Impact of Irradiation on Post-Surgical Residuals of WHO Grade I Meningioma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Treatments
2.3. Follow-Up Workflow and Recurrences
2.4. Endpoints and Statistical Analysis
3. Results
3.1. Baseline Data
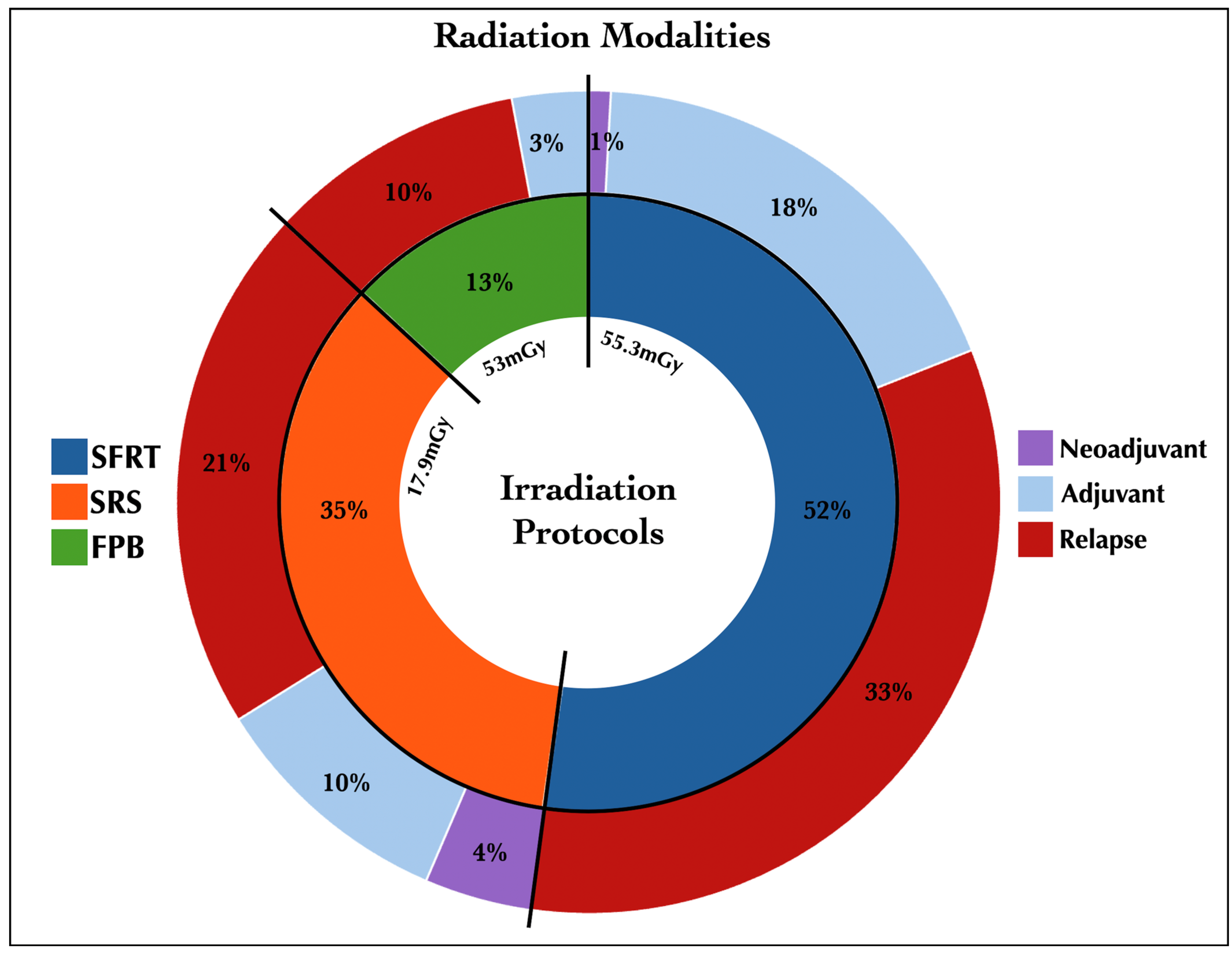
3.2. Irradiation Protocols
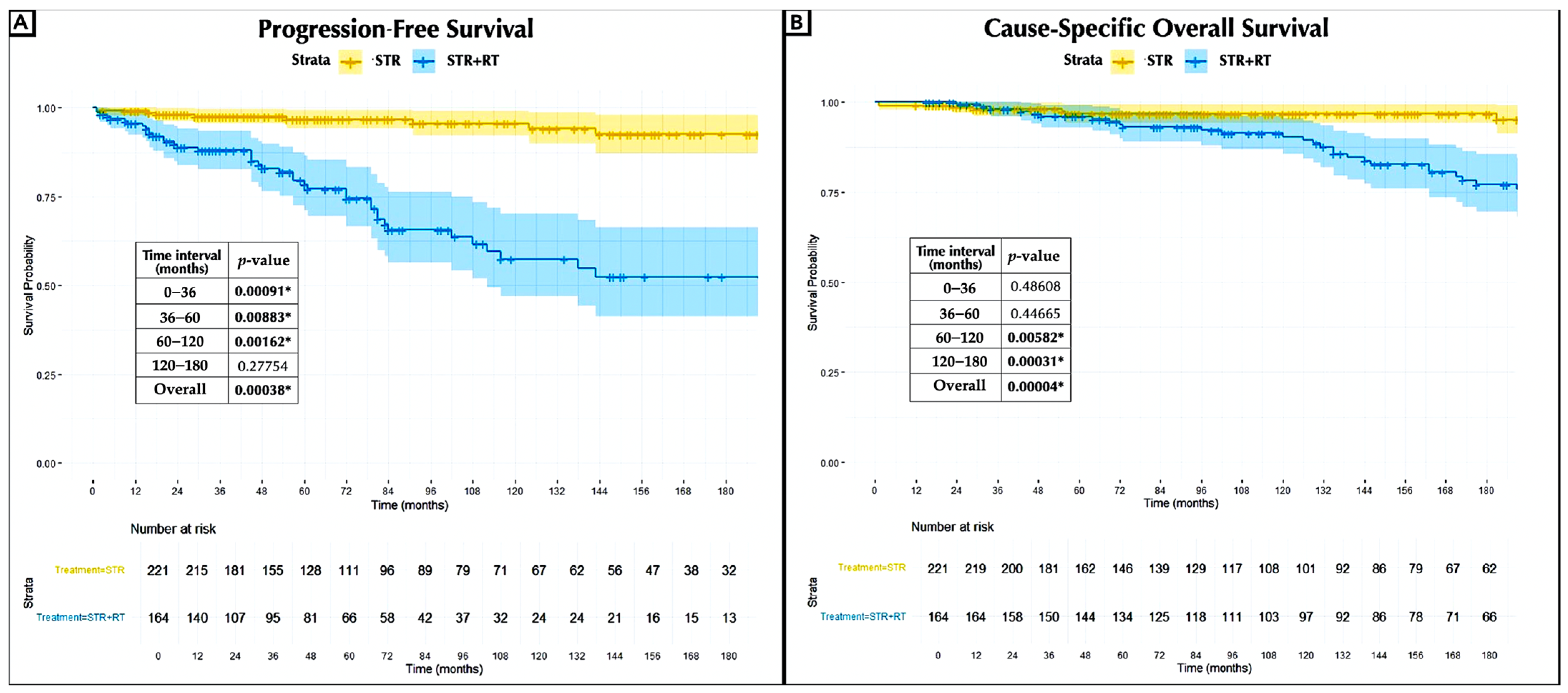
3.3. Recurrence Rates
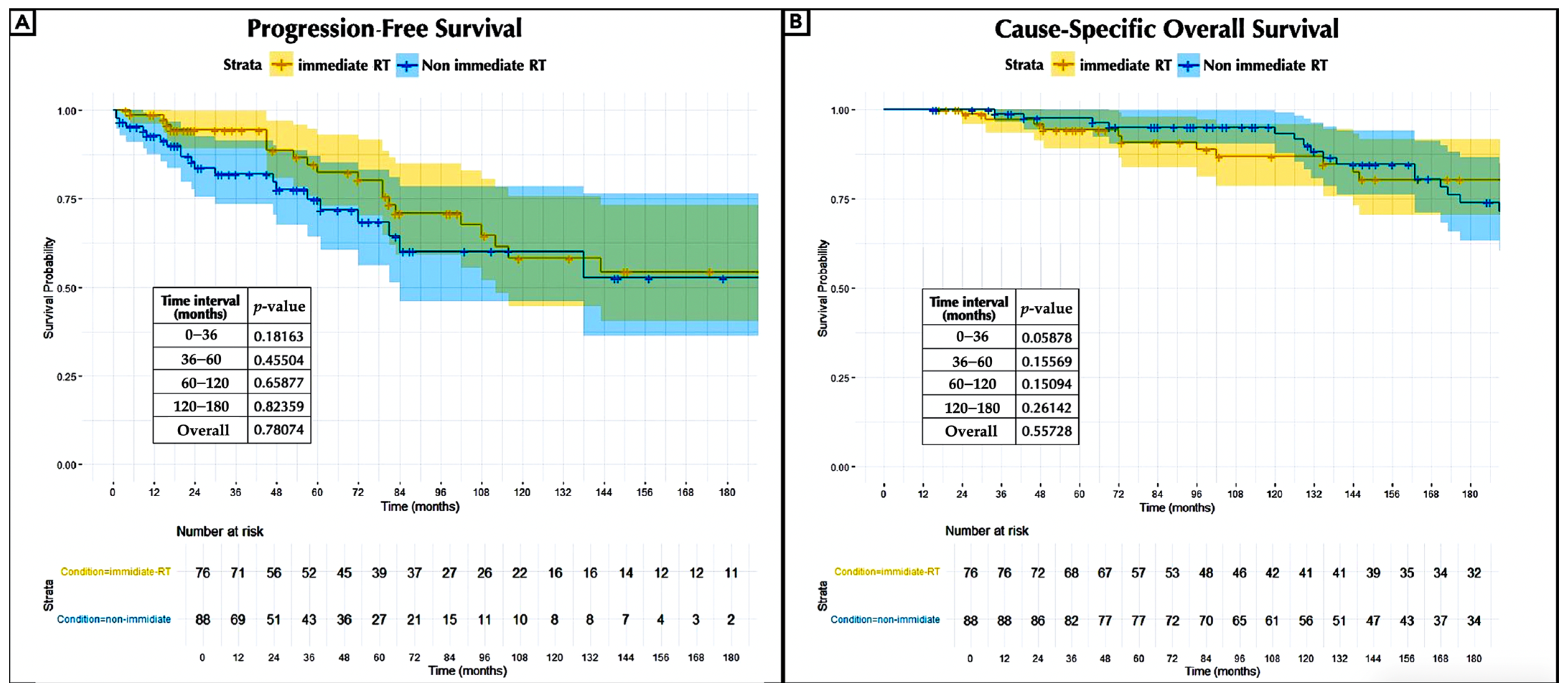
3.4. Extended Long-Term Outcomes
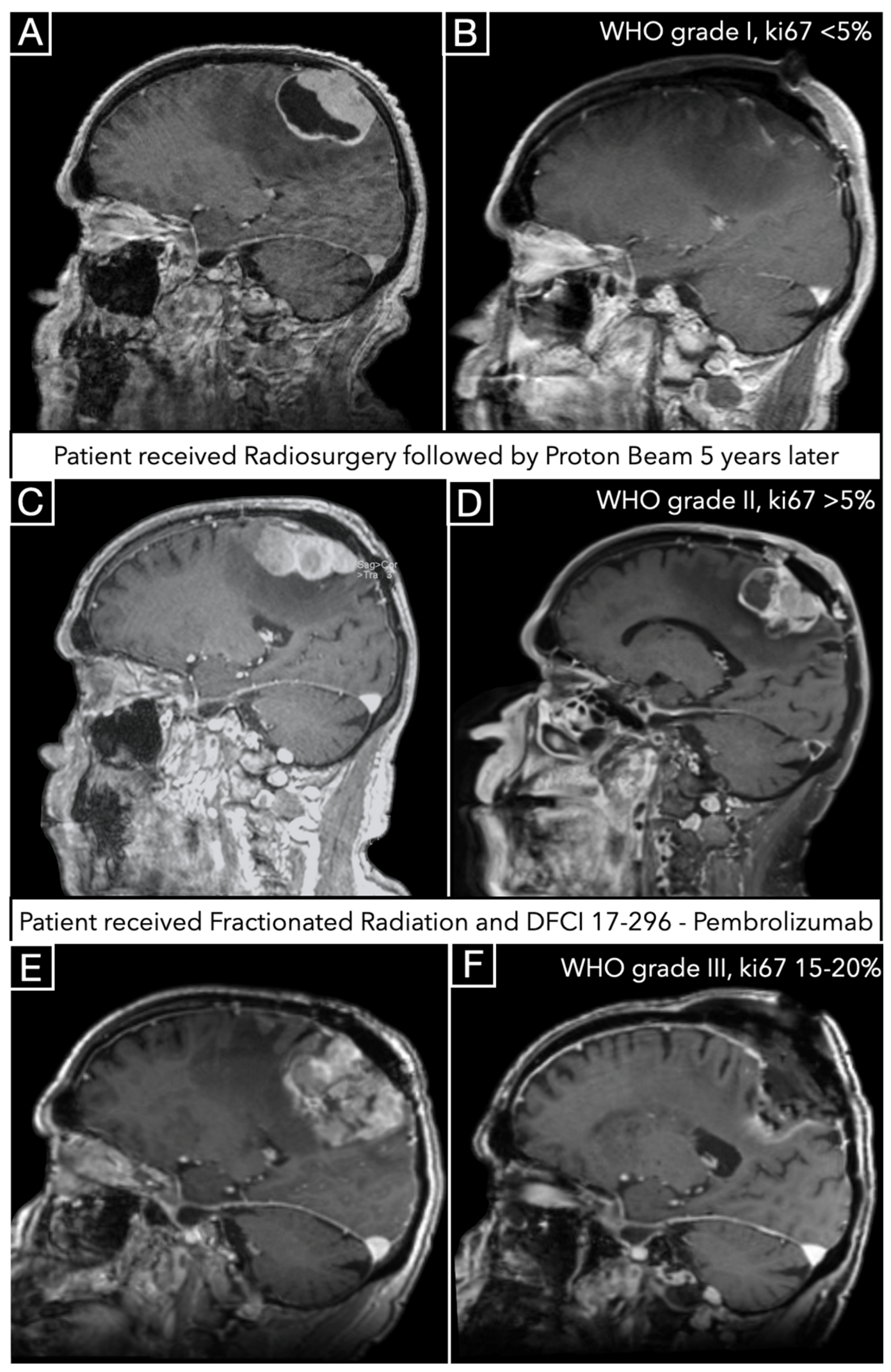
3.5. Illustrative Case
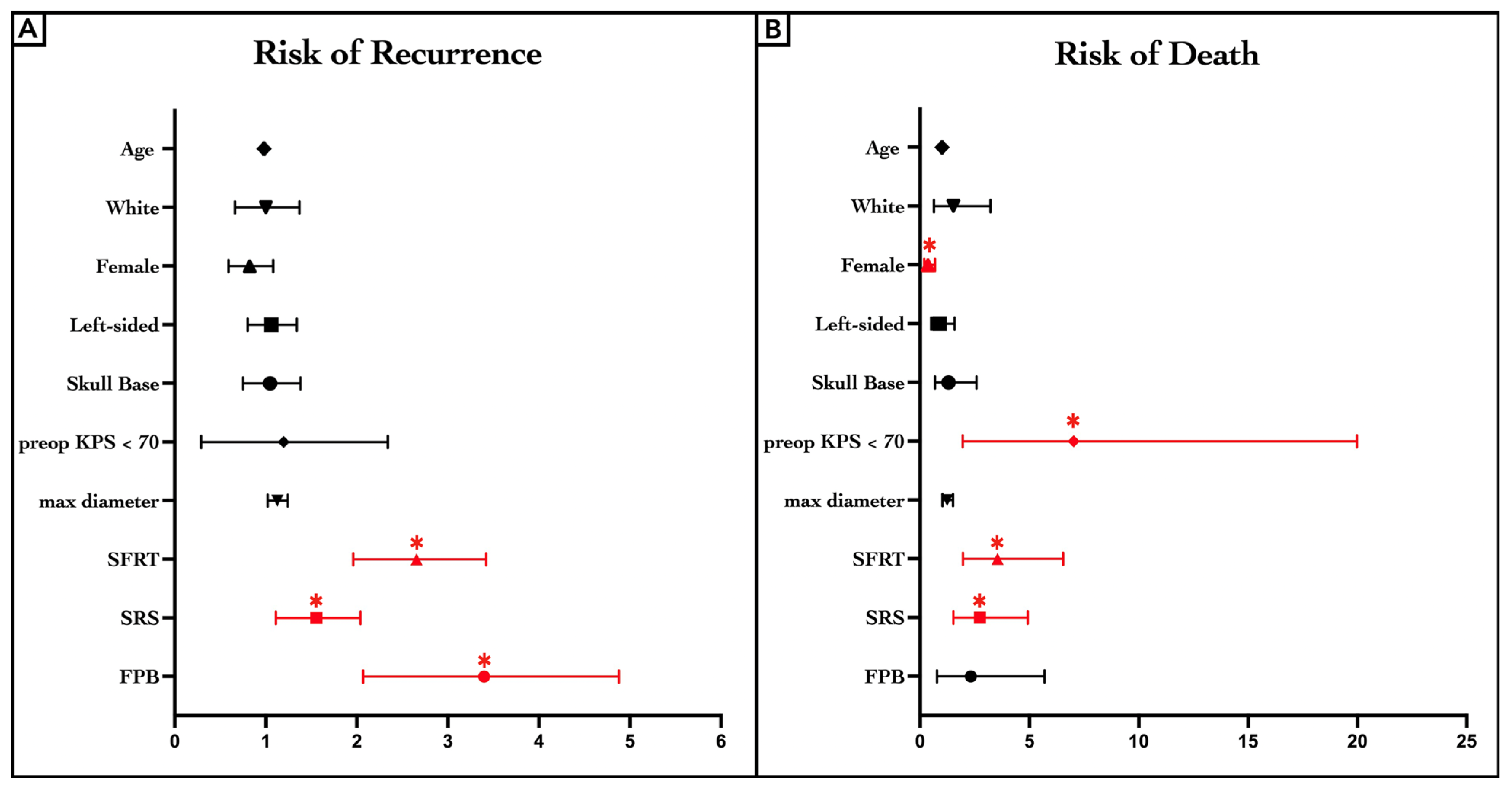
3.6. Bonferroni-Adjusted Cox-PH Regression Model
3.7. Clinical Endpoints and Complications
4. Discussion
4.1. Meningioma and Radiotherapy
4.2. Progression-Free Survival and Clinical Control
4.3. Survival Rates
4.4. Early vs. Delayed Adjuvant Radiotherapy
4.5. Malignant Progression
4.6. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| FPB | Fractionated Proton Beam Therapy |
| Hz | Hazard Ratio |
| MRI | Magnetic Resonance Imaging |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| RBE | Relative Biological Effectiveness |
| RT | Radiation Therapy |
| SFRT | Fractionated Stereotactic Conformal Radiotherapy |
| SRS | Stereotactic Radiosurgery |
| STR | Subtotal Resection |
References
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncol. 2021, 23, iii1–iii105. [Google Scholar] [CrossRef]
- Simpson, D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Mirimanoff, R.O.; Dosoretz, D.E.; Linggood, R.M.; Ojemann, R.G.; Martuza, R.L. Meningioma: Analysis of recurrence and progression following neurosurgical resection. J. Neurosurg. 