Post-Coarctectomy Pseudoaneurysm with Recurrent Coarctation Treated with Open Surgery: A Comprehensive Literature Review and Case Report
Abstract
1. Introduction
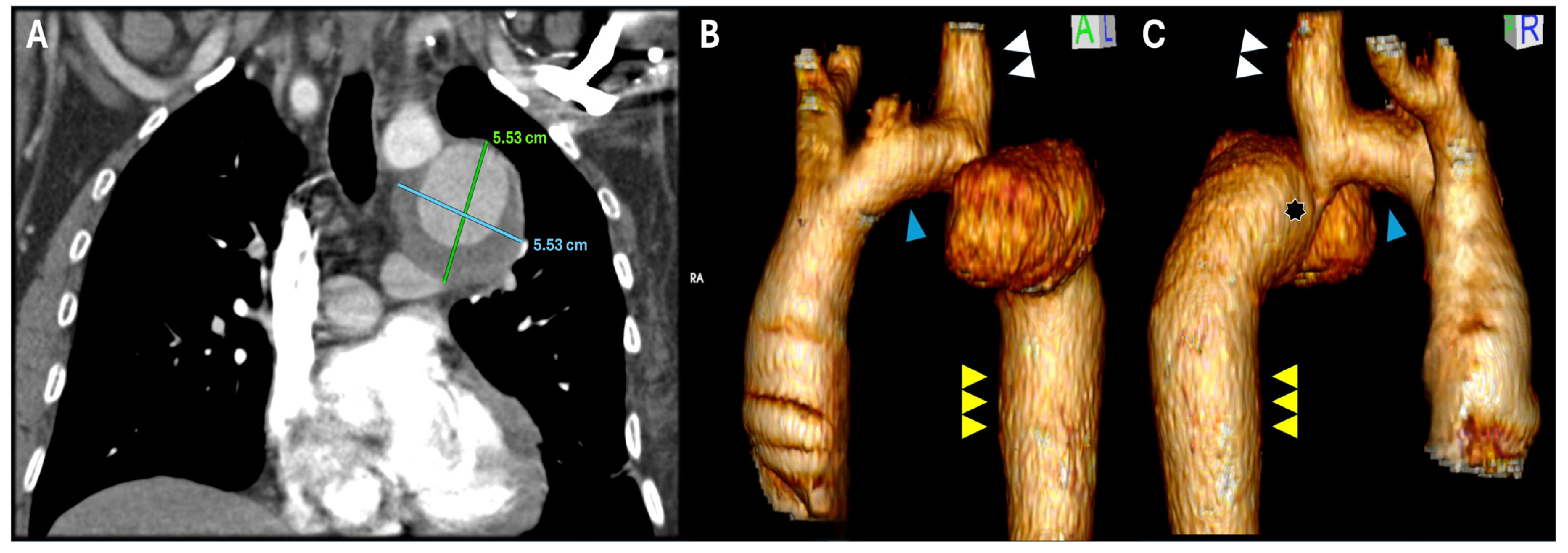
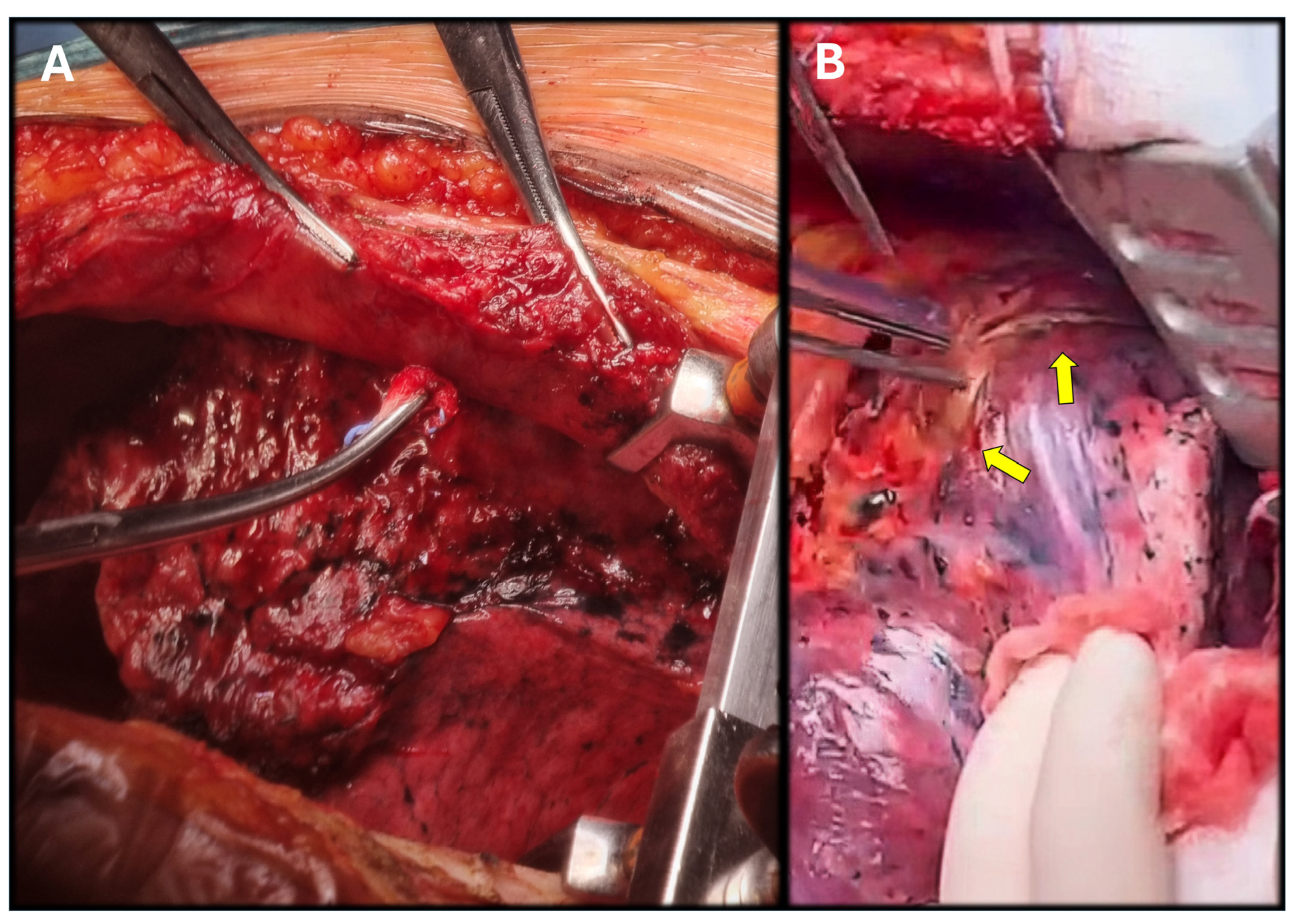
2. Case Report Description
3. Comprehensive Literature Review
3.1. Post-Aortic Coarctation Aneurysms (pCoAA)
3.2. Recoarctation
4. Discussion
5. Conclusions
6. Future Directions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CoA | Aortic coarctation |
| pCoAA | Post-aortic coarctation aneurysm |
| LVEF | Left ventricular ejection fraction |
| LSA | Left subclavian artery |
| OSR | Open surgical repair |
| BAV | Bicuspid aortic valve |
| SMEP | Somatosensory-evoked potentials |
| CSF | Cerebral–spinal fluid |
| CTA | Computerized tomography angiography |
| MRI | Magnetic resonance imaging |
| CPBP | Cardiopulmonary bypass |
| DHCA | Deep hypothermic circulatory arrest |
| LHBP | Left heart bypass |
| TEVAR | Thoracic endovascular aortic repair |
| CMD | Custom-made device |
References
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. Peer Review Committee Members. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Hafen, L.; Shutze, W.P.; Potluri, S.; Squiers, J.J.; DiMaio, J.M.; Brinkman, W.T. Heart team approach for comprehensive management of aortic coarctation in the adult. Ann. Cardiothorac. Surg. 2022, 11, 37–45. [Google Scholar] [CrossRef]
- Velayudhan, B.; Idhrees, A.M. Coarctation of aorta in adults: A narrative review of surgical and endovascular management. J. Vis. Surg. 2021, 7, 17. [Google Scholar] [CrossRef]
- von Kodolitsch, Y.; Aydin, A.M.; Bernhardt, A.M.; Habermann, C.; Treede, H.; Reichenspurner, H.; Meinertz, T.; Dodge-Khatami, A. Aortic aneurysms after correction of aortic coarctation: A systematic review. Vasa 2010, 39, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Melissano, G.; Canaud, L.; Pacini, D.; Bilman, V.; Erben, Y.; Oo, A.Y.; Riambau, V.; Pedro, L.M.; Oderich, G.S.; Estrera, A.L.; et al. International Rare Aortic Conditions (IRAC) Consortium. Surgical and endovascular treatment of late postcoarctation repair aortic aneurysms: Results from an international multicenter study. J. Vasc. Surg. 2022, 76, 1449–1457.e4. [Google Scholar] [CrossRef]
