Abstract
Background: Re-excision after breast-conserving surgery (BCS) is routinely recommended when positive margins are found. However, secondary surgery often reveals no residual disease, exposing patients to unnecessary interventions that compromise cosmetic outcomes, increase costs, and reduce quality of life. This study investigates clinicopathological predictors of a residual tumour to identify low-risk patients who may safely avoid re-excision. Methods: We conducted a retrospective cohort study of 135 patients who underwent reoperation for positive margins following BCS at the Breast Unit of Fondazione Policlinico Universitario A. Gemelli IRCCS in Rome, between 2019 and 2024. Data on patient demographics, tumour characteristics, and histopathological findings were analyzed using univariate and multivariate models to identify predictors of residual disease. Results: A residual tumour was detected in 66 of 135 patients (48.9%). In the remaining 69 cases (51.1%), no residual disease was found, indicating that re-excision may have been unnecessary. Multifocality (p < 0.01), lymphovascular invasion (LVI) (p < 0.05), and involvement of ≥2 margins (p < 0.05) were independently associated with the residual tumour. Patients with unifocal disease, absence of LVI, and a single positive margin had a significantly lower risk of residual disease. Conclusions: Over half of re-excisions performed for positive margins may be avoidable. A risk-adapted approach incorporating tumour focality, LVI status, and margin involvement can help identify patients for whom secondary surgery may offer limited benefits. These findings support a more individualized strategy to margin management in BCS aimed at reducing overtreatment without compromising oncologic safety.
1. Introduction
Breast-conserving surgery (BCS) has become the gold standard in the treatment of early-stage breast cancer, offering survival rates comparable to a mastectomy while preserving breast appearance and improving patient quality of life [1]. To achieve oncologic outcomes equivalent to a mastectomy, BCS must be accompanied by appropriate adjuvant therapies and meticulous surgical technique, particularly in margin management [2,3].
Traditionally, a clear rim of uninvolved tissue between the tumour and inked surgical margins was required to ensure an adequate resection. However, current guidelines define a positive margin as the presence of tumor cells directly at the inked edge (‘ink on tumor’) [4]. Positive margins are associated with a higher likelihood of residual disease and approximately double the risk of local recurrence, even when favourable tumor biology or adjuvant therapy is present [5].
Meta-analyses estimate that 8–10% of BCS cases result in positive margins. This rate is now a key quality indicator in breast cancer surgery. According to EUSOMA standards, high-performing centres should aim for a positive margin rate below 10% [6].
Guidelines recommend a second surgery, either re-excision or completion mastectomy, in cases of positive margins. However, this comes at a cost: secondary surgery can negatively affect cosmetic results, delay systemic therapies, increase perioperative risk, and significantly impact patients’ psychological well-being. Moreover, growing interest in surgical de-escalation and precision oncology has prompted a critical re-evaluation of whether re-excision is always necessary.
Several retrospective studies have shown that over 50% of re-excised specimens reveal no residual tumour, suggesting that a substantial proportion of reoperations may be oncologically unnecessary [7]. Despite this, reliable predictors of residual disease remain underexplored and inconsistently reported.
This study aims to assess the incidence of residual tumours following a margin re-excision and to identify clinicopathological factors predictive of residual disease. The ultimate goal is to define a subset of patients at low risk, in whom reoperation may be safely avoided.
2. Materials and Methods
2.1. Study Design and Patients
This retrospective cohort study included 135 female patients who underwent BCS followed by a second surgical procedure (re-excision or completion mastectomy) due to positive margins at the Breast Unit of the Fondazione Policlinico Universitario A. Gemelli IRCCS in Rome, Italy, between January 2019 and December 2024.
Patients were eligible if they
- -
- Had histologically confirmed invasive breast cancer;
- -
- Underwent BCS as a primary surgical treatment;
- -
- Required a second operation due to positive surgical margins on final pathology.
Exclusion criteria were
- -
- Reoperations performed for postoperative complications, recurrence, or patient preference unrelated to margin status;
- -
- Missing or incomplete histopathological or clinical data.
2.2. Margin Definition and Surgical Technique
Positive margins for invasive carcinoma were defined according to current guidelines as the presence of tumour cells at the inked surface of the specimen (‘ink on tumor’).
Preoperative localization of non-palpable lesions was performed using ultrasound- or mammography-guided skin marking. Surgical margins were assessed intraoperatively in selected cases using specimen radiography or gross pathological examination. All surgical decisions were made by a multidisciplinary team (MDT) including breast surgeons, radiologists, pathologists, oncologists, and radiation oncologists, and were shared with the patient.
