Sedation and Analgesia for Intubation, LISA, and INSURE Procedures in Israeli NICUs: Caregivers’ Practices and Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Results
- Responder Demographics:
- Pharmacologic Agents Used:
- Elective Intubation vs. LISA/INSURE:
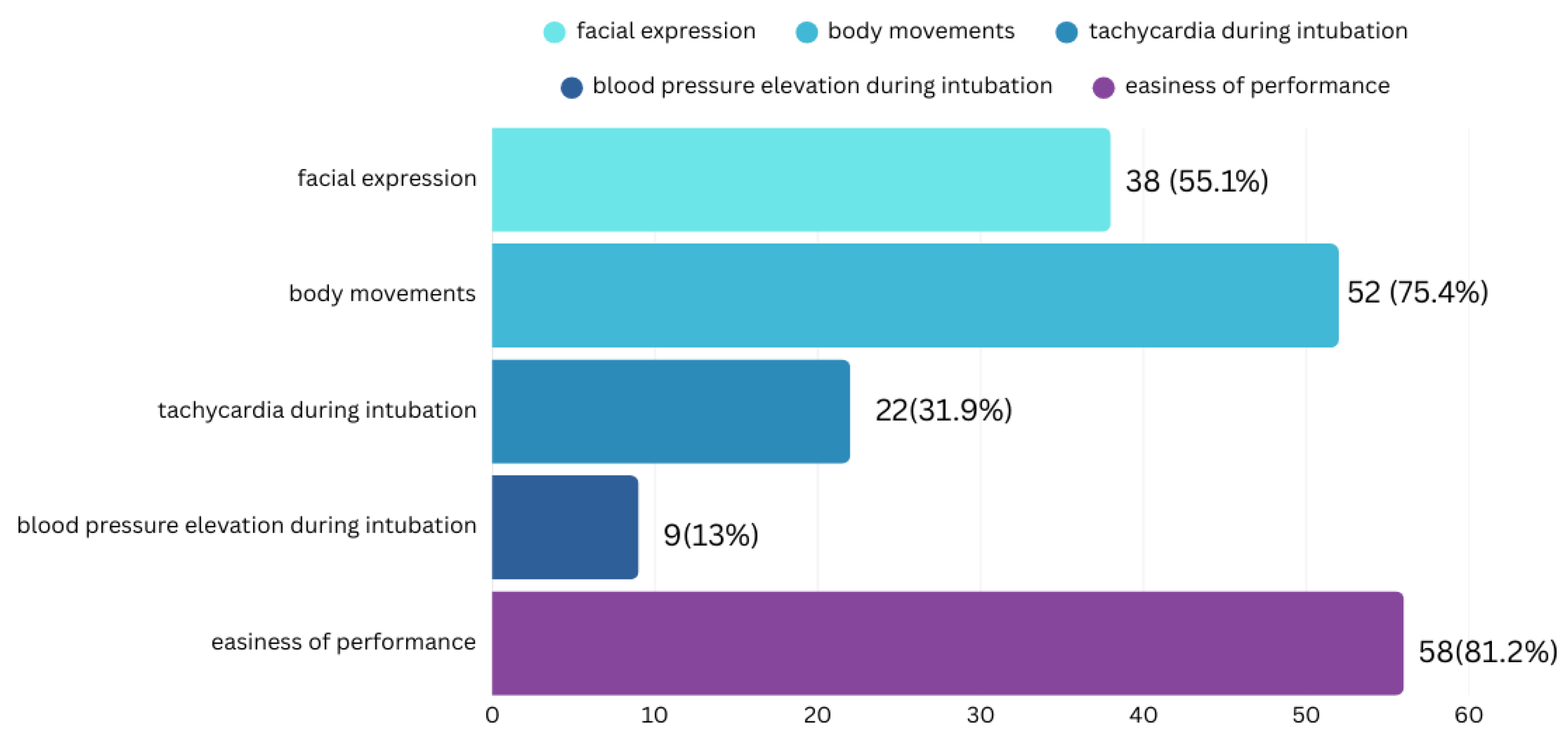
- Premedication Effectiveness and Procedure:
- Premedication Complications:
- Standardized Protocols:
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NICU | Neonatal Intensive Care Unit |
| LISA | Less Invasive Surfactant Administration |
| INSURE | Intubation–Surfactant–Extubation |
| ELBW | Extremely Low-Birth-Weight (preterm infants weighing ≤ 1000 g at birth) |
| VLBW | Very-Low-Birth-Weight (infants weighting ≤ 1500 g at birth) |
| RDS | Respiratory Distress Syndrome |
References
- Anand, K.J.; Hickey, P.R. Pain and its effects in the human neonate and fetus. N. Engl. J. Med. 1987, 317, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Krauss, B. Management of acute pain and anxiety in children undergoing procedures in the emergency department. Pediatr. Emerg. Care 2001, 13, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Vinall, J.; Miller, S.P.; Chau, V.; Brummelte, S.; Synnes, A.R.; Grunau, R.E. Neonatal pain in relation to postnatal growth in infants born very preterm. Pain 2012, 153, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ranger, M.; Chau, C.M.; Garg, A.; Woodward, T.S.; Beg, M.F.; Bjornson, B.; Poskitt, K.; Fitzpatrick, K.; Synnes, A.R.; Miller, S.P.; et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS ONE 2013, 8, e76702. [Google Scholar] [CrossRef]
- Tortora, D.; Severino, M.; Di Biase, C.; Malova, M.; Parodi, A.; Minghetti, D.; Traggiai, C.; Uccella, S.; Boeri, L.; Morana, G.; et al. Early pain exposure influences functional brain connectivity in very preterm neonates. Front. Neurosci. 2019, 13, 418. [Google Scholar] [CrossRef]
- Grunau, R.E.; Tu, M.T. Long-term consequences of pain in human neonates. Pain 2020, 161 (Suppl. S1), S76–S85. [Google Scholar] [CrossRef]
- Cruz, M.D.; Fernandes, A.M.; Oliveira, C.R. Epidemiology of painful procedures performed in neonates: A systematic review of observational studies. Eur. J. Pain 2016, 20, 1167–1175. [Google Scholar] [CrossRef]
- Whyte, S.; Birrell, G.; Wyllie, J.; Woolf, A. Premedication before intubation in UK neonatal units. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F406–F407. [Google Scholar] [CrossRef]
- Mari, J.; Franczia, P.; Margas, W.; Rutkowski, J.; Bebrysz, M.; Bokiniec, R.; Seliga-Siwecka, J. International consensus is needed on premedication for non-emergency neonatal intubation after survey found wide-ranging policies and practices in 70 countries. Acta Paediatr. 2020, 109, 472–479. [Google Scholar] [CrossRef]
- Sweet, D.; Carnelli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European consensus guidelines on the management of respiratory distress syndrome—2019 update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Sanghera, R.; Boyle, E.M. New techniques, new challenges—The dilemma of pain management for less invasive surfactant administration? Pediatr. Neonatal Pain 2020, 3, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Aldana-Aguirre, J.C.; Pinto, M.; Featherstone, R.M.; Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F17–F23. [Google Scholar] [CrossRef] [PubMed]
