Thoracic Endovascular Aortic Repair Using a Branched Endograft Versus Open Arch Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
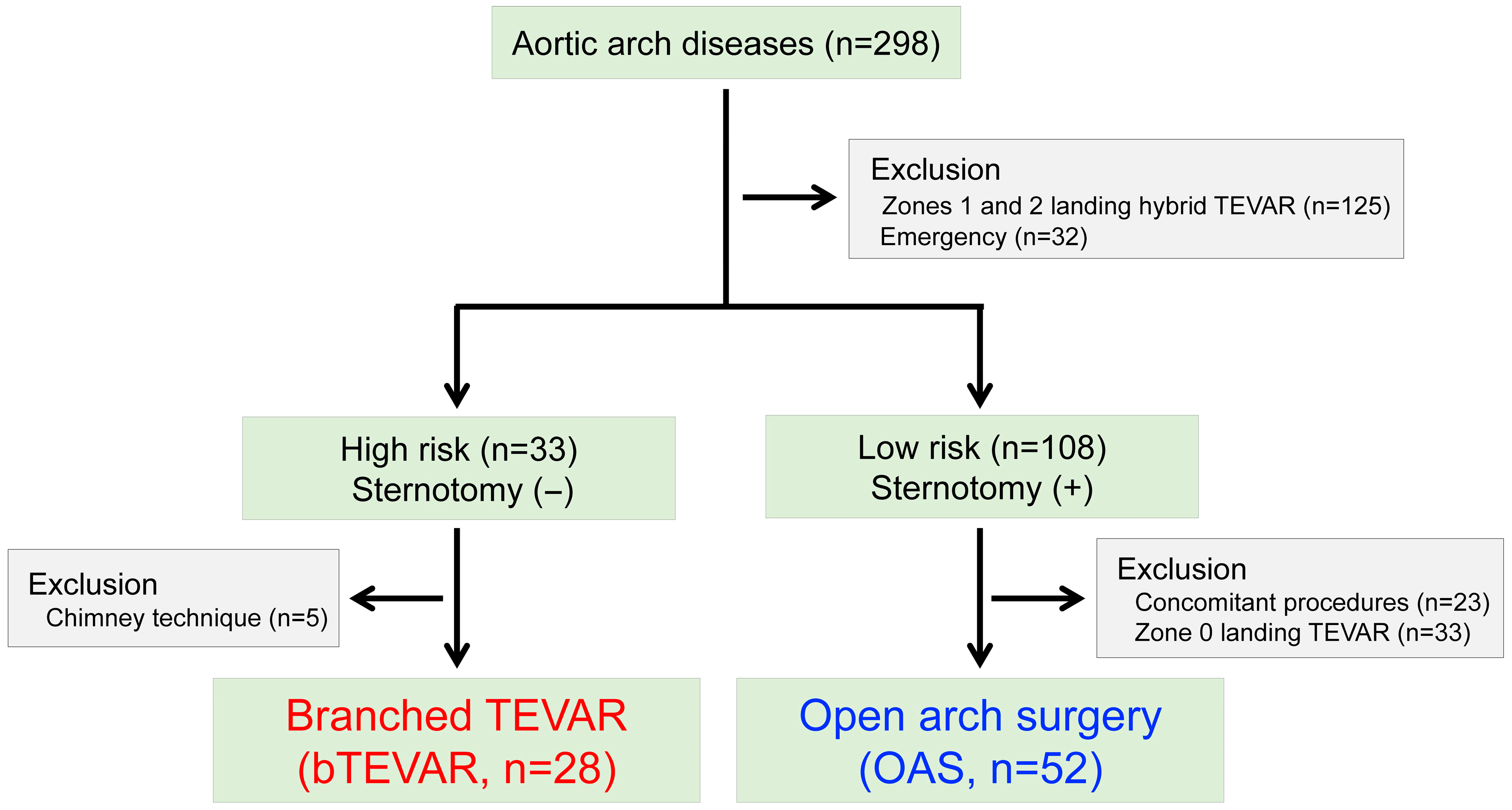
2.2. Study Population
2.3. Surgical Procedures
2.3.1. Branched TEVAR (n = 28, 35.0%)
2.3.2. Open Arch Surgery (OAS; n = 65.0%)
2.4. Follow-Up
2.5. Endpoints
2.6. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
3.2. Operative and Early (30-Day) Outcomes
3.3. Late Outcomes
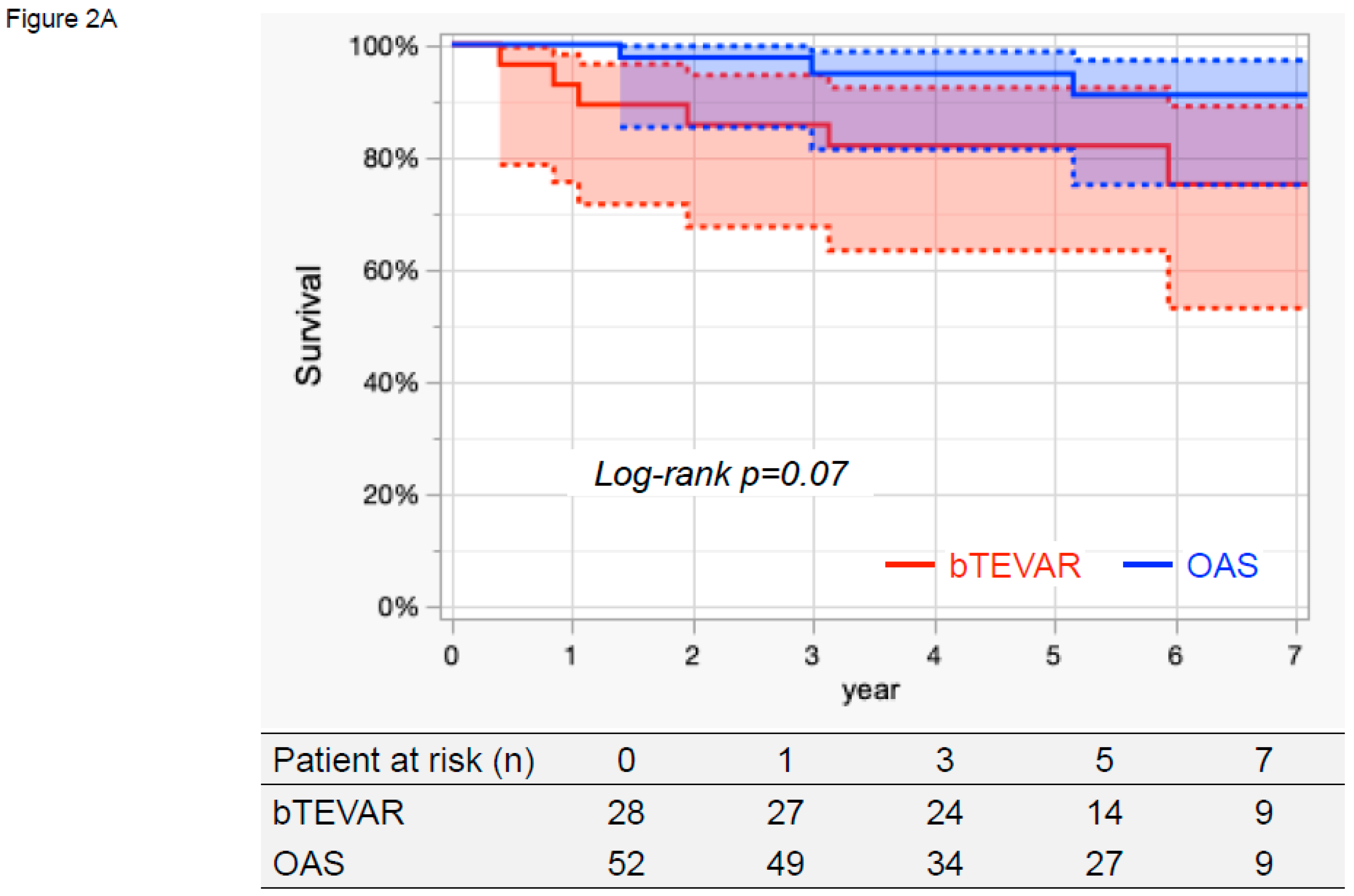
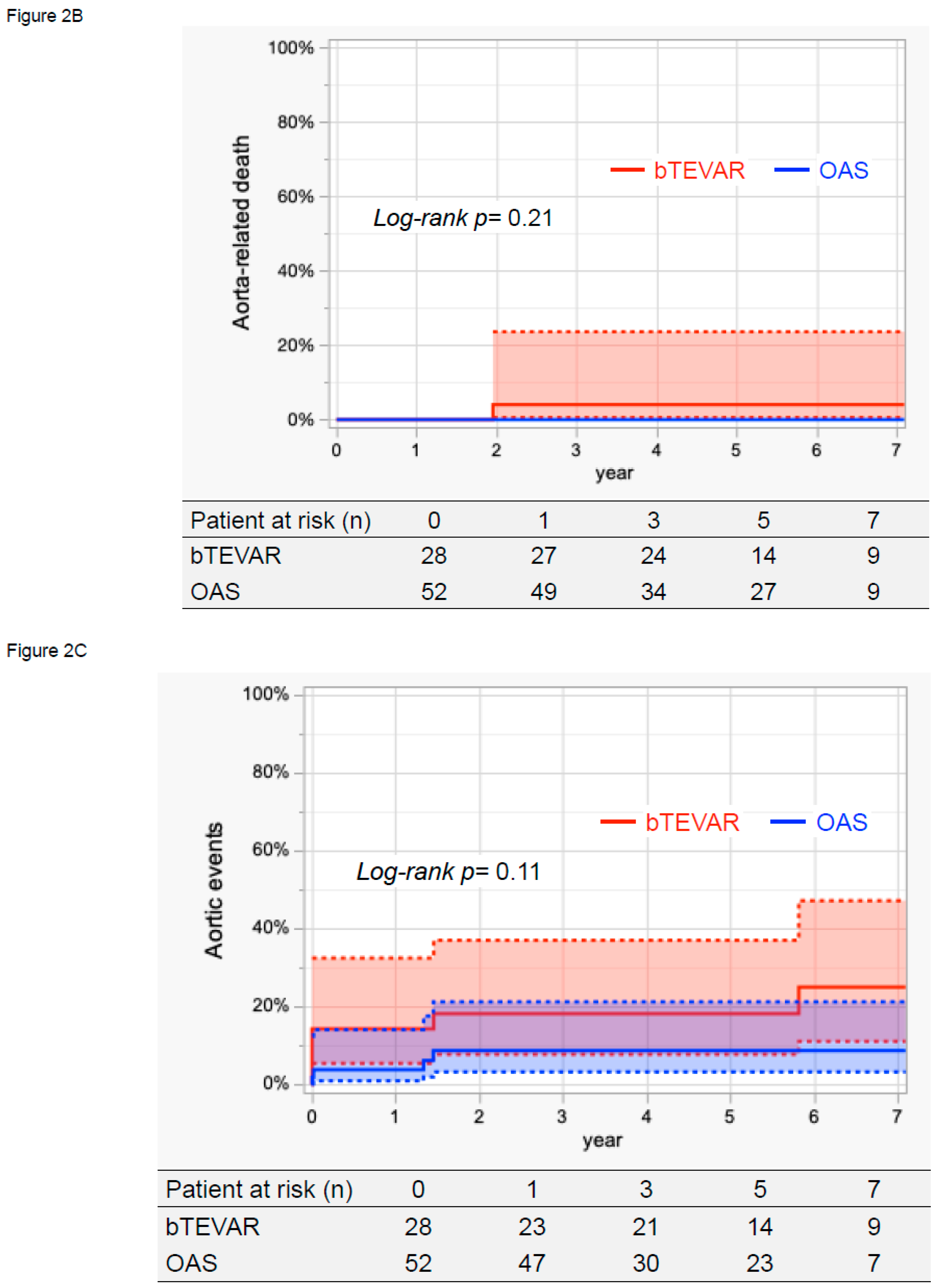
3.4. Survival, Aorta-Related Death, and Aortic Events
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kudo, T.; Kuratani, T.; Shirakawa, Y.; Shimamura, K.; Kin, K.; Sakamoto, T.; Shijo, T.; Watanabe, Y.; Masada, K.; Sakaniwa, R.; et al. Effectiveness of Proximal Landing Zones 0, 1, and 2 Hybrid Thoracic Endovascular Aortic Repair: A Single Centre 12 Year Experience. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 410–420. [Google Scholar] [CrossRef]
- Shimizu, H.; Hirahara, N.; Motomura, N.; Miyata, H.; Takamoto, S. Current status of cardiovascular surgery in Japan, 2015 and 2016: Analysis of data from Japan Cardiovascular Surgery Database. 4-Thoracic aortic surgery. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Shimamoto, T.; Esaki, J.; Komiya, T.; Ohno, N.; Nakayama, S.; Paku, M.; Hidaka, Y.; Morita, S.; Marui, A.; et al. Comparison of open and hybrid endovascular repair for aortic arch: A multi-centre study of 1052 adult patients. Eur. J. Cardio-Thorac. Surg. 2024, 66, ezae377. [Google Scholar] [CrossRef] [PubMed]
- Seike, Y.; Matsuda, H.; Fukuda, T.; Inoue, Y.; Omura, A.; Uehara, K.; Sasaki, H.; Kobayashi, J. Total arch replacement versus debranching thoracic endovascular aortic repair for aortic arch aneurysm: What indicates a high-risk patient for arch repair in octogenarians? Gen. Thorac. Cardiovasc. Surg. 2018, 66, 263–269. [Google Scholar] [CrossRef]
