.; Kytö, V. Long-term outcomes of mechanical versus biological valve prosthesis in native mitral valve infective endocarditis. Scand. Cardiovasc. J. 2022, 56, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Salsano, A.; Di Mauro, M.; Labate, L.; Della Corte, A.; Presti, F.L.; De Bonis, M.; Trumello, C.; Rinaldi, M.; Stura, E.C.; Dato, G.A.; et al. Survival and Recurrence of Endocarditis following Mechanical vs. Biological Aortic Valve Replacement for Endocarditis in Patients Aged 40 to 65 Years: Data from the INFECT-Registry. J. Clin. Med. 2024, 13, 153. [Google Scholar] [CrossRef]
- Salaun, E.; Clavel, M.A.; Rodés-Cabau, J.; Pibarot, P. Bioprosthetic aortic valve durability in the era of transcatheter aortic valve implantation. Heart 2018, 104, 1323–1332. [Google Scholar] [CrossRef]
- Pibarot, P.; Herrmann, H.C.; Wu, C.; Hahn, R.T.; Otto, C.M.; Abbas, A.E.; Chambers, J.; Dweck, M.R.; Leipsic, J.A.; Simonato, M.; et al. Standardized definitions of bioprosthetic valve dysfunction following aortic or mitral valve replacement: JACC state-of-the art review. JACC 2022, 80, 545–561. [Google Scholar] [CrossRef]
- Ackermann, P.; Marin-Cuartas, M.; Weber, C.; De La Cuesta, M.; Lichtenberg, A.; Petrov, A.; Hagl, C.; Aubin, H.; Matschke, K.; Diab, M.; et al. Sex-related differences in patients with infective endocarditis requiring cardiac surgery: Insights from the CAMPAIGN Study Group. Eur. J. Cardiothorac. Surg. 2024, 66, ezae292. [Google Scholar] [CrossRef]
- Lazam, S.; Vanoverschelde, J.-L.; Tribouilloy, C.; Grigioni, F.; Suri, R.M.; Avierinos, J.-F.; de Meester, C.; Barbieri, A.; Rusinaru, D.; Russo, A.; et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: Analysis of a large, prospective, multicenter, international registry. Circulation 2017, 135, 410–422. [Google Scholar] [CrossRef]
- Miura, T.; Obase, K.; Ariyoshi, T.; Matsumaru, I.; Yokose, S.; Nakaji, S.; Tasaki, Y.; Shimada, T.; Miyamoto, J.; Eishi, K. Impact of Lesion Localization on Durability of Mitral Valve Repair in Infective Endocarditis. Ann. Thorac. Surg. 2020, 109, 1335–1342. [Google Scholar] [CrossRef]
- Solari, S.; De Kerchove, L.; Tamer, S.; Aphram, G.; Baert, J.; Borsellino, S.; Mastrobuoni, S.; Navarra, E.; Noirhomme, P.; Astarci, P.; et al. Active infective mitral valve endocarditis: Is a repair-oriented surgery safe and durable? Eur. J. Cardiothorac. Surg. 2019, 55, 256–262. [Google Scholar] [CrossRef]