To identify the surgical margins in both standard and oncoplastic procedures, our shared institutional protocol includes the placement of metallic clips at the resected margins of the tumour cavity. This approach aims to facilitate potential margin re-shaving or the delivery of an adjuvant radiotherapy boost, if needed.
Adjuvant radiotherapy (including boost) was administered according to institutional protocols and MDT decisions, considering patient age, margin status, and tumour biology.
2.3. Data Collection and Variables
Demographic, clinical, imaging, surgical, and histopathologic data were collected from electronic medical records. The following variables were analyzed:
- -
- Patient-related factors: Age at diagnosis (≤50 vs. >50 years), breast density (BI-RADS classification), preoperative MRI usage, and presence of microcalcifications.
- -
- Tumour characteristics: Size (TNM classification), histological type and grade (modified Bloom–Richardson), immunophenotype (ER, PR, HER2, and Ki-67), lymph node status, presence of an extensive intraductal component (EIC), lymphovascular invasion (LVI), and multifocality.
- -
- Margin-related variables: Number of positive margins (1 vs. ≥2), margin width (if available), and type of second surgery (re-excision vs. mastectomy).
- -
- Residual disease: Defined as the presence of invasive carcinoma and/or DCIS in the re-excision specimen.
In multifocal tumours, the largest lesion was considered for size and histopathological assessment. The EIC was defined as present when >25% of the tumour contained intraductal components.
2.4. Statistical Analysis
Univariate analysis was performed using a Fisher’s exact test or Chi-square test, as appropriate, to evaluate associations between clinical and pathological variables and the presence of a residual tumour at re-excision.
Variables with p ≤ 0.05 in univariate analysis were entered into a multivariate logistic regression model using a backward stepwise method to identify independent predictors. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated.
All statistical analyses were conducted using IBM SPSS Statistics version 24.0 (IBM Corp., Armonk, NY, USA). A p-value ≤ 0.05 was considered statistically significant.
3. Results
Between January 2019 and December 2024, a total of 135 patients who underwent breast-conserving surgery (BCS) followed by a second surgical procedure for positive margins were included in the study. The mean patient age was 52.7 years (range: 28–85). Patient and tumour characteristics are summarized in Table 1.

Table 1.
Factors associated with residual tumour after re-excision for positive margins.
Of the 135 patients who required reoperation, 124 (91.9%) underwent re-quadrantectomy, while 11 (8.1%) underwent a completion mastectomy. A residual tumour was identified in 66 patients, corresponding to an overall residual disease rate of 48.9% (Figure 1). This indicates that 51.1% of re-excision specimens showed no residual disease and were potentially unnecessary.
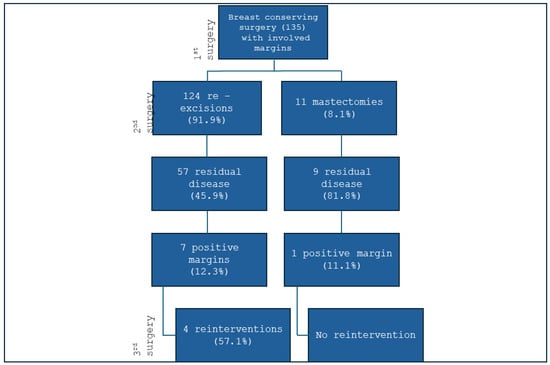
Figure 1.
Surgical treatment of patients who underwent re-excision for positive margins.
3.1. Univariate Analysis
A univariate logistic regression identified the following variables as significantly associated with the presence of a residual tumour in re-excision specimens:
- -
- Multifocality: OR 3.06, 95% CI: 1.47–6.35, and p = 0.0027;
- -
- LVI: OR 5.97, 95% CI: 2.10–17.02, and p < 0.001;
- -
- Number of positive margins (≥2): OR 2.30, 95% CI: 1.48–3.59, and p < 0.001.
These associations were based on histopathologic findings from the initial conservative surgical specimens.