- Cramer, J.W.; Ginde, S.; Bartz, P.J.; Tweddell, J.S.; Litwin, S.B.; Earing, M.G. Aortic aneurysms remain a significant source of morbidity and mortality after use of Dacron(®) patch aortoplasty to repair coarctation of the aorta: Results from a single center. Pediatr. Cardiol. 2013, 34, 296–301. [Google Scholar] [CrossRef]
- Knyshov, G.V.; Sitar, L.L.; Glagola, M.D.; Atamanyuk, M.Y. Aortic aneurysms at the site of the repair of coarctation of the aorta: A review of 48 patients. Ann. Thorac. Surg. 1996, 61, 935–939. [Google Scholar] [CrossRef]
- Chiesa, R.; Melissano, G.; Civilini, E.; Bertoglio, L.; Rinaldi, E.; Marone, E.M.; Tshomba, Y. Video-atlas of open thoracoabdominal aortic aneurysm repair. Ann. Cardiothorac. Surg. 2012, 1, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, R.; Rinaldi, E.; Kahlberg, A.; Tinaglia, S.; Santoro, A.; Colacchio, G.; Melissano, G. Outcomes following Management of Complex Thoracoabdominal Aneurysm by an Open Approach. J. Clin. Med. 2023, 12, 3193. [Google Scholar] [CrossRef] [PubMed]
- Deniz, E.; Bobylev, D.; Krüger, H.; Salman, J.; Zubarevich, A.; Martens, A.; Kaufeld, T.; Schmack, B.; Weymann, A.; Ruhparwar, A.; et al. The Fate of the Aorta after Coarctation Repair: Open Surgical Replacement of Descending Aorta in a High-Volume Unit. J. Clin. Med. 2024, 13, 5345. [Google Scholar] [CrossRef]
- Raza, S.; Aggarwal, S.; Jenkins, P.; Kharabish, A.; Anwer, S.; Cullington, D.; Jones, J.; Dua, J.; Papaioannou, V.; Ashrafi, R.; et al. Coarctation of the Aorta: Diagnosis and Management. Diagnostics 2023, 13, 2189. [Google Scholar] [CrossRef]
- Salciccioli, K.B.; Zachariah, J.P. Coarctation of the Aorta: Modern Paradigms Across the Lifespan. Hypertension 2023, 80, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 1494–1563. [Google Scholar] [CrossRef]
- Duijnhouwer, A.; van den Hoven, A.; Merkx, R.; Schokking, M.; van Kimmenade, R.; Kempers, M.; Dijk, A.V.; de Boer, M.J.; Roos-Hesselink, J. Differences in Aortopathy in Patients with a Bicuspid Aortic Valve with or without Aortic Coarctation. J. Clin. Med. 2020, 9, 290. [Google Scholar] [CrossRef]
- Arya, B.; Maskatia, S.A. Coarctation of the aorta: Prenatal assessment, postnatal management and neonatal outcomes. Semin. Perinatol. 2022, 46, 151584. [Google Scholar] [CrossRef]
- Vasile, C.M.; Laforest, G.; Bulescu, C.; Jalal, Z.; Thambo, J.B.; Iriart, X. From Crafoord’s End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions. J. Clin. Med. 2023, 12, 7350. [Google Scholar] [CrossRef]