- Helmers, M.R.; Fowler, C.; Kim, S.T.; Shin, M.; Han, J.J.; Arguelles, G.; Bryski, M.; Hargrove, W.C.; Atluri, P. Repair of Isolated Native Mitral Valve Endocarditis: A Propensity Matched Study. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 490–499. [Google Scholar] [CrossRef]
- Awad, A.K.; Wilson, K.; Elnagar, M.A.; Elbadawy, M.A.; Fathy, M.H. To repair or to replace in mitral valve infective endocarditis? an updated meta-analysis. J. Cardiothorac. Surg. 2024, 19, 247. [Google Scholar] [CrossRef]
- Tomšič, A.; de Weger, A.; van der Stoel, M.; Klautz, R.J.M.; Palmen, M. A Nationwide Study on Mitral Valve Repair vs Replacement for Active Endocarditis. Cardiothoracic Surgery Registration Committee of the Netherlands Heart Registration. Ann. Thorac. Surg. 2024, 117, 120–126. [Google Scholar] [CrossRef] [PubMed]
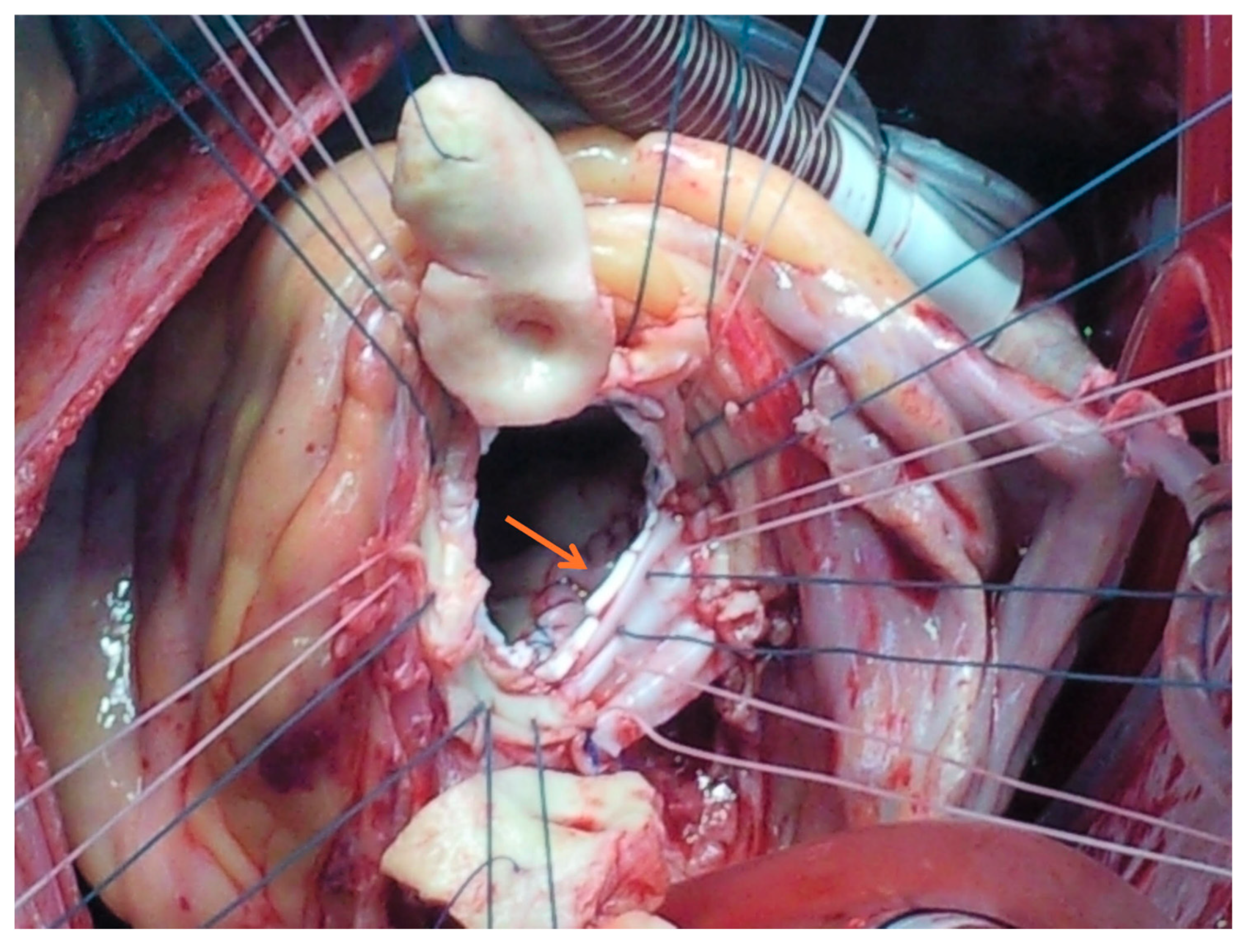
| Parameter † | Overall (n = 177) | Simple (n = 129) | Complex (n = 48) | p-Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean ± SD (years) | 59.6 ± 13.8 | 58.56 ± 14.4 | 62.42 ± 11.4 | 0.09 | |
| Male gender, n (%) | 127 (71.8%) | 91 (70.5%) | 36 (75.0%) | 0.56 | |
| Comorbidities | |||||
| DM | 43 (24.3%) | 26 (20.2%) | 17 (35.4%) | 0.03 | |
| HTN | 76 (42.9%) | 53 (41.1%) | 23 (47.9%) | 0.41 | |
| COPD | 10 (5.6%) | 6 (4.7%) | 4 (8.3%) | 0.46 | |
| PVD | 7 (4.0%) | 6 (4.7%) | 1 (2.1%) | 0.67 | |
| Atrial fibrillation | 30 (16.9%) | 21 (16.3%) | 9 (18.8%) | 0.66 | |
| IVDU | 4 (2.3%) | 2 (1.6%) | 2 (4.2%) | 0.30 | |
| Previous stroke (old) | 7 (4.0%) | 5 (3.9%) | 2 (4.2%) | 0.45 | |
| Predisposing cardiac condition | Myxomatous valve | 24 (13.6%) | 20 (15.5%) | 4 (8.3%) | 0.21 |
| BAV | 19 (10.7%) | 9 (7.0%) | 10 (20.8%) | 0.008 | |
| RHD | 4 (2.3%) | 4 (3.1%) | 0 (0.0%) | 0.57 | |
| Valve stenosis/regurgitation | 19 (10.7%) | 15 (11.6%) | 4 (8.3%) | 0.53 | |
| Other | 5 (2.8%) | 3 (2.3%) | 2 (4.2%) | 0.61 | |
| Renal Failure | Chronic | 19 (11.2%) | 12 (9.5%) | 7 (16.3%) | 0.26 |
| Dialysis | 11 (6.2%) | 5 (3.9%) | 6 (12.5%) | 0.07 | |
| LV dysfunction | Preserved (EF > 50%) | 154 (87.0%) | 115 (89.1%) | 39 (81.3%) | 0.20 |
| Mild (EF 40–50%) | 16 (9.0%) | 10 (7.8%) | 6 (12.5%) | ||
| Moderate (EF 30–40%) | 2 (1.1%) | 2 (1.6%) | 0 (0.0%) | ||
| Severe (EF < 30%) | 5 (2.8%) | 2 (1.6%) | 3 (6.3%) | ||
| Clinical presentation | |||||