3.2. Multivariate Analysis
In the multivariate logistic regression model, two variables remained independently associated with the presence of a residual tumour (Figure 2, Table 2):
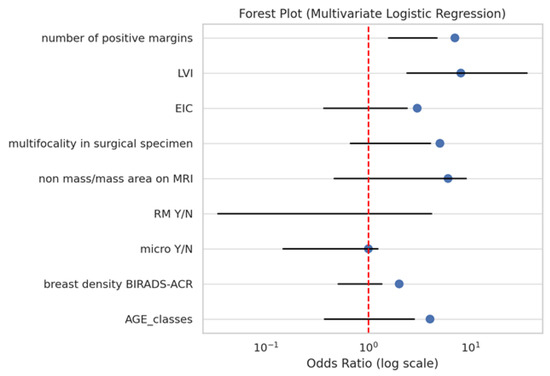
Figure 2.
Forest plot from multivariate logistic regression for predictors of residual tumour.

Table 2.
Factors significantly associated with residual tumour after re-excision for positive margins in the logistic regression analysis model.
- -
- LVI: OR 9.21, 95% CI: 2.39–35.47, and p = 0.0013;
- -
- Number of positive margins (≥2): OR 2.73, 95% CI: 1.58–4.69, and p < 0.001.
Although multifocality was significant in the univariate analysis, it did not retain independent significance in the multivariate model. This suggests potential collinearity or confounding, particularly with LVI, which may serve as a stronger marker of biological aggressiveness.
The multivariate model demonstrated a good discriminative performance with an area under the ROC curve (AUC) of 0.81, indicating a strong ability to distinguish between patients with and without a residual tumour.
4. Discussion
The rate of surgical re-excision for infiltrated margins varies in the literature from 10% to 57% [7], depending on the definition of the tumour margin width used. Margin status is considered the most important predictor of local recurrence, but the correct surgical resection margin remains a matter of debate. While a wide resection margin was deemed necessary in the past, today, the guidelines state that it is sufficient to have no ink on the tumour [4]. Several meta-analyses [5,8] have shown that while a higher recurrence rate occurs with ink on the tumour versus margins of at least 1 mm, widths greater than 1 mm offer no statistical benefit. The main conclusion is that there is no justification for margins > 1 mm in patients undergoing BCS [8]. Conversely, a recent meta-analysis by Bundred and colleagues [9] provided enough data to compare ‘no ink on tumour’ with 1 mm. They concluded that a minimum negative margin should be set at 1 mm instead of ‘no ink on tumour’ and recommended revising international guidelines accordingly. Additionally, a recent review [8] reinforced this viewpoint, suggesting that guidelines should redefine a negative margin as 1 mm instead of ‘no ink on tumour’ in the context of BCT. The broader implications of this change require thorough examination. The total number of patients affected may be considerable, especially in busy breast units, leading to more re-excision procedures that could strain healthcare systems and diminish patient quality of life. Additional surgeries introduce risks of complications, cosmetic issues, delays in adjuvant therapies, and increased costs. Moreover, 30–70% of patients undergoing re-excision for inadequate margins show no remaining disease [10]. These findings align with the current SSO-ASTRO-ASCO consensus guidelines, which emphasize the importance of minimizing unnecessary re-excisions without compromising oncologic outcomes [4,11]. The necessity for individualized re-excision strategies is also highlighted in more recent data, indicating that residual disease may still be present despite margin clearance, particularly in aggressive tumour subtypes [12,13].
In our study, 51.5% of the patients with positive surgical margins displayed no evident residual cancer during reoperation. This finding is congruent with data reported in most of the existing literature [14,15,16], although considerable variability exists [17,18,19]. Several factors may clarify the high absence rate of residual tumours in surgical reinterventions. Research indicates a significant occurrence of ‘false positives’ due to technical mistakes, such as ink penetrating through cracks in the surgical specimen, which can create a misleading impression of margin infiltration [20]. Other studies point to the ‘pancake phenomenon,’ where the specimen flattens between excision and evaluation, combined with the shrinking effect of formalin, which may result in the tumour appearing nearer to the excision margin than it truly is in vivo [21,22]. Another explanation involves how surgery impacts the remaining breast tissue, potentially lowering the likelihood of residual disease by destroying remaining tumour cells directly through diathermy or inducing an inflammatory reaction [23]. In some cases, the residual tumour may appear in the re-excision specimen but may not be detected in the reviewed slides, as it is widely acknowledged that examining the entire tissue is not practically possible in a sample [20]. Ultimately, from a surgical perspective, conducting a targeted re-excision of the infiltrated margin presents challenges, especially in recent years due to the growing prevalence of oncoplastic remodelling, which involves the extensive mobilization of glandular flaps [23,24].
In our study, over half of the re-excisions were deemed unnecessary given that no residual disease was identified. We examined various clinicopathologic factors to determine their effectiveness in identifying patients with positive or close margins who could safely avoid additional surgery.