- Frankel, W.C.; Roselli, E.E. Strategies for Complex Reoperative Aortic Arch Reconstruction in Patients With Congenital Heart Disease. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2023, 26, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, B.K.; Jose, R.; Kader, N.P.; Varma, P.K. Coarctation of aorta aneurysm with aberrant right subclavian artery and single carotid artery: Surgical and perfusion strategies. J. Thorac. Cardiovasc. Surg. 2019, 157, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Mansourian, M.; Sabri, M.R.; Ghaderian, M.; Karimi, R.; Roustazadeh, R. Follow-up outcomes and effectiveness of stent implantation for aortic coarctation: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102513. [Google Scholar] [CrossRef]
- von Kodolitsch, Y.; Aydin, M.A.; Koschyk, D.H.; Loose, R.; Schalwat, I.; Karck, M.; Cremer, J.; Haverich, A.; Berger, J.; Meinertz, T.; et al. Predictors of aneurysmal formation after surgical correction of aortic coarctation. J. Am. Coll. Cardiol. 2002, 39, 617–624. [Google Scholar] [CrossRef]
- Tilea, I.; Hadadi, L.; Serban, R.C.; Tilea, B. Hybrid repair of a very late, post-aortic coarctation surgery thoracic aneurysm: A case report. J. Med. Case Rep. 2012, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, A.; Villemain, O.; Gaudin, R.; Méot, M.; Raisky, O.; Bonnet, D. Risk factors of mortality and recoarctation after coarctation repair in infancy. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 469–475. [Google Scholar] [CrossRef]
- Beckmann, E.; Jassar, A.S. Coarctation repair—Redo challenges in the adults: What to do? J. Vis. Surg. 2018, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Soetisna, T.W.; Pajala, F.B.; Wartono, D.A.; Hanafy, D.A.; Tjubandi, A. Surgical technique challenges in adult aortic coarctation patients: A case series. Int. J. Surg. Case Rep. 2025, 134, 111718. [Google Scholar] [CrossRef]
- Di Domenico, R.; Fargion, A.T.; Speziali, S.; Petroni, R.; Villani, F.; Esposito, D.; Pratesi, C. Hybrid Surgical Approach to a Giant Post-Coarctation Aortic Aneurysm. J. Endovasc. Ther. 2021, 28, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, I.N.; Simeonov, P. Endovascular treatment of postoperative aortic coarctation aneurysms-a single center experience. Front. Cardiovasc. Med. 2024, 11, 1441867. [Google Scholar] [CrossRef]