| Critical state | 33 (18.6%) | 21 (16.3%) | 12 (25.0%) | 0.18 | |
| Recent Stroke | 30 (16.9%) | 22 (17.1%) | 8 (16.7%) | 0.85 | |
| Atrioventricular block | 6 (3.4%) | 0 (0.0%) | 6 (12.5%) | <0.001 | |
| Acute renal failure | 19 (10.7%) | 10 (7.8%) | 9 (18.8%) | 0.03 | |
| Valve involved | Aortic | 71 (40.1%) | 46 (35.7%) | 25 (52.1%) | 0.014 |
| Mitral | 90 (50.8%) | 74 (57.4%) | 16 (33.3%) | ||
| Both | 16 (9.0%) | 9 (7.0%) | 7 (14.6%) | ||
| WBC | 10.29 ± 5.28 | 9.57 ± 4.09 | 12.22 ± 7.31 | 0.02 | |
| Creatinine | 1.43 ± 1.61 | 1.19 ± 1.11 | 2.05 ± 2.42 | 0.02 | |
| Hemoglobin | 10.86 ± 1.90 | 11.06 ± 1.94 | 10.34 ± 1.71 | 0.03 | |
| Causative agent | S. aureus | 31 (17.5%) | 20 (15.5%) | 11 (22.9%) | 0.25 |
| Strep. species | 65 (36.7%) | 49 (38.0%) | 16 (33.3%) | 0.57 | |
| Enterococcus | 13 (7.3%) | 13 (10.1%) | 0 (0%) | 0.02 | |
| CONS | 20 (11.3%) | 13 (10.1%) | 7 (14.6%) | 0.40 | |
| HACEK | 5 (2.8%) | 2 (1.6%) | 3 (6.3%) | 0.12 | |
| Other | 16 (9.0%) | 11 (8.5%) | 5 (10.4%) | 0.77 | |
| Negative culture | 17 (9.6%) | 11 (8.5%) | 6 (12.5%) | 0.40 | |
| Healed | 10 (5.6%) | 10 (7.8%) | 0 (0.0%) | 0.06 | |
| Indication | Uncontrolled infection | 37 (20.9%) | 19 (14.7%) | 18 (37.5%) | <0.001 |
| Emboli | 22 (12.4%) | 17 (13.2%) | 5 (10.4%) | 0.62 | |
| CHF/valve dysfunction | 118 (66.7%) | 93 (72.1%) | 25 (52.1%) | 0.01 | |
| Vegetation size (mm) | 14.12 ± 5.74 (n = 110) | 13.99 ± 5.8 (n = 78) | 14.44 ± 5.66 (n = 32) | 0.71 | |
| Operative data | |||||
| Valve type | Tissue | 122 (68.9%) | 85 (65.9%) | 37 (77.1%) | 0.15 |
| Mechanical | 30 (16.9%) | 20 (15.5%) | 10 (20.8%) | 0.40 | |
| Repair | 32 (18.1%) | 26 (20.2%) | 6 (12.5%) | 0.24 | |
| Concomitant procedures | 58 (32.8%) | 46 (35.7%) | 12 (25.0%) | 0.18 | |
| CABG | 22 (12.4%) | 20 (15.5%) | 2 (4.2%) | 0.34 | |
| MV repair | 2 (1.1%) | 1 (0.8%) | 1 (2.1%) | ||
| TV annuloplasty | 16 (9.0%) | 13 (10.1%) | 3 (6.3%) | ||
| Ablation/excision of LAA | 9 (5.1%) | 6 (4.7%) | 3 (6.3%) | ||
| Other | 9 (5.1%) | 6 (4.7%) | 3 (6.3%) | ||
| All (n = 177) | Simple (n = 129) | Complex (n = 48) | p-Value | ||
|---|---|---|---|---|---|
| In hospital | Operative mortality | 21 (11.9%) | 12 (9.3%) | 9 (18.8%) | 0.08 |
| Length of stay—days (median, interquartile range) | 15 (10–22) | 15 (9.5–22) | 15 (11–21.75) | 0.67 | |
| ICU stay (median, days) | 2 (1–4) | 2 (1–3.25) | 2 (1–6.75) | 0.04 | |
| Stroke | 10 (5.6%) | 8 (6.2%) | 2 (4.2%) | 0.73 | |
| Pacemaker implantation | 11 (6.2%) | 5 (3.9%) | 6 (12.5%) | 0.07 | |
| Acute renal failure | 27 (15.3%) | 16 (12.4%) | 11 (22.9%) | 0.08 | |
| Prolonged ventilation | 28 (15.8%) | 15 (11.6%) | 13 (27.1%) | 0.01 | |
| Late | Wound infection | 3 (1.9%) | 3 (2.6%) | 0 (0.0%) | 0.56 |
| Re-endocarditis | 4 (2.5%) | 2 (1.7%) | 2 (5.1%) | 0.17 | |
| Reoperation d/t re-endocarditis | 1 (0.6%) | 0 (0.0%) | 1 (2.6%) | 0.27 | |
| Re-intervention for structural valve degeneration † | 6 (5.9%) | 6 (8.2%) | 0 (0.0%) | 0.18 | |
| p-Value | Hazard Ratio | 95% C.I. | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Complex | 0.167 | 1.675 | 0.806 | 3.482 |
| Age > 65 | 0.015 | 2.380 | 1.179 | 4.801 |
| Female gender | 0.042 | 1.933 | 1.023 | 3.656 |
| Chronic renal failure/dialysis | <0.01 | 3.566 | 1.798 | 7.075 |
| Mechanical valve | 0.225 | 0.455 | 0.128 | 1.621 |
| Diabetes mellitus | 0.304 | 1.481 | 0.700 | 3.134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shavit, R.; Orvin, K.; Shaked, H.; Rubchevsky, V.; Shapira, Y.; Kornowski, R.; Sharony, R. Surgery for Complex vs. Simple Native Left-Sided Endocarditis: Insights from an Extended Follow-Up on Survival, Recurrent Infection, and Valve Durability. J. Clin. Med. 2025, 14, 5870. https://doi.org/10.3390/jcm14165870
Shavit R, Orvin K, Shaked H, Rubchevsky V, Shapira Y, Kornowski R, Sharony R. Surgery for Complex vs. Simple Native Left-Sided Endocarditis: Insights from an Extended Follow-Up on Survival, Recurrent Infection, and Valve Durability. Journal of Clinical Medicine. 2025; 14(16):5870. https://doi.org/10.3390/jcm14165870
Chicago/Turabian StyleShavit, Reut, Katia Orvin, Hila Shaked, Victor Rubchevsky, Yaron Shapira, Ran Kornowski, and Ram Sharony. 2025. "Surgery for Complex vs. Simple Native Left-Sided Endocarditis: Insights from an Extended Follow-Up on Survival, Recurrent Infection, and Valve Durability" Journal of Clinical Medicine 14, no. 16: 5870. https://doi.org/10.3390/jcm14165870
APA StyleShavit, R., Orvin, K., Shaked, H., Rubchevsky, V., Shapira, Y., Kornowski, R., & Sharony, R. (2025). Surgery for Complex vs. Simple Native Left-Sided Endocarditis: Insights from an Extended Follow-Up on Survival, Recurrent Infection, and Valve Durability. Journal of Clinical Medicine, 14(16), 5870. https://doi.org/10.3390/jcm14165870