- Milewski, R.K.; Szeto, W.Y.; Pochettino, A.; Moser, G.W.; Moeller, P.; Bavaria, J.E. Have hybrid procedures replaced open aortic arch reconstruction in high-risk patients? A comparative study of elective open arch debranching with endovascular stent graft placement and conventional elective open total and distal aortic arch reconstruction. J. Thorac. Cardiovasc. Surg. 2010, 140, 590–597. [Google Scholar] [CrossRef]
- Tokuda, Y.; Oshima, H.; Narita, Y.; Abe, T.; Araki, Y.; Mutsuga, M.; Fujimoto, K.; Terazawa, S.; Yagami, K.; Ito, H.; et al. Hybrid versus open repair of aortic arch aneurysms: Comparison of postoperative and mid-term outcomes with a propensity score-matching analysis. Eur. J. Cardio-Thorac. Surg. 2016, 49, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Settepani, F.; Cappai, A.; Basciu, A.; Barbone, A.; Tarelli, G. Outcome of open total arch replacement in the modern era. J. Vasc. Surg. 2016, 63, 537–545. [Google Scholar] [CrossRef]
- Shah, P.J.; Estrera, A.L.; Miller, C.C., III.; Lee, T.Y.; Irani, A.D.; Meada, R.; Safi, H.J. Analysis of ascending and transverse aortic arch repair in octogenarians. Ann. Thorac. Surg. 2008, 86, 774–779. [Google Scholar] [CrossRef]
- Hiraoka, A.; Saito, K.; Chikazawa, G.; Totsugawa, T.; Tamura, K.; Ishida, A.; Sakaguchi, T.; Yoshitaka, H. Modified predictive score based on frailty for mid-term outcomes in open total aortic arch surgery. Eur. J. Cardio-Thorac. Surg. 2018, 54, 42–47. [Google Scholar] [CrossRef]
- Seike, Y.; Matsuda, H.; Fukuda, T.; Hori, Y.; Inoue, Y.; Omura, A.; Uehara, K.; Sasaki, H.; Kobayashi, J. Is debranching thoracic endovascular aortic repair acceptable as the first choice for arch aneurysm in the elderly? Interact. Cardiovasc. Thorac. Surg. 2019, 29, 101–108. [Google Scholar] [CrossRef]