In the present study, tumour multifocality emerged as a significant risk factor for residual disease following breast-conserving surgery in the univariate analysis. Specifically, 66% of patients with multifocal breast cancer were found to have residual disease, compared to only 38.8% of patients with unifocal tumours. These findings align with those reported by Sabel et al. [25], who identified multifocality as the sole variable significantly associated with residual disease in both univariate and multivariate analyses. In their cohort, over 50% of patients with multifocal tumours had residual disease upon re-excision, in contrast to fewer than 25% of patients with unifocal disease [25]. Further evidence supports these observations. Gurdal et al. reported that patients with multifocal tumours had a more than fivefold increased risk of residual disease at re-excision or mastectomy compared to those with unifocal tumours (OR = 5.2, 95% CI: 2.6–10.4) [7]. Additionally, a meta-analysis by Sun et al. demonstrated that patients with multifocal or multicentric breast cancer undergoing breast-conserving therapy had significantly higher rates of local recurrence compared to those with unifocal tumours [26]. Similarly, Saarela et al. found that multifocality was significantly associated with positive histologic margins and residual disease in re-excisions (p = 0.001), recommending routine re-excision in such cases [27]. Rath et al. also identified multifocality as an independent predictor of residual tumour in multivariate analysis (p = 0.002), alongside tumour size and presence of DCIS [28]. These findings support our data and suggest that tumour multifocality should be considered a strong indicator for the presence of residual disease, potentially guiding more aggressive surgical management.
In our study, the number of involved margins following BCS was significantly associated with residual disease upon re-excision in both univariate and multivariate analyses. Specifically, a residual tumour was detected in 84% of cases with three involved margins, 52.8% with two, and 26.5% with one.
These results are consistent with the prior literature highlighting the prognostic value of the extent of margin involvement. Simpson et al. evaluated 220 patients undergoing re-excision and found a clear linear trend between the number of involved margins and the likelihood of residual disease: patients with a single involved margin had a significantly lower residual tumour rate than those with two or more. Their multivariate analysis confirmed this trend as statistically significant (p < 0.001), supporting the number of involved margins as an independent predictor of residual disease [23]. Vos et al. analyzed 499 patients and stratified margin involvement into four categories: negative, close, focally positive (≤4 mm of tumour at the ink), and extensively positive (>4 mm). They found that residual disease was present in 50% of cases with focally positive margins and 70% of those with extensively positive margins. Although not reported per number of margins, the extent of involvement directly paralleled our stratification, indicating that a more extensive or multifocal margin positivity significantly raises the risk of residual disease [29]. Similarly, Patterson et al. conducted a large retrospective cohort study on 1001 patients with DCIS, reporting that both the number and type of involved margins were significantly associated with residual disease on re-excision. The statistical significance for a number of positive margins remained robust in multivariate analysis (p = 0.004), and the presence of multiple involved margins substantially increased the probability of residual disease [30]. In our analysis, we included only patients diagnosed with invasive breast carcinoma. However, data emerging from the literature suggest that a similar approach could also be applied to cases of DCIS.
In our study, the presence of LVI on the final histopathological examination was significantly associated with the presence of residual disease following a margin re-excision after breast-conserving surgery in both univariate and multivariate analyses. Specifically, 80.8% of patients with LVI had a residual tumour at reoperation, compared to only 41.3% of patients without LVI. This pronounced difference highlights the potential role of LVI as a reliable indicator of microscopic disease spread beyond the primary tumour bed.
Prior studies strongly support this observation. Alrahbi et al. reported that LVI was significantly associated with residual tumours after margin re-excision in breast-conserving surgery [31]. In their retrospective cohort of 720 patients, LVI, along with an extensive intraductal component and the involvement of more than one radial margin, emerged as a key predictor of residual disease. Similarly, Ozmen et al. found a statistically significant association between LVI and residual tumours in re-excision specimens (p = 0.029), reinforcing its role as a marker for occult microscopic spread [32]. Although not always statistically significant, other studies have consistently supported this association. For example, Atalay and Irkkan noted that LVI approached significance (p = 0.064) as a predictive factor for a residual tumour following re-excision in patients with positive margins [33]. Together, these findings align with our results and suggest that the presence of LVI should be considered a strong risk factor for residual disease, potentially guiding the decision to perform margin re-excision.
Several studies have demonstrated a correlation between a younger age and an increased risk of a residual tumour following surgical re-excision for positive margins [34,35]. Our findings indicate that age, categorized as below or above 50 years, did not reach statistical significance as a predictor of residual tumours.
Our results regarding age are consistent with those of Mendoza-Rojas et al., who found no significant association between age and residual tumours in their cohort of early-stage breast cancer patients undergoing re-excision [36]. However, other studies have reported that a younger age, particularly under 45 years, is an independent risk factor for residual disease following breast-conserving surgery [34,35]. These discrepancies may be attributed to variations in study populations, surgical techniques, and approaches for younger patients.
According to our dataset, HER2 3+ and Luminal B subtypes were more frequently observed among patients with a residual tumour, suggesting a possible trend that did not achieve statistical significance. This evidence is supported by findings from Atalay and Irkkan, who identified HER2 positivity as a significant predictor of residual tumours in re-excision specimens [36]. However, other studies, such as that by Haideri et al., have not found consistent associations between hormone receptor status or HER2 expression and residual tumours, suggesting that these factors may be influenced by additional variables such as tumour size or margin width [37].
Limitations of the Study
This study has several limitations that should be acknowledged. First, the retrospective and single-centre nature of the analysis may introduce selection bias and limit the generalizability of our findings to other institutions with different surgical protocols and patient populations. Second, the relatively small sample size may have reduced the statistical power to detect subtle associations, especially in subgroup analyses. Moreover, certain potentially relevant factors—such as the exact width and anatomical location of positive margins, the type of oncoplastic technique used, and the extent of glandular remodelling—were not systematically captured or included in the multivariate model.
Another limitation of our study is the absence of systematic data collection on the use of a radiotherapy boost as an alternative to re-excision. Although some evidence suggests that high-dose boosts may provide adequate local control, particularly in patients with focally positive margins [38], our retrospective analysis did not consistently capture whether boost radiotherapy was employed as a compensatory strategy in these cases. This observation could serve as a valuable starting point for further and more in-depth investigations.
Furthermore, we did not investigate long-term oncological outcomes such as local recurrence or disease-free survival, which could reinforce the clinical impact of avoiding re-excision in low-risk patients. Although our findings highlight key clinicopathological predictors of residual tumours, these results should be interpreted within the context of these limitations.
Another limitation of our study is the exclusion of cases with a preoperative diagnosis of pure DCIS from the analysis. Our aim is therefore to investigate, in future studies, the correlation between residual disease and predictive prognostic factors in cases diagnosed with DCIS without preoperative evidence of an invasive component.
Importantly, these limitations also represent valuable opportunities for future research. Prospective, multicentre, and potentially randomized studies are needed to validate our proposed risk-adapted approach and to determine whether omission of re-excision in selected patients is both oncologically safe and beneficial in terms of quality of life, cosmesis, and resource utilization.
5. Conclusions
This study suggests that a significant proportion of re-excisions following breast-conserving surgery for positive margins may be oncologically unnecessary with over 50% of patients showing no residual tumour. These findings highlight the need for a more nuanced risk-adapted approach to margin management.
Our results identify LVI, tumour multifocality, and the number of involved margins as strong predictors of residual disease. In particular, LVI and multiple positive margins retained independent significance in a multivariate analysis. Conversely, patients with unifocal tumours, no LVI, and only one involved margin had a notably lower likelihood of residual disease.
However, these findings stem from a single-centre, retrospective cohort and should not be interpreted as a basis for immediate changes in clinical practice. Instead, our results serve as a proof-of-concept that supports the rationale for designing prospective, multicentre—and potentially randomized—studies aimed at validating a risk-adapted strategy for margin re-excision.
In the era of precision medicine, rather than adopting a uniform threshold for reoperation, surgical decisions should be guided by a combination of pathological and clinical risk factors. This tailored approach can reduce unnecessary procedures, preserve cosmetic outcomes, avoid treatment delays, and optimize resource use without compromising oncological safety.
6. Key Implications
- Routine re-excision after positive margins may lead to overtreatment in up to 50% of cases.
- LVI and multiple margin involvement are strong independent predictors of residual tumours.
- A risk-adapted approach to re-excision should be incorporated into multidisciplinary surgical decision-making.
- Clinical guidelines should evolve to consider tumour biology and patient-specific factors alongside margin status.