1985, 62, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zülch, K.J. Roentgen sensitivity of cerebral tumours and so-called late irradiation necrosis of the brain. Acta. Radiol. Ther. Phys. Biol. 1969, 8, 92–110. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-Oncol. 2021, 23, 1821–1834. [Google Scholar] [CrossRef]
- Nabors, B.; Portnow, J.; Hattangadi-Gluth, J.; Horbinski, C. NCCN CNS tumor guidelines update for 2023. Neuro-Oncol. 2023, 25, 2114–2116. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, A.; Walier, M.; Régis, J.; Liščák, R.; Motti, E.; Lindquist, C.; Kemeny, A.; Kitz, K.; Lippitz, B.; Martínez Álvarez, R.; et al. Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurg 2012, 70, 32–39. [Google Scholar] [CrossRef]
- Rogers, C.L.; Pugh, S.L.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Barani, I.J.; Braunstein, S.; Bovi, J.A.; et al. Low-risk meningioma: Initial outcomes from NRG Oncology/RTOG 0539. Neuro-Oncol. 2023, 25, 137–145. [Google Scholar] [CrossRef]
- Bailey, P. The results of roentgen therapy on brain tumors. Am. J. Roentgenol. Radium Ther. 1925, 13, 48–53. [Google Scholar]
- Cushing, H.; Eisenhardt, L. Meningiomas, Their Classification, Regional Behaviour, Life History, and Surgical End Results. Bull Med. Libr. Assoc. 1938, 27, 185. [Google Scholar]
- McWhirter, R. Radiation treatment of cerebral tumours: Joint Discussion No. 3. Proc. R. Soc. Med. 1946, 39, 673–680. [Google Scholar]
- McWhirter, R. Tumors of the brain and spinal cord. In Practice in Radiotherapy; Windeyer, B.W., Carling, E.R., Smithers, D.W., Eds.; Butterworth & Co., Ltd.: London, UK, 1955. [Google Scholar]
- King, D.L.; Chang, C.H.; Pool, J.L. Radiotherapy in the Management of Meningiomas. Acta. Radiologica. Diagn. 1966, 5, 26–33. [Google Scholar] [CrossRef]
- Mathiesen, T.; Kihlström, L.; Karlsson, B.; Lindquist, C. Potential complications following radiotherapy for meningiomas. Surg. Neurol. 2003, 60, 193–198. [Google Scholar] [CrossRef]
- Bucy, P.C. Editorial: Removal of involved bone with meningiomas. Surg. Neurol. 1982, 17, 416. [Google Scholar] [CrossRef]
- Wara, W.M.; Sheline, G.E.; Newman, H.; Townsend, J.J.; Boldrey, E.B. Radiation therapy of meningiomas. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1975, 123, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, B.J.; Wara, W.M.; Wilson, C.B.; Larson, D.A. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J. Neurosurg. 1994, 80, 195–201. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, D.G.; Kim, J.W.; Han, J.H.; Chung, H.T.; Paek, S.H. Long-Term Outcomes After Gamma Knife Radiosurgery for Benign Meningioma: A Single Institution’s Experience with 424 Patients. Neurosurgery 2018, 83, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, B.; Celtikci, E.; Bulduk, E.B.; Borcek, A.O.; Kurt, G.; Kaymaz, M.; Aykol, S.; Emmez, H. Stereotactic Radiosurgery after Subtotal Resection of Critically-Located Grade I Meningioma: A Single-Center Experience and Review of Literature. Turk. Neurosurg. 2021, 31, 519–529. [Google Scholar] [CrossRef]
- Oya, S.; Ikawa, F.; Ichihara, N.; Wanibuchi, M.; Akiyama, Y.; Nakatomi, H.; Mikuni, N.; Narita, Y. Effect of adjuvant radiotherapy after subtotal resection for WHO grade I meningioma: A propensity score matching analysis of the Brain Tumor Registry of Japan. J. Neuro-Oncol. 2021, 153, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, A.L.; Glassman, G.E.; Dagan, R.; Rao, D.; Fiester, P.J.; Tavanaieour, D.; Morris, C.G.; Indelicato, D.J.; Mendenhall, W.M. Long-term outcomes of fractionated proton beam therapy for benign or radiographic intracranial meningioma. J. Neuro-Oncol. 2023, 161, 481–489. [Google Scholar] [CrossRef]
- Mesic, J.B.; Hanks, G.E.; Doggett, R.L. The value of radiation therapy as an adjuvant to surgery in intracranial meningiomas. Am. J. Clin. Oncol. 1986, 9, 337–340. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Starke, R.M.; Kano, H.; Barnett, G.H.; Mathieu, D.; Chiang, V.; Yu, J.B.; Hess, J.; McBride, H.L.; Honea, N.; et al. Gamma Knife radiosurgery for posterior fossa meningiomas: A multicenter study. J. Neurosurg. 2015, 122, 1479–1489. [Google Scholar] [CrossRef]
- Mathiesen, T.; Lindquist, C.; Kihlström, L.; Karlsson, B. Recurrence of cranial base meningiomas. Neurosurgery 1996, 39, 2–7. [Google Scholar] [CrossRef]
- Pettersson-Segerlind, J.; Orrego, A.; Lönn, S.; Mathiesen, T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurg. 2011, 76, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.; Grainger, A.; Walton, L.; Silcocks, P.; Radatz, M.; Kemeny, A. Risk of malignancy after gamma knife stereotactic radiosurgery. Neurosurgery 2007, 60, 60–65. [Google Scholar] [CrossRef]
- Kondziolka, D.; Patel, A.D.; Kano, H.; Flickinger, J.C.; Lunsford, L.D. Long-term Outcomes After Gamma Knife Radiosurgery for Meningiomas. Am. J. Clin. Oncol. 2016, 39, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Mathieu, D.; Lunsford, L.D.; Martin, J.J.; Madhok, R.; Niranjan, A.; Flickinger, J.C. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery 2008, 62, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Morris, C.G.; Amdur, R.J.; Foote, K.D.; Friedman, W.A. Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer 2003, 98, 1473–1482. [Google Scholar] [CrossRef]
- Tanzler, E.; Morris, C.G.; Kirwan, J.M.; Amdur, R.J.; Mendenhall, W.M. Outcomes of WHO Grade I Meningiomas Receiving Definitive or Postoperative Radiotherapy. Int. J. Radiat. Oncol.·Biol.·Phys. 2011, 79, 508–513. [Google Scholar] [CrossRef]
- Shibui, S. Brain Tumor Registry of Japan (2001–2004). Neurol Med. Chir. 2014, 54, 9–102. [Google Scholar]
- Mathiesen, T.; Pettersson-Segerlind, J.; Kihlström, L.; Ulfarsson, E. Meningiomas engaging major venous sinuses. World Neurosurg. 2014, 81, 116–124. [Google Scholar] [CrossRef]
- Bowden, G.; Faramand, A.; Mallella, A.; Wei, Z.; Patel, K.; Niranjan, A.; Lunsford, L.D. Does the Timing of Radiosurgery after Grade 1 Meningioma Resection Affect Long-Term Outcomes? Stereotact. Funct. Neurosurg. 2021, 99, 506–511. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Kadri, P.A.; Pravdenkova, S.; Sawyer, J.R.; Stangeby, C.; Husain, M. Malignant progression in meningioma: Documentation of a series and analysis of cytogenetic findings. J. Neurosurg. 2004, 101, 210–218. [Google Scholar] [CrossRef]
- McGovern, S.L.; Aldape, K.D.; Munsell, M.F.; Mahajan, A.; DeMonte, F.; Woo, S.Y. A comparison of World Health Organization tumor grades at recurrence in patients with non-skull base and skull base meningiomas. J. Neurosurg. 2010, 112, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, D.; Zhang, Z.; Li, Y.; Lin, Y.; Wang, G.; Zong, Y.; Liu, E. Gamma Knife radiosurgery for intracranial benign meningiomas: Follow-up outcome in 130 patients. Neurosurg. Focus 2019, 46, E7. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, J.R.; Husain, M.; Lukacs, J.L.; Stangeby, C.; Binz, R.L.; Al-Mefty, O. Telomeric fusion as a mechanism for the loss of 1p in meningioma. Cancer Genet. Cytogenet. 2003, 145, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.; Knisely, J.; Symons, M.; Ruggieri, R. Radioresistance of Brain Tumors. Cancers 2016, 8, 42. [Google Scholar] [CrossRef]
- Couldwell, W.T.; Cole, C.D.; Al-Mefty, O. Patterns of skull base meningioma progression after failed radiosurgery. J. Neurosurg. 2007, 106, 30–35. [Google Scholar] [CrossRef]
- Landry, A.P.; Wang, J.Z.; Liu, J.; Patil, V.; Gui, C.; Patel, Z.; Ajisebutu, A.; Ellenbogen, Y.; Wei, Q.; Singh, O.; et al. Development and validation of a molecular classifier of meningiomas. Neuro-Oncol. 2025, 27, 1258–1269. [Google Scholar] [CrossRef]
- Sahm, F.; Aldape, K.D.; Brastianos, P.K.; Brat, D.J.; Dahiya, S.; von Deimling, A.; Giannini, C.; Gilbert, M.R.; Louis, D.N.; Raleigh, D.R.; et al. cIMPACT-NOW update 8: Clarifications on molecular risk parameters and recommendations for WHO grading of meningiomas. Neuro-Oncol. 2025, 27, 319–330. [Google Scholar] [CrossRef]
| Data | Value |
|---|---|
| Timeframe | 1990 through 2023 |
| Number of patients | 432 |
| Number of meningiomas | 436 |
| In female patients, n. (%) | 319 (73%) |
| In male patients, n. (%) | 117 (27%) |
| Mean age (years ± SD) | 69 ± 13.9 |
| Race, n. (%) | |
| Caucasian | 363 (83%) |
| Black | 21 (5%) |
| Asian | 7 (2%) |
| Hispanic | 21 (5%) |
| Not available | 24 (5%) |
| Side, n. (%) | |
| Left | 198 (45%) |
| Right | 185 (43%) |
| Median/bilateral | 53 (12%) |
| Location, n. (%) | |
| Vault | 146 (33%) |
| Skull Base | |
| Anterior | 150 (34%) |
| Middle | 81 (19%) |
| Posterior | 57 (13%) |
| Intraventricular | 2 (1%) |
| Average maximum diameter (cm ± SD) | 3.4 ± 1.4 |
| Preoperative Karnofsky Performance Scale score | |
| ≤70 | 40 (9%) |
| >70 | 396 (91%) |
| Pretreatment neurological deficits | 296 (68%) |
| Pretreatment hydrocephalus | 6 (1%) |
| Intraoperative complications, n. (%) | |
| Vascular injury/hemorrhage/ischemia | 9 (2%) |
| Irradiated, n. (%) | 176 (40%) |
| MIB-11/Ki-67, n. (%) | |
| ≤5% | 163 (37%) |
| >5% | 64 (15%) |
| Not available | 209 (48%) |
| Genetic profile, n. (%) | |
| Benign/monosomy 22 | 78 (18%) |
| Malignant multiple mutations | 25 (6%) |
| Not available | 333 (76%) |
| Data | STR (n. 260) | STR + RT (n. 176) |
|---|---|---|
| Actual median PFS (months/years) | 59/4.9 | 47.5/3.9 |
| Actuarial PFS (%) | ||
| 3 years | 91% | 59% |
| 5 years | 85% | 43% |
| 10 years | 77% | 23% |
| 15 years | 70% | 16% |
| Actual median cause-specific OS (months/years) | 112/9.3 | 150/12.5 |
| Actuarial cause-specific OS (%) | ||
| 3 years | 98% | 98% |
| 5 years | 97.6% | 97% |
| 10 years | 97.6% | 93% |
| 15 years | 97.6% | 85% |
| 20 years | 96% | 76% |
| Mortality rate (%) | ||
| Overall | 4% | 26% |
| 36 months | 10% | 21% |
| 36–60 months | 6% | 18% |
| 60–120 months | 0% | 16% |
| 120–180 months | 0% | 45% |
| Clinical control n. (%) | 225 (87%) | 68 (37%) |
| Malignant progression n. (%) | 2 (1%) | 49 (28%) |
| to WHO II | 2 (1%) | 39 (22%) |
| to WHO III | / | 3 (2%) |
| to WHO II–III | / | 7 (4%) |
| New malignant mutations n. (%) | 5 (2%) | 19 (11%) |
| KPS at the last follow-up | ||
| ≤70 | 23 (9%) | 59 (34%) |
| >70 | 237 (21%) | 117 (66%) |
| Postoperative complications n. (%) | ||
| Vascular | 20 (8%) | 4 (2%) |
| Cranial nerve palsy | 26 (10%) | 18 (10%) |
| Abscess/wound infection | 2 (1%) | 3 (2%) |
| Hydrocephalus/CSF leak | 10 (4%) | 2 (1%) |
| Motor impairment | 4 (2%) | 3 (2%) |
| Seizure | 7 (3%) | 2 (1%) |
| RT complications n. (%) | ||
| Radiation necrosis | / | 67 (38%) |
| T2 signal hyperintensity | / | 118 (67%) |
| Pituitary dysfunction | / | 29 (17%) |
| Visual impairment | / | 53 (30%) |
| Hydrocephalus | / | 22 (13%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giotta Lucifero, A.; Almefty, R.; Al-Mefty, O. Impact of Irradiation on Post-Surgical Residuals of WHO Grade I Meningioma. J. Clin. Med. 2025, 14, 5829. https://doi.org/10.3390/jcm14165829
Giotta Lucifero A, Almefty R, Al-Mefty O. Impact of Irradiation on Post-Surgical Residuals of WHO Grade I Meningioma. Journal of Clinical Medicine. 2025; 14(16):5829. https://doi.org/10.3390/jcm14165829
Chicago/Turabian StyleGiotta Lucifero, Alice, Rami Almefty, and Ossama Al-Mefty. 2025. "Impact of Irradiation on Post-Surgical Residuals of WHO Grade I Meningioma" Journal of Clinical Medicine 14, no. 16: 5829. https://doi.org/10.3390/jcm14165829
APA StyleGiotta Lucifero, A., Almefty, R., & Al-Mefty, O. (2025). Impact of Irradiation on Post-Surgical Residuals of WHO Grade I Meningioma. Journal of Clinical Medicine, 14(16), 5829. https://doi.org/10.3390/jcm14165829