- Kudo, T.; Kuratani, T.; Shimamura, K.; Sawa, Y. Early and midterm results of thoracic endovascular aortic repair using a branched endograft for aortic arch pathologies: A retrospective single-center study. JTCVS Tech. 2020, 4, 17–25. [Google Scholar] [CrossRef]
- Basha, A.M.; Moore, R.D.; Rommens, K.L.; Herget, E.J.; McClure, R.S. A Systematic Review of Total Endovascular Aortic Arch Repair: A Promising Technology. Can. J. Cardiol. 2023, 39, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Spath, P.; Campana, F.; Tsilimparis, N.; Gallitto, E.; Pini, R.; Faggioli, G.; Caputo, S.; Gargiulo, M. Outcomes of Fenestrated and Branched Endografts for Partial and Total Endovascular Repair of the Aortic Arch—A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 106–116. [Google Scholar] [CrossRef] [PubMed]
- van Bakel, T.M.; de Beaufort, H.W.; Trimarchi, S.; Marrocco-Trischitta, M.M.; Bismuth, J.; Moll, F.L.; Patel, H.J.; van Herwaarden, J.A. Status of branched endovascular aortic arch repair. Ann. Cardiothorac. Surg. 2018, 7, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Preventza, O.; Tan, C.W.; Orozco-Sevilla, V.; Euhus, C.J.; Coselli, J.S. Zone zero hybrid arch exclusion versus open total arch replacement. Ann. Cardiothorac. Surg. 2018, 7, 372–379. [Google Scholar] [CrossRef]
- Shiraya, S.; Nakamura, Y.; Harada, S.; Kishimoto, Y.; Onohara, T.; Otsuki, Y.; Kurashiki, T.; Horie, H.; Nishimura, M. Debranching thoracic endovascular aortic repair for distal aortic arch aneurysm in elderly patients aged over 75 years old. J. Cardiothorac. Surg. 2020, 15, 13. [Google Scholar] [CrossRef]