Author Contributions
Conceptualization: S.D., B.C. and M.S. Formal Analysis: F.D.L., E.D.G., A.F., E.P., F.G., M.N., C.A. and L.S. Supervision: G.F., A.D.L., S.M., and A.M.S. Validation: A.D.L., S.M. and G.F. Visualization: E.D.G., E.P. and M.S. Writing—original draft: S.D. and B.C. Writing—review and editing: L.S., A.F., M.S., F.D.L., F.G., F.M., M.N., C.A., E.D.G. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In accordance with the guidelines by the Fondazione Policlinico Universitario A. Gemelli IRCCS Ethics Committee, ethical review and approval were not required due to the retrospective, non-profit, and non-pharmacological nature of the study. This study was approved by the Institutional Review Board of Fondazione Policlinico Universitario Agostino Gemelli in Rome IRCCS, as it was carried out in full compliance with ethical and scientific regulations, as well as with the guidelines relating to the protection of personal data and the privacy of the patients involved.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
All the authors declare no relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in the manuscript. The authors declare that they have no conflicts of interest.
References
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- Franceschini, G.; Sanchez, A.M.; Di Leone, A.; Magno, S.; Moschella, F.; Accetta, C.; Natale, M.; Di Giorgio, D.; Scaldaferri, A.; D’Archi, S.; et al. Update on the surgical management of breast cancer. Ann. Ital. Chir. 2015, 86, 89–99. [Google Scholar]
- Franceschini, G.; Visconti, G.; Terribile, D.; Fabbri, C.; Magno, S.; Di Leone, A.; Salgarello, M.; Masetti, R. The role of oxidized regenerate cellulose to prevent cosmetic defects in oncoplastic breast surgery. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 966–971. [Google Scholar]
- Moran, M.S.; Schnitt, S.J.; Giuliano, A.E.; Harris, J.R.; Khan, S.A.; Horton, J.; Klimberg, S.; Chavez-MacGregor, M.; Freedman, G.; Houssami, N.; et al. Society of Surgical Oncology–American Society for Radiation Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Stages I and II Invasive Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Macaskill, P.; Marinovich, M.L.; Dixon, J.M.; Irwig, L.; Brennan, M.E.; Solin, L.J. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur. J. Cancer 2010, 46, 3219–3232. [Google Scholar] [CrossRef]
- Rubio, I.T.; Marotti, L.; Biganzoli, L.; Aristei, C.; Athanasiou, A.; Campbell, C.; Cardoso, F.; Cardoso, M.J.; Coles, C.E.; Eicher, M.; et al. EUSOMA quality indicators for non-metastatic breast cancer: An update. Eur. J. Cancer 2024, 198, 113500. [Google Scholar] [CrossRef]
- Gurdal, S.O.; Karanlik, H.; Cabioglu, N.; Ozcinar, B.; Yavuz, E.; Tuzlali, S.; Ozmen, V. Positive or close margins in breast conserving surgery: Is re-excision always necessary? Eur. J. Surg. Oncol. 2012, 38, 399–406. [Google Scholar] [CrossRef]
- Rakha, E.A.; Quinn, C.; Masannat, Y.A.; Lee, A.H.S.; Tan, P.H.; Karakatsanis, A.; Matrai, Z.T.; Al Shaibani, S.H.M.; Gehani, S.A.; Shaaban, A.; et al. Revisiting surgical margins for invasive breast cancer patients treated with breast conservation therapy—Evidence for adopting a 1 mm negative width. Eur. J. Surg. Oncol. 2024, 50, 108573. [Google Scholar] [CrossRef]
- Bundred, J.R.; Michael, S.; Stuart, B.; Cutress, R.I.; Beckmann, K.; Holleczek, B.; Dahlstrom, J.E.; Gath, J.; Dodwell, D.; Bundred, N.J. Margin status and survival outcomes after breast cancer conservation surgery: Prospectively registered systematic review and meta-analysis. BMJ 2022, 378, e070346. [Google Scholar] [CrossRef]
- Franceschini, G.; D’Archi, S.; Di Leone, A.; Masetti, R. Positive margins after breast-conserving surgery: Is it possible to hang up the scalpel in the era of precision medicine? Updates Surg. 2025, 77, 265–266. [Google Scholar] [CrossRef]