- Price, J.D.; LaPar, D.J. The Challenges of Redo Aortic Coarctation Repair in Adults. Curr. Cardiol. Rep. 2019, 21, 99. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ohuchi, Y.; Yata, S.; Adachi, A.; Endo, M.; Takasugi, S.; Fujii, S.; Hashimoto, M.; Kaminou, T.; Ogawa, T.; et al. Compressed Amplatzer Vascular Plug II Embolization of the Left Subclavian Artery for Thoracic Endovascular Aortic Repair is Efficient and Safety Method Comparable to Conventional Coil Embolization. Yonago Acta Med. 2019, 62, 24–29. [Google Scholar] [CrossRef]
- Wahlgren, C.M.; Aylwin, C.; Davenport, R.A.; Davidovic, L.B.; DuBose, J.J.; Gaarder, C.; Heim, C.; Jongkind, V.; Jørgensen, J.; Kakkos, S.K.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2025 Clinical Practice Guidelines on the Management of Vascular Trauma. Eur. J. Vasc. Endovasc. Surg. 2025, 69, 179–237. [Google Scholar] [CrossRef]
- Contrella, B.N.; Sabri, S.S.; Tracci, M.C.; Stone, J.R.; Kern, J.A.; Upchurch, G.R.; Matsumoto, A.H.; Angle, J.F. Outcomes of Coverage of the Left Subclavian Artery during Endovascular Repair of the Thoracic Aorta. J. Vasc. Interv. Radiol. 2015, 26, 1609–1614. [Google Scholar] [CrossRef]
- Konstantinou, N.; Debus, E.S.; Vermeulen, C.F.W.; Wipper, S.; Diener, H.; Larena-Avellaneda, A.; Kölbel, T.; Tsilimparis, N. Cervical Debranching in the Endovascular Era: A Single Centre Experience. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 34–40. [Google Scholar] [CrossRef]
- Bellamkonda, K.S.; Yousef, S.; Nassiri, N.; Dardik, A.; Guzman, R.J.; Geirsson, A.; Chaar, C.I.O. Trends and outcomes of thoracic endovascular aortic repair with open concomitant cervical debranching. J. Vasc. Surg. 2021, 73, 1205–1212.e3. [Google Scholar] [CrossRef]
- Aru, R.G.; Tyagi, S.C.; Minion, D.J.; Orr, N.T.; Bounds, M.C. Carotid-Carotid Transposition for Zone 1 Thoracic Endovascular Aortic Repair. Ann. Vasc. Surg. 2021, 76, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Squiers, J.J.; DiMaio, J.M.; Schaffer, J.M.; Baxter, R.D.; Gable, C.E.; Shinn, K.V.; Harrington, K.; Moore, D.O.; Shutze, W.P.; Brinkman, W.T.; et al. Surgical debranching versus branched endografting in zone 2 thoracic endovascular aortic repair. J. Vasc. Surg. 2022, 75, 1829–1836.e3. [Google Scholar] [CrossRef] [PubMed]
- Makaloski, V.; Tsilimparis, N.; Rohlffs, F.; Heidemann, F.; Debus, E.S.; Kölbel, T. Endovascular total arch replacement techniques and early results. Ann. Cardiothorac. Surg. 2018, 7, 380–388. [Google Scholar] [CrossRef]
- Katsargyris, A.; Uthayakumar, V.; Marques de Marino, P.; Botos, B.; