Clinical Characteristics and Prognosis in Spontaneous Isolated Abdominal Aortic Dissection Based on the Dissection Length
Abstract
1. Introduction
2. Methods
Data Collection and Classifications
3. Statistical Analysis
4. Results
4.1. Patient Characteristics and Clinical Presentation
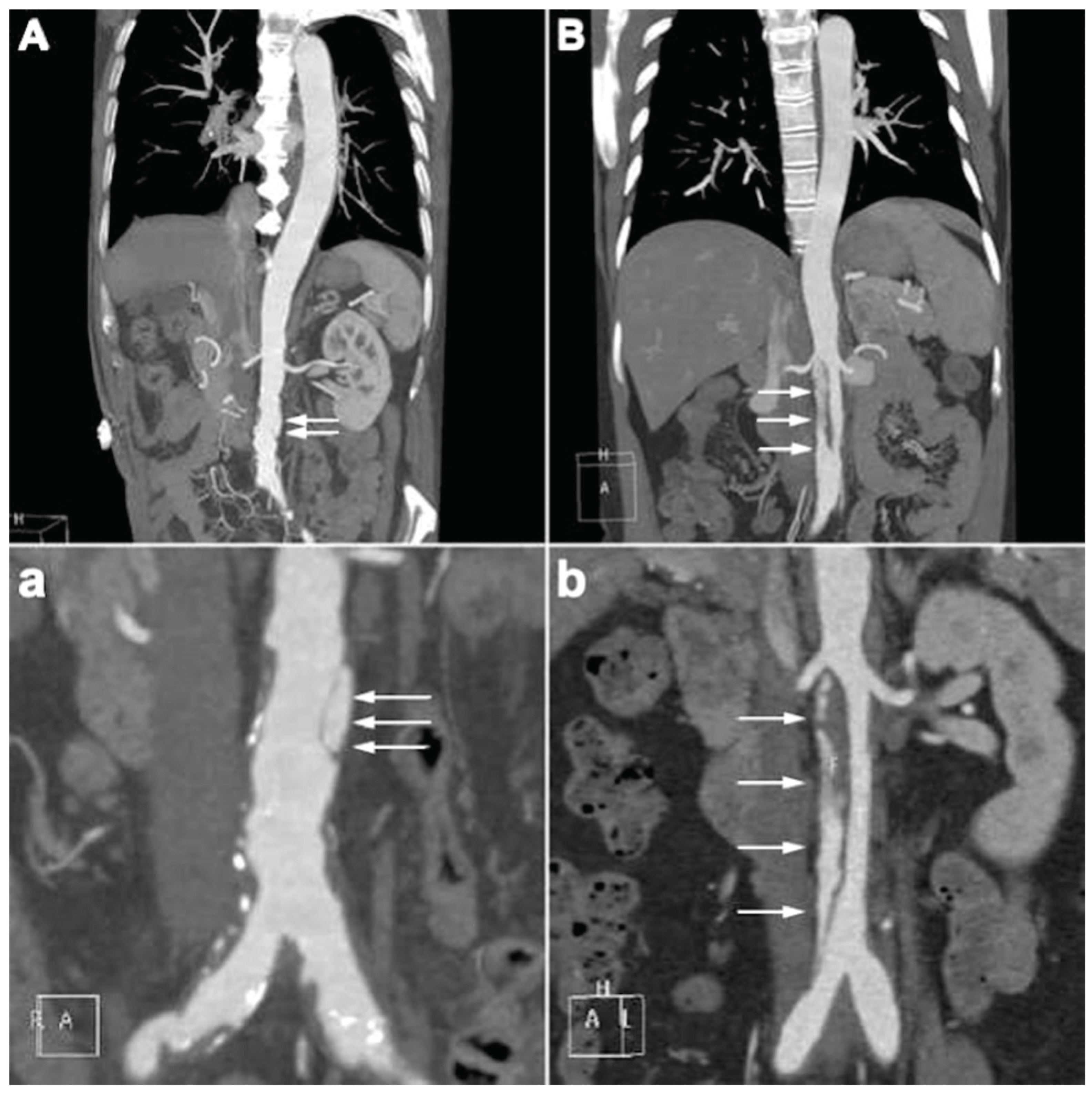
4.2. CTA Imaging Findings
4.3. Management and In-Hospital Outcomes
4.4. Long-Term Outcomes
5. Discussion
6. Conclusions
7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsubouchi, H.; Onishi, H.; Maeno, K.; Nakagaichi, M.; Tsukushi, I.; Kitano, Y.; Makino, Y.; Hayashi, H.; Terasawa, H.; Kabuto, H. Study of Risk Factors and Image Findings of Isolated Abdominal Incidentally Detected Aortic Dissection. J. Clin. Ultras. 2025, 53, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, S.; Tsai, T.; Eagle, K.A.; Isselbacher, E.M.; Froehlich, J.; Cooper, J.V.; Rampoldi, V.; Upchurch, G.R., Jr.; International Registry of Acute Aortic Dissection (IRAD) investigators. Acute abdominal aortic dissection: Insight from the International Registry of Acute Aortic Dissection (IRAD). J. Vasc. Surg. 2007, 46, 913–919.e911. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Nishina, T.; Nishio, I.; Asano, M.; Noda, K.; Ueno, Y. Endovascular stent-graft repair for spontaneous dissection of infra-renal abdominal aorta. Ann. Vasc. Surg. 2010, 24, 955.e1–955.e4. [Google Scholar] [CrossRef] [PubMed]
- Böckler, D.; Massoni, C.B.; Geisbüsch, P.; Hakimi, M.; von Tengg-Kobligk, H.; Hyhlik-Dürr, A. Single-center experience in the management of spontaneous isolated abdominal aortic dissection. Langenbecks Arch. Surg. 2016, 401, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.P.; Bernardes, M.V.; dos Santos Souza, J.E.; Pereira, R.M. Isolated abdominal aortic dissection in a young female patient. J. Vasc. Surg. 2016, 63, 243. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-P.; Liu, F.-E.; Duan, Q.; Ye, R.; Xiao, J.-Q. Spontaneous isolated dissection at renal upper abdominal aortic: A rare case report. Int. J. Clin. Exp. Med. 2015, 8, 8190. [Google Scholar] [PubMed]
- Farber, A.; Wagner, W.H.; Cossman, D.V.; Cohen, J.L.; Walsh, D.B.; Fillinger, M.F.; Cronenwett, J.L.; Lauterbach, S.R.; Levin, P.M. Isolated dissection of the abdominal aorta: Clinical presentation and therapeutic options. J. Vasc. Surg. 2002, 36, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Mózes, G.; Gloviczki, P.; Park, W.M.; Schultz, H.L.; Andrews, J.C. Spontaneous dissection of the infrarenal abdominal aorta. Semin. Vasc. Surg. 2002, 15, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Cannavale, A.; O’Sullivan, G.J.; Gazzetti, M.; Cirelli, C.; Lucatelli, P.; Santoni, M.; Catalano, C. Endovascular repair of acute and chronic aortic type B dissections: Main factors affecting aortic remodeling and clinical outcome. JACC Cardiovasc. Interv. 2016, 9, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-q.; Li, D.-l.; Lai, M.-c.; Chen, X.-d.; Jin, W.; Zhang, H.-k.; Li, M. Endovascular treatment of isolated abdominal aortic dissection and postoperative aortic remodeling. J. Vasc. Surg. 2015, 61, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Kouvelos, G.N.; Vourliotakis, G.; Arnaoutoglou, E.; Papa, N.; Avgos, S.; Peroulis, M.; Papadopoulos, G.; Matsagkas, M.I. Endovascular treatment for isolated acute abdominal aortic dissection. J. Vasc. Surg. 2013, 58, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef] [PubMed]
- Jonker, F.H.; Schlösser, F.J.; Moll, F.L.; Muhs, B.E. Dissection of the abdominal aorta. Current evidence and implications for treatment strategies: A review and meta-analysis of 92 patients. J. Endovasc. Ther. 2009, 16, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.M.; Pearce, S.W.; Xiao, R.; Oo, A.Y.; Xiao, Q. Matrix metalloproteinase in abdominal aortic aneurysm and aortic dissection. Pharmaceuticals 2019, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Jawadi, N.; Bisdas, T.; Torsello, G.; Stavroulakis, K.; Donas, K.P. Endovascular treatment of isolated abdominal aortic dissections: Long-term results. J. Endovasc. Ther. 2014, 21, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiao, Y.; Li, W.; He, C.; Zhang, H.; Zhang, T. Management of isolated abdominal aortic dissection: Indications and strategies for treatment. Ann. Vasc. Surg. 2024, 99, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Vandy, F.; Upchurch, G.R., Jr. Endovascular aneurysm repair: Current status. Circ. Cardiovasc. Interv. 2012, 5, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, T.; Guo, D.; Xu, X.; Chen, B.; Jiang, J.; Yang, J.; Shi, Z.; Zhu, T.; Shi, Y. Endovascular treatment of acute and chronic isolated abdominal aortic dissection. Vascular 2018, 26, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zafar, M.; Qiu, J.; Huang, Y.; Chen, Y.; Yu, C.; Elefteriades, J.A. A systematic review and meta-analysis of isolated abdominal aortic dissection. J. Vasc. Surg. 2019, 70, 2046–2053.e6. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Y.; Jiang, J. Thoracic endovascular aortic repair or best medical therapy for uncomplicated type B aortic dissection? A meta-analysis. J. Cardiovasc. Surg. 2015, 63, 288–298. [Google Scholar] [CrossRef]




| Total | f-SIAAD | e-SIAAD | p-Value | ||
|---|---|---|---|---|---|
| (n = 159) | (n = 85) | (n = 74) | |||
| Age | 57.7 ± 12.4 | 63.74 ± 10.97 | 50.70 ± 10.10 | 0.000 | |
| Sex | Female | 40 (25.16) | 21 (24.71) | 19 (25.68) | 0.888 |
| Male | 119 (74.84) | 64 (75.29) | 55 (74.32) | ||
| Smoking | No | 109 (68.55) | 61 (71.76) | 48 (64.86) | 0.350 |
| Yes | 50 (31.45) | 24 (28.24) | 26 (35.14) | ||
| Alcohol | No | 124 (77.99) | 64 (75.29) | 60 (81.08) | 0.380 |
| Yes | 35 (22.01) | 21 (24.71) | 14 (18.92) | ||
| Hypertension | No | 47 (29.56) | 18 (21.18) | 29 (39.19) | 0.013 |
| Yes | 112 (70.44) | 67 (78.82) | 45 (60.81) | ||
| Diabetes mellitus | No | 132 (83.02) | 60 (70.59) | 72 (97.3) | 0.000 |
| Yes | 27 (16.98) | 25 (29.41) | 2 (2.7) | ||
| Hyperlipidemia | No | 128 (80.5) | 62 (72.94) | 66 (89.19) | 0.010 |
| Yes | 31 (19.5) | 23 (27.06) | 8 (10.81) | ||
| Chronic renal insufficiency | No | 133 (83.65) | 66 (77.65) | 67 (90.54) | 0.028 |
| Yes | 26 (16.35) | 19 (22.35) | 7 (9.46) | ||
| Coronary artery disease | No | 103 (64.78) | 38 (44.71) | 65 (87.84) | 0.000 |
| Yes | 56 (35.22) | 47 (55.29) | 9 (12.16) | ||
| COPD | No | 154 (96.86) | 81 (95.29) | 73 (98.65) | 0.227 |
| Yes | 5 (3.14) | 4 (4.71) | 1 (1.35) | ||
| SIAAD-related symptoms | No | 44 (27.67) | 33 (38.82) | 11 (14.86) | 0.0037 |
| Yes | 115 (72.33) | 52 (61.18) | 63 (85.14) | ||
| Chest Pain | No | 115 (72.33) | 65 (76.47) | 50 (67.57) | 0.211 |
| Yes | 44 (27.67) | 20 (23.53) | 24 (32.43) | ||
| Abdominal Pain | No | 97 (61.01) | 59 (69.41) | 38 (51.35) | 0.020 |
| Yes | 62 (38.99) | 26 (30.59) | 36 (48.65) | ||
| Back Pain N | No | 128 (80.5) | 72 (84.71) | 56 (75.68) | 0.152 |
| Yes | 31 (19.5) | 13 (15.29) | 18 (24.32) | ||
| Lumbar Pain | No | 132 (83.02) | 77 (90.59) | 55 (74.32) | 0.006 |
| Yes | 27 (16.98) | 8 (9.41) | 19 (25.68) | ||
| Acute chronicity | 0.000 | ||||
| Acute-SIAAD | 83 (52.2) | 29 (34.12) | 54 (72.97) | ||
| Chronic-SIAAD | 76 (47.8) | 56 (65.88) | 20 (27.03) | ||