- American Society of Breast Surgeons (ASBrS). Consensus Statement on Breast Cancer Surgical Margins; American Society of Breast Surgeons: Columbia, MD, USA, 2016. [Google Scholar]
- Cheun, J.-H.; Kim, H.-K.; Lee, H.-B.; Han, W.; Moon, H.-G. Residual Risk of Ipsilateral Tumor Recurrence in Patients Who Achieved Clear Lumpectomy Margins After Repeated Resection. J. Breast Cancer 2023, 26, 558. [Google Scholar] [CrossRef] [PubMed]
- Nardone, L.; Valentini, V.; Marino, L.; De Santis, M.C.; Terribile, D.; Franceschini, G.; Balducci, M.; Mantini, G.; Mattiucci, G.; Mulè, A.; et al. A Feasibility Study of Neo-Adjuvant Low-Dose Fractionated Radiotherapy with Two Different Concurrent Anthracycline-Docetaxel Schedules in Stage IIA/B-IIIA Breast Cancer. Tumori 2012, 98, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Chism, D.B.; Freedman, G.M.; Li, T.; Anderson, P.R. Re-excision of margins before breast radiation—Diagnostic or therapeutic? Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.F.; Hill, A.D.K.; Quinn, C.M.; McDermott, E.W.; O’Higgins, N. A Pathologic Assessment of Adequate Margin Status in Breast-Conserving Therapy. Ann. Surg. Oncol. 2006, 13, 333–339. [Google Scholar] [CrossRef]
- Neuschatz, A.C.; DiPetrillo, T.; Steinhoff, M.; Safaii, H.; Yunes, M.; Landa, M.; Chung, M.; Cady, B.; Wazer, D.E. The value of breast lumpectomy margin assessment as a predictor of residual tumor burden in ductal carcinoma in situ of the breast. Cancer 2002, 94, 1917–1924. [Google Scholar] [CrossRef]
- Findlay-Shirras, L.J.; Outbih, O.; Muzyka, C.N.; Galloway, K.; Hebbard, P.C.; Nashed, M. Predictors of Residual Disease After Breast Conservation Surgery. Ann. Surg. Oncol. 2018, 25, 1936–1942. [Google Scholar] [CrossRef]
- Mimouni, M.; Lecuru, F.; Rouzier, R.; Lotersztajn, N.; Heitz, D.; Cohen, J.; Fauconnier, A.; Huchon, C. Reexcision for positive margins in breast cancer: A predictive score of residual disease. Surg. Oncol. 2015, 24, 129–135. [Google Scholar] [CrossRef]
- Lai, H.-W.; Huang, R.H.; Wu, Y.T.; Chen, C.J.; Chen, S.T.; Lin, Y.J.; Chen, D.R.; Lee, C.W.; Wu, H.K.; Lin, H.Y.; et al. Clinicopathologic factors related to surgical margin involvement, reoperation, and residual cancer in primary operable breast cancer—An analysis of 2050 patients. Eur. J. Surg. Oncol. 2018, 44, 1725–1735. [Google Scholar] [CrossRef]
- Héquet, D.; Bricou, A.; Delpech, Y.; Barranger, E. Surgical Management Modifications Following Systematic Additional Shaving of Cavity Margins in Breast-Conservation Treatment. Ann. Surg. Oncol. 2011, 18, 114–118. [Google Scholar] [CrossRef]
- Graham, R.A.; Homer, M.J.; Katz, J.; Rothschild, J.; Safaii, H.; Supran, S. The pancake phenomenon contributes to the inaccuracy of margin assessment in patients with breast cancer. Am. J. Surg. 2002, 184, 89–93. [Google Scholar] [CrossRef]
- Zaidi, M.; Khan, S.; Farooqi, N.B.; Abbas, K.; Idrees, R. Effect of Formalin Fixation on Surgical Margins in Breast Cancer Surgical Specimen. Int. J. Breast Cancer 2014, 2014, 121838. [Google Scholar] [CrossRef]
- Simpson, D.J.; Allan, J.; McFall, B. Factors predicting residual disease on re-excision after breast conserving surgery. Surgeon 2022, 20, e149–e157. [Google Scholar] [CrossRef] [PubMed]
- Mason, E.J.; Di Leone, A.; Franco, A.; D’archi, S.; Rianna, C.; Sanchez, A.M.; Murando, F.; Accetta, C.; Scardina, L.; Terribile, D.A.; et al. Oncoplastic Breast Surgery versus Conservative Mastectomy in the Management of Large Ductal Carcinoma In Situ (DCIS): Surgical, Oncological, and Patient-Reported Outcomes. Cancers 2022, 14, 5624. [Google Scholar] [CrossRef] [PubMed]