- Dakour-Aridi, H.; Yin, K.; Hussain, F.; Locham, S.; Azizzadeh, A.; Malas, M.B. Outcomes of intact thoracic endovascular aortic repair in octogenarians. J. Vasc. Surg. 2021, 74, 882–892.E1. [Google Scholar] [CrossRef]
- Frola, E.; Mortola, L.; Ferrero, E.; Ferri, M.; Apostolou, D.; Quaglino, S.; Maione, M.; Gaggiano, A. Multicenter Comparison of Aortic Arch Aneurysms and Dissections Zone 0 Hybrid and Total Endovascular Repair. Cardiovasc. Interv. Radiol. 2023, 46, 1674–1683. [Google Scholar] [CrossRef]
- Ikeno, Y.; Yokawa, K.; Yamanaka, K.; Inoue, T.; Tanaka, H.; Okada, K.; Okita, Y. Total arch replacement in octogenarians and nonagenarians: A single-center 18-year experience. J. Thorac. Cardiovasc. Surg. 2020, 160, 346–356.E1. [Google Scholar] [CrossRef]
- Hachiro, K.; Kinoshita, T.; Suzuki, T.; Asai, T. Total arch replacement in octogenarians. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 283–290. [Google Scholar] [CrossRef]
- Chung, J.; Stevens, L.M.; Chu, M.W.A.; Dagenais, F.; Peterson, M.D.; Boodhwani, M.; Bozinovski, J.; El-Hamamsy, I.; Yamashita, M.H.; Atoui, R.; et al. The impact of age on patients undergoing aortic arch surgery: Evidence from a multicenter national registry. J. Thorac. Cardiovasc. Surg. 2021, 162, 759–766.E1. [Google Scholar] [CrossRef] [PubMed]
- Akhmerov, A.; Shah, A.S.; Gupta, N.; Tulloch, A.W.; Gewertz, B.; Azizzadeh, A. Thoracic Endovascular Aortic Repair in Octogenarians and Nonagenarians. Ann. Vasc. Surg. 2020, 68, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Miyata, H.; Motomura, N.; Takamoto, S.; Japan Cardiovascular Surgery Database, O. A study of brain protection during total arch replacement comparing antegrade cerebral perfusion versus hypothermic circulatory arrest, with or without retrograde cerebral perfusion: Analysis based on the Japan Adult Cardiovascular Surgery Database. J. Thorac. Cardiovasc. Surg. 2015, 149, S65–S73. [Google Scholar] [CrossRef] [PubMed]
| Total Cohort (n = 80) | bTEVAR n = 28 (35.0%) | OAS n = 52 (65.0%) | p Value | |
|---|---|---|---|---|
| Age (years) | 73 (66–79) | 81 (73–83) | 70 (63–76) | <0.001 |
| Male, n (%) | 62 (77.5) | 17 (60.7) | 45 (83.8) | 0.008 |
| Aortic pathologies | ||||
| Aneurysm, n (%) | 49 (61.3) | 22 (78.6) | 27 (51.9) | 0.030 |
| Dissection, n (%) | 31 (38.8) | 6 (21.4) | 25 (48.1) | |
| Preoperative complications | ||||
| Cerebrovascular disease, n (%) | 12 (15.0) | 7 (25.0) | 5 (9.6) | 0.10 |
| Coronary artery disease, n (%) | 10 (12.5) | 7 (25.0) | 3 (5.8) | 0.028 |
| CKD stage ≥ 4, n (%) | 10 (12.5) | 6 (21.4) | 4 (7.7) | 0.09 |
| COPD, n (%) | 18 (22.5) | 10 (35.7) | 8 (15.4) | 0.038 |
| Ejection fraction (%) | 66 (60–71) | 66 (58–72) | 66 (60–71) | 0.74 |
| Prior median sternotomy, n (%) | 10 (12.5) | 3 (10.7) | 7 (13.5) | 1.00 |
| EuroSCORE 2 (%) | 3.6 (2.2–6.4) | 6.6 (5.7–8.9) | 2.4 (1.8–3.7) | <0.001 |
| Total Cohort (n = 80) | bTEVAR n = 28 (35.0%) | OAS n = 52 (65.0%) | p Value | |
|---|---|---|---|---|
| Procedure success, n (%) | 80 (100) | 28 (100) | 68 (100) | 1.00 |
| Operative time (minutes) | 354 (240–435) | 222 (194–258) | 409 (354–473) | <0.001 |
| 30-day mortality, n (%) | 0 | 0 | 0 | 1.00 |
| Hospital mortality, n (%) | 0 | 0 | 0 | 1.00 |
| Hospital stay (days) | 18 (14–25) | 12 (9–20) | 19 (17–25) | <0.001 |
| Early aortic events | ||||
| Complications | ||||
| Stroke, n (%) | 4 (5.0) | 4 (14.3) | 0 | 0.013 |
| Disabling stroke, n (%) | 2 (2.5) | 2 (7.1) | 0 | 0.12 |
| Minor stroke, n (%) | 2 (2.5) | 2 (7.1) | 0 | 0.12 |
| Spinal cord injury, n (%) | 1 (1.3) | 0 | 1 (1.9) | 1.00 |
| RTAD, n (%) | 0 | 0 | 0 | 1.00 |
| Distal SINE, n (%) | 1 (1.3) | 0 | 1 (1.9) | 1.00 |