- Herting, E.; Härtel, C.; Göpel, W. Less invasive surfactant administration (LISA): Chances and limitations. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F655–F659. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Chonat, S.; Gowda, H.; Clarke, P.; Curley, A. Use of premedication for intubation in tertiary neonatal units in the United Kingdom. Paediatr. Anaesth. 2009, 19, 1178–1182. [Google Scholar] [CrossRef]
- Wheeler, B.; Broadbent, R.; Reith, D. Premedication for neonatal intubation in Australia and New Zealand: A survey of current practice. J. Paediatr. Child. Health 2012, 48, 34–39. [Google Scholar] [CrossRef]
- Durrmeyer, X.; Daoud, P.; Decombert, F.; Boileau, P.; Renolleau, S.; Zana-Taib, E.; Saizou, C.; Lapillonne, A.; Granier, M.; Durand, P.; et al. Premedication for neonatal endotracheal intubation: Results from the epidemiology of procedural pain in neonates study. Pediatr. Crit. Care Med. 2013, 14, e169–e175. [Google Scholar] [CrossRef]
- Muniraman, H.K.; Yaari, J.; Hand, I. Premedication use before nonemergent intubation in the newborn infant. Am. J. Perinatol. 2015, 32, 89–94. [Google Scholar] [CrossRef]
- Raban, M.S.; Joolay, Y.; Horn, A.R.; Harrison, M.C. Newborns should be receiving premedication before elective intubation. S. Afr. Med. J. 2014, 104, 873. [Google Scholar] [CrossRef]
- Dekker, J.; Lopriore, E.; Rijken, M.; Rijntjes-Jacobs, E.; Smith-Wintjens, V.; Te Pas, A. Sedation during minimal invasive surfactant therapy in preterm infants. Neonatology 2016, 109, 308–313. [Google Scholar] [CrossRef]
- Dekker, J.; Lopriore, E.; van Zanten, H.A.; Tan, R.N.G.B.; Hooper, S.B.; Te Pas, A.B. Sedation during minimal invasive surfactant therapy: A randomized controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F378–F383. [Google Scholar] [CrossRef]
- Fernandez, C.; Boix, H.; Camba, F.; Comuñas, J.J.; Castillo, F. Less invasive surfactant administration in Spain: A survey regarding its practice, the target population, and premedication use. Am. J. Perinatol. 2020, 37, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Mimoglu, E.; Joyce, K.; Basma, M.; Sathiyamurthy, S.; Banerjee, J. Variability of neonatal premedication practices for endotracheal intubation and LISA in the UK (NeoPRINT survey). Early Hum. Dev. 2023, 183, 105808. [Google Scholar] [CrossRef] [PubMed]
- Durrmeyer, X.; Walter-Nicolet, E.; Chollat, C.; Chabernaud, J.L.; Chery Tardy, A.C.; Berenguer, D.; Bedu, A.; Zayat, N.; Roué, J.M.; Beissel, A.; et al. Premedication before laryngoscopy in neonates: Evidence based statement from the French society of neonatology (SFN). Front. Pediatr. 2023, 10, 1075184. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, P.; Szpecht, D.; Hożejowski, R. Premedication practices for less invasive surfactant administration—Results from a nationwide cohort study. J. Matern. Fetal Neonatal Med. 2020, 35, 4750–4754. [Google Scholar] [CrossRef]
- Van Alfen-van der, A.A.; Hopman, J.C.; Klaessens, J.H.; Feuth, T.; Sengers, R.C.; Liem, K.D. Effects of midazolam and morphine on cerebral oxygenation and hemodynamics in ventilated premature infants. Biol. Neonate 2006, 90, 197–202. [Google Scholar] [CrossRef]