- Sabel, M.S.; Rogers, K.; Griffith, K.; Jagsi, R.; Kleer, C.G.; Diehl, K.A.; Breslin, T.M.; Cimmino, V.M.; Chang, A.E.; Newman, L.A. Residual disease after re-excision lumpectomy for close margins. J. Surg. Oncol. 2009, 99, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, L.; Zhou, X.; Wang, Z.; Li, Y.; Sun, Q. Local Recurrence and Survival Outcomes of Multifocal/Multicentric Breast Cancer After Breast Conserving Therapy: A systematic Review and Meta-Analysis. Clin. Breast Cancer 2025, 25, e229–e239.e9. [Google Scholar] [CrossRef]
- Saarela, A.; Rissanen, T.; Lähteenmäki, K.; Soini, Y.; Haukipuro, K.; Kaarela, O.; Kiviniemi, H. Wire-guided excision of non-palpable breast cancer: Determinants and correlations between radiologic and histologic margins and residual disease in re-excisions. Breast 2001, 10, 28–34. [Google Scholar] [CrossRef]
- Rath, M.G.; Uhlmann, L.; Heil, J.; Domschke, C.; Roth, Z.; Sinn, H.-P.; Marme, F.; Scharf, A.; Schneeweiss, A.; Kieser, M.; et al. Predictors of Residual Tumor in Breast-Conserving Therapy. Ann. Surg. Oncol. 2015, 22, 451–458. [Google Scholar] [CrossRef]
- Vos, E.; Gaal, J.; Verhoef, C.; Brouwer, K.; van Deurzen, C.; Koppert, L. Focally positive margins in breast conserving surgery: Predictors, residual disease, and local recurrence. Eur. J. Surg. Oncol. 2017, 43, 1846–1854. [Google Scholar] [CrossRef]
- Patterson, R.; Guest, M.; Shenouda, M.; Pareek, V.; Galloway, K.; Bucher, O.; Hebbard, P.; Nashed, M. Predictors of residual disease after breast conservation surgery for ductal carcinoma in situ: A retrospective study. J. Cancer Res. Ther. 2024, 20, 844–849. [Google Scholar] [CrossRef]
- Alrahbi, S.; Chan, P.M.; Ho, B.C.; Seah, M.D.; Chen, J.J.; Tan, E.Y. Extent of Margin Involvement, Lymphovascular Invasion, and Extensive Intraductal Component Predict for Residual Disease After Wide Local Excision for Breast Cancer. Clin. Breast Cancer 2015, 15, 219–226. [Google Scholar] [CrossRef]
- Ozmen, V.; Gurdal, S.O.; Muslumanoglu, M.; Igci, A.; Tuzlali, S.S.; Ozcinar, B.; Canbay, E.; Kecer, M.; Dagoglu, T. Predictive factor for residual tumor after lumpectomy for close margins. J. Clin. Oncol. 2009, 27, e11538. [Google Scholar] [CrossRef]
- Atalay, C.; Irkkan, C. Predictive Factors for Residual Disease in Re-Excision Specimens After Breast-Conserving Surgery: Predictive Factors for Residual Disease after Breast-Conserving Surgery. Breast J. 2012, 18, 339–344. [Google Scholar] [CrossRef]
- Voguet, L.; Hébert, T.; Levêque, J.; Acker, O.; Mesbah, H.; Marret, H.; Porée, P.; Body, G. Patient age and positive margins are predictive factors of residual tumor on mastectomy specimen after conservative treatment for breast cancer. Breast 2009, 18, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Wazer, D.E.; Schmidt-Ullrich, R.K.; Ruthazer, R.; DiPetrillo, T.; Boyle, T.; Kanski, J.; Safaii, H. The influence of age and extensive intraductal component histology upon breast lumpectomy margin assessment as a predictor of residual tumor. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-rojas, J.J.; Bautista-Hernández, M.Y.; Quintero-Beuló, G.; Santoyo-Sánchez, A.; Ramos-Peñafiel, C.O. Surgical-pathologic correlation to assess the margin status in wide local excision for early-stages breast cancer. Ginecol. Obstet. Mex. 2015, 83, 88–95. [Google Scholar] [PubMed]
- Haideri, N.A.; Moormeier, J.; Arora, N. Positive predictive factors for residual disease in re-excision specimens of early stage breast cancer. J. Clin. Oncol. 2006, 24, 10604. [Google Scholar] [CrossRef]
- Uomori, T.; Horimoto, Y.; Ueki, Y.; Ishizuka, Y.; Onagi, H.; Hayashi, T.; Watanabe, J.; Shikama, N. Efficacy of radiation therapy in Japanese patients with positive margins after breast-conserving surgery. Jpn. J. Clin. Oncol. 2024, 54, 556–561. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).