| Endoleaks | ||||
| Type 1a, n (%) | 0 | 0 | 0 | 1.00 |
| Type 1b, n (%) | 0 | 0 | 0 | 1.00 |
| Type 1c, n (%) | 0 | 0 | 0 | 1.00 |
| Type 2, n (%) | 0 | 0 | 0 | 1.00 |
| Type 3, n (%) | 0 | 0 | 0 | 1.00 |
| Total Cohort (n = 80) | bTEVAR n = 28 (35.0%) | OAS n = 52 (65.0%) | p Value | |
|---|---|---|---|---|
| Late aortic events | ||||
| Complications | ||||
| Stroke, n (%) | 0 | 0 | 0 | 1.00 |
| Aneurysm rupture, n (%) | 2 (2.5) | 2 (7.1) | 0 | 0.12 |
| RTAD, n (%) | 0 | 0 | 0 | 1.00 |
| Distal SINE, n (%) | 1 (1.3) | 0 | 1 (1.9) | 1.00 |
| Prosthetic infection, n (%) | 0 | 0 | 0 | 1.00 |
| Endoleaks | ||||
| Type 1a, n (%) | 0 | 0 | 0 | 1.00 |
| Type 1b, n (%) | 1 (1.3) | 1 (4.6) | 0 | 0.35 |
| Type 1c, n (%) | 0 | 0 | 1.00 | |
| Type 2, n (%) | 0 | 0 | 1.00 | |
| Type 3, n (%) | 1 (1.3) | 1 (4.6) | 0 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, T.; Kuratani, T.; Sawa, Y.; Miyagawa, S. Thoracic Endovascular Aortic Repair Using a Branched Endograft Versus Open Arch Surgery. J. Clin. Med. 2025, 14, 5837. https://doi.org/10.3390/jcm14165837
Kudo T, Kuratani T, Sawa Y, Miyagawa S. Thoracic Endovascular Aortic Repair Using a Branched Endograft Versus Open Arch Surgery. Journal of Clinical Medicine. 2025; 14(16):5837. https://doi.org/10.3390/jcm14165837
Chicago/Turabian StyleKudo, Tomoaki, Toru Kuratani, Yoshiki Sawa, and Shigeru Miyagawa. 2025. "Thoracic Endovascular Aortic Repair Using a Branched Endograft Versus Open Arch Surgery" Journal of Clinical Medicine 14, no. 16: 5837. https://doi.org/10.3390/jcm14165837
APA StyleKudo, T., Kuratani, T., Sawa, Y., & Miyagawa, S. (2025). Thoracic Endovascular Aortic Repair Using a Branched Endograft Versus Open Arch Surgery. Journal of Clinical Medicine, 14(16), 5837. https://doi.org/10.3390/jcm14165837