- Welzing, L.; Kribs, A.; Eifinger, F.; Huenseler, C.; Obertheur, A.; Roth, B. Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm neonates. Paediatr. Anaesth. 2010, 20, 605–611. [Google Scholar] [CrossRef]
- Morag, I.; Gover, A.; Domba, S.; Poldi, S.; Drombus, A.; Fried, I.; Avava Campino, G.; Zilberstein, D.; Berkowitz, M.; Berlin, M.; et al. Israeli National Clinical Guidelines for Neonatal Pain Management. Available online: https://ima-contentfiles.s3.amazonaws.com/clinical_103_PreventPainNewborn.pdf (accessed on 29 May 2025).
- Klotz, D.; Porcaro, U.; Fleck, T.; Fuchs, H. European perspective on less invasive surfactant administration—A survey. Eur. J. Pediatr. 2017, 176, 147–154. [Google Scholar] [CrossRef]
- Sk, H.; Saha, B.; Mukherjee, S.; Hazra, A. Premedication with fentanyl for less invasive surfactant application (LISA): A randomized controlled trial. J. Trop. Pediatr. 2022, 68, fmac019. [Google Scholar] [CrossRef]
- Merative, Micromedex NeoFax and Pediatrics. Merative. Available online: https://www.merative.com/clinical-decision-support/neofax-and-pediatrics (accessed on 29 May 2025).
- Descamps, C.; Chevallier, M.; Ego, A.; Pin, I.; Epiard, C.; Debillon, T. Propofol for sedation during less invasive surfactant administration in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 10, F465. [Google Scholar] [CrossRef]
- Brotelande, C.; Milési, C.; Combes, C.; Durand, S.; Badr, M.; Cambonie, G. Premedication with ketamine or propofol for less invasive surfactant administration (LISA): Observational study in the delivery room. Eur. J. Pediatr. 2021, 180, 3053–3058. [Google Scholar] [CrossRef]
- Bourgoin, L.; Caeymaex, L.; Decobert, F.; Jung, C.; Danan, C.; Durrmeyer, X. Administering atropine and ketamine before less invasive surfactant administration resulted in low pain scores in a prospective study of premature neonates. Acta Paediatr. 2018, 107, 1184–1190. [Google Scholar] [CrossRef]
- Chavallier, M.; Durrmeyer, X.; Ego, A.; Debillon, T.; PROLISA Study Group. Propofol versus placebo (with rescue with ketamine) before less invasive surfactant administration: Study protocol for a multicenter, double blind, placebo controlled trial (PROLISA). BMC Pediatr. 2020, 20, 199. [Google Scholar] [CrossRef]
- Nissimov, S.; Kohn, A.; Keidar, R.; Livne, A.; Shemer, M.; Gover, A.; Hershkovich-Shporen, C.; Berkovitch, M.; Morag, I. Dexmedetomidine for Less Invasive Surfactant Administration: A Pilot Study. Pediatr. Drugs 2025, 27, 247–255. [Google Scholar] [CrossRef]
- Kelly, M.A.; Finer, N.N. Nasotracheal intubation in the neonate: Physiologic responses and effects of atropine and pancuronium. J. Pediatr. 1984, 105, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.C., Jr. Miller’s Basics of Anesthesia, 8th ed.; Elsevier: Philadelphia, PA, USA, 2023; pp. 42–43. [Google Scholar]
- Rosow, C. Anesthetic drug interaction: An overview. J. Clin. Anesth. 1997, 9 (Suppl. S6), 27S–32S. [Google Scholar] [CrossRef] [PubMed]
- Kudchadkar, S.; Shaffner, M. Pharmacologic recipes for tracheal intubation in the PICU: What’s on the menu? Pediatr. Crit. Care Med. 2015, 16, 290–292. [Google Scholar] [CrossRef]
- Tarquinio, K.M.; Howell, J.; Montgomery, V.; Turner, D.A.; Hsing, D.D.; Parker, M.M.; Brown CA3rd Walls, R.M.; Nadkarni, V.M.; Nishisaki, A. Current medication practice and tracheal intubation safety outcomes from a prospective multicenter observational cohort study. Pediatr. Crit. Care Med. 2015, 16, 210–218. [Google Scholar] [CrossRef]
- Barrington, K.J.; Finer, N.N.; Ethches, P.C. Succinylcholine and atropine for premedication of the newborn infant before nasotracheal intubation: A randomized, controlled trial. Crit. Care Med. 1989, 17, 1293–1296. [Google Scholar] [CrossRef]
- Kumar, P.; Denson, S.E.; Mancuso, T.J. Premedication for nonemergency Endotracheal Intubation in the Neonate. Pediatrics 2010, 125, 608–615. [Google Scholar] [CrossRef]
- DeMartino, C.; Neches, S.; Gray, M.M.; Sawyer, T.; Johnston, L. The use of premedication for intubating very low birth weight infants in the neonatal intensive care unit: Results of a neonatal survey. Am. J. Perinatol. 2025; Epub ahead of printing. [Google Scholar] [CrossRef]

| Number of Responders | Yes | No | Sometimes | |
|---|---|---|---|---|
| Give premedication before elective intubation. | 69 | 66 (95.7%) | 3 (4.3%) | |
| Give premedication before emergent intubation. | 69 | 4 (6%) | 43 (62.3%) | 22 (31.7%) |
| Give premedication before LISA/INSURE. | 67 | 44 (65.7%) | 23 (34.3%) | |
| Give premedication before elective intubation in ELBW. | 69 | 52 (75.4%) | 17 (24.6%) | |
| Use muscle relaxants as a part of premedication before intubation. | 68 | 1 (1.5%) | 56 (82.3%) | 11 (16.2%) |
| Use atropine as part of premedication. | 69 | 30 (43.5%) | 39 (56.5%) | |
| Department heads indicate having written premedication protocols. | 27 | 20 (74.1%) | 7 (25.9%) | |
| Caregivers think that there is a need for uniform premedication protocols. | 69 | 47 (68.1%) | 22 (31.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoabi Safadi, R.; Gover, A.; Tal Shahar, N.; Shoris, I.; Toropine, A.; Iofe, A.; Bader, D.; Shnaider, M.; Riskin, A. Sedation and Analgesia for Intubation, LISA, and INSURE Procedures in Israeli NICUs: Caregivers’ Practices and Perspectives. J. Clin. Med. 2025, 14, 5865. https://doi.org/10.3390/jcm14165865
Zoabi Safadi R, Gover A, Tal Shahar N, Shoris I, Toropine A, Iofe A, Bader D, Shnaider M, Riskin A. Sedation and Analgesia for Intubation, LISA, and INSURE Procedures in Israeli NICUs: Caregivers’ Practices and Perspectives. Journal of Clinical Medicine. 2025; 14(16):5865. https://doi.org/10.3390/jcm14165865
Chicago/Turabian StyleZoabi Safadi, Rasha, Ayala Gover, Naama Tal Shahar, Irit Shoris, Arina Toropine, Adir Iofe, David Bader, Morya Shnaider, and Arieh Riskin. 2025. "Sedation and Analgesia for Intubation, LISA, and INSURE Procedures in Israeli NICUs: Caregivers’ Practices and Perspectives" Journal of Clinical Medicine 14, no. 16: 5865. https://doi.org/10.3390/jcm14165865
APA StyleZoabi Safadi, R., Gover, A., Tal Shahar, N., Shoris, I., Toropine, A., Iofe, A., Bader, D., Shnaider, M., & Riskin, A. (2025). Sedation and Analgesia for Intubation, LISA, and INSURE Procedures in Israeli NICUs: Caregivers’ Practices and Perspectives. Journal of Clinical Medicine, 14(16), 5865. https://doi.org/10.3390/jcm14165865