| Dissection as initial diagnosis on admission | No | 82 (51.5) | 64 (75.29) | 23 (31.08) | 0.000 |
| Yes | 77 (48.43) | 21 (24.71) | 51 (68.92) | ||
| Total | f-SIAAD | e-SIAAD | p-Value | ||
|---|---|---|---|---|---|
| (n = 159) | (n = 85) | (n = 74) | |||
| Location of the primary entry site | |||||
| Supraceliac | No | 120 (75.47) | 74 (87.06) | 46 (62.16) | 0.000 |
| Yes | 39 (24.53) | 11 (12.94) | 28 (37.84) | ||
| Paravisceral | No | 142 (89.31) | 80 (94.12) | 62 (83.78) | 0.035 |
| Yes | 17 (10.69) | 5 (5.88) | 12 (16.22) | ||
| Infrarenal | No | 56 (35.22) | 16 (18.82) | 40 (54.05) | 0.000 |
| Yes | 103 (64.78) | 69 (81.18) | 34 (45.95) | ||
| SIAAD length, mm | 68.44 ± 56.37 | 24.48 ± 10.92 | 118.93 ± 43.65 | 0.000 | |
| SIAAD maximum diameter, mm | 27.89 ± 11.72 | 24.41 ± 11.28 | 31.89 ± 10.99 | 0.000 | |
| Involvement | |||||
| Celiac trunk artery | No | 152 (95.6) | 80 (94.12) | 72 (97.3) | 0.330 |
| Yes | 7 (4.4) | 5 (5.88) | 2 (2.7) | ||
| Superior mesenteric artery | No | 155 (97.48) | 83 (97.65) | 72 (97.3) | 0.888 |
| Yes | 4 (2.52) | 2 (2.35) | 2 (2.7) | ||
| Renal artery | No | 127 (79.87) | 75 (88.24) | 52 (70.27) | 0.005 |
| Yes | 32 (20.13) | 10 (11.76) | 22 (29.73) | ||
| Inferior mesenteric artery | No | 137 (86.71) | 80 (95.24) | 57 (77.03) | 0.001 |
| Yes | 21 (13.29) | 4 (4.76) | 17 (22.97) | ||
| Common iliac artery | No | 79 (49.69) | 63 (74.12) | 16 (21.62) | 0.000 |
| Yes | 80 (50.31) | 22 (25.88) | 58 (78.38) | ||
| False lumen thrombosis | No | 117 (73.58) | 74 (87.05) | 43 (58.10) | 0.0002 |
| Yes | 42 (26.41) | 11 (12.94) | 31 (41.89) | ||
| Arteriosclerosis | No | 81 (50.94) | 24 (28.24) | 57 (77.03) | 0.000 |
| Yes | 78 (49.06) | 61 (71.76) | 17 (22.97) | ||
| AAA | No | 119 (74.84) | 73 (85.88) | 46 (62.16) | 0.001 |
| Yes | 40 (25.16) | 12 (14.12) | 28 (37.84) | ||
| PAU | No | 110 (69.18) | 47 (55.29) | 63 (85.14) | 0.000 |
| Yes | 49 (30.82) | 38 (44.71) | 11 (14.86) | ||
| Pleural effusion | No | 122 (76.73) | 71 (83.53) | 51 (68.92) | 0.030 |
| Yes | 37 (23.27) | 14 (16.47) | 23 (31.08) | ||
| Total | f-SIAAD | e-SIAAD | p-Value | ||
|---|---|---|---|---|---|
| (n = 159) | (n = 85) | (n = 74) | |||
| In-hospital treatment | |||||
| Medication | No | 108 (67.92) | 53 (62.35) | 55 (74.32) | 0.272 |
| Yes | 48 (30.19) | 29 (34.12) | 19 (25.68) | ||
| Endovascular repair | No | 55 (34.59) | 34 (40) | 21 (28.38) | 0.307 |
| Yes | 104 (65.41) | 51 (60) | 53 (71.62) | ||
| Open surgery | No | 155 (97.48) | 83 (97.65) | 72 (97.3) | 0.99 |
| Yes | 4 (2.52) | 2 (2.35) | 2 (2.7) | ||
| In-hospital medical therapy | |||||
| Aspirin | No | 126 (79.25) | 60 (70.59) | 66 (89.19) | 0.004 |
| Yes | 33 (20.75) | 25 (29.41) | 8 (10.81) | ||
| Clopidogrel | No | 141 (88.68) | 70 (82.35) | 71 (95.95) | 0.007 |
| Yes | 18 (11.32) | 15 (17.65) | 3 (4.05) | ||
| Statins | No | 89 (55.97) | 41 (48.24) | 48 (64.86) | 0.035 |
| Yes | 70 (44.03) | 44 (51.76) | 26 (35.14) | ||
| βblocker | No | 58 (36.48) | 31 (36.47) | 27 (36.49) | 0.998 |
| Yes | 101 (63.52) | 54 (63.53) | 47 (63.51) | ||
| CCB | No | 33 (20.75) | 18 (21.18) | 15 (20.27) | 0.888 |
| Yes | 126 (79.25) | 67 (78.82) | 59 (79.73) | ||
| ACEI/ARB | No | 104 (65.41) | 52 (61.18) | 52 (70.27) | 0.229 |
| Yes | 55 (34.59) | 33 (38.82) | 22 (29.73) | ||
| In-hospital outcome | |||||
| Hospitalization time | 11.4 ± 8 | 12 ± 8 | 11 ± 6 | 0.819 | |
| In-hospital mortality | No | 152 (95.60) | 79 (92.94) | 73 (98.65) | 0.08 |
| Yes | 7 (4.40) | 6 (7.06) | 1 (1.35) | ||
| Patient Number | Sex/Age | SIAAD Type | SIAAD-Related Symptoms | Acute Chronicity | Cardiovascular Risk Factor | AAA | Comorbidity | Treatment | Follow-Up (Months) | Time of Death Month | Death Cause |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/67 | Extensive | Chest pain | Chronic | CAD | No | No | Endovascular Repair | 88 | Unknown | Unknown |
| 2 | M/68 | Focal | Abdominal pain | Chronic | HTN, CAD, Smo | No | COPD, DLBCL | Conservative | 29 | 28 | Respiratory failure |
| 3 | M/43 | Extensive | Abdominal pain, back pain | Acute | No | No | No | Conservative | In-hospital mortality | In-hospital mortality | Acute aortic rupture |
| 4 | M/82 | Focal | Asymptomatic | Chronic | HTN, HL, CAD, Smo | No | AMI | Conservative | In-hospital mortality | In-hospital mortality | Heart failure |
| 5 | M/66 | Focal | Asymptomatic | Chronic | CAD | Yes | CRI | Conservative | In-hospital mortality | In-hospital mortality | Advanced lung cancer |
| 6 | M/75 | Focal | Asymptomatic | Chronic | CAD, COPD, Smo | No | HCC | Conservative | 15 | 8 | Liver cancer |
| 7 | F/59 | Focal | Abdominal pain | Chronic | DM | No | No | Conservative | In-hospital mortality | In-hospital mortality | Acute liver failure |
| 8 | M/70 | Focal | Abdominal pain | Acute | HTN | Yes | CRI | Open Surgery | In-hospital mortality | In-hospital mortality | AAA rupture |
| 9 | M/72 | Focal | Back pain | Chronic | HTN, CAD | Yes | MI | Open Surgery | 129 | Unknown | Unknown |
| 10 | M/73 | Focal | Asymptomatic | Chronic | HTN | No | CRI | Conservative | 133 | 31 | Unknown |
| 11 | M/85 | Focal | Asymptomatic | Chronic | HTN, HL, DM, Smo | No | AI | Conservative | 129 | 11 | Heart failure |
| 12 | M/69 | Focal | Chest pain | Chronic | HTN, CAD | No | AMI | Conservative | 11 | 11 | re-AMI |
| 13 | M/77 | Focal | Abdominal pain | Acute | HTN, CAD | No | No | Conservative | In-hospital mortality | In-hospital mortality | SMA dissection leading to multiorgan failure |
| 14 | M/55 | Focal | Chest pain, back pain | Acute | HTN | Yes | No | Conservative | 109 | 4 | AAA rupture |
| 15 | M/71 | Focal | Abdominal pain | Acute | HTN | Yes | RAI | Conservative | In-hospital mortality | In-hospital mortality | AAA rupture |
| 16 | M/65 | Focal | Back pain | Acute | HTN, DM, CAD, Smo | No | No | Conservative | 128 | 34 | Unknown |
| 17 | F/70 | Focal | Abdominal pain, chest pain | Chronic | HTN, CAD, COPD | Yes | No | Conservative | 148 | 32 | Unknown |
| 18 | M/52 | Extensive | Abdominal pain, chest pain | Acute | HTN | Yes | COPD | Conservative | 82 | 41 | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaiea, S.; He, X.; Mansai, H.; Aldali, F.; Hashem, A.; Heng, Y.; Zeng, H. Clinical Characteristics and Prognosis in Spontaneous Isolated Abdominal Aortic Dissection Based on the Dissection Length. J. Clin. Med. 2025, 14, 5849. https://doi.org/10.3390/jcm14165849
Shaiea S, He X, Mansai H, Aldali F, Hashem A, Heng Y, Zeng H. Clinical Characteristics and Prognosis in Spontaneous Isolated Abdominal Aortic Dissection Based on the Dissection Length. Journal of Clinical Medicine. 2025; 14(16):5849. https://doi.org/10.3390/jcm14165849
Chicago/Turabian StyleShaiea, Saddam, Xingwei He, Hussen Mansai, Fatima Aldali, Abdulwahab Hashem, Ye Heng, and Hesong Zeng. 2025. "Clinical Characteristics and Prognosis in Spontaneous Isolated Abdominal Aortic Dissection Based on the Dissection Length" Journal of Clinical Medicine 14, no. 16: 5849. https://doi.org/10.3390/jcm14165849
APA StyleShaiea, S., He, X., Mansai, H., Aldali, F., Hashem, A., Heng, Y., & Zeng, H. (2025). Clinical Characteristics and Prognosis in Spontaneous Isolated Abdominal Aortic Dissection Based on the Dissection Length. Journal of Clinical Medicine, 14(16), 5849. https://doi.org/10.3390/jcm14165849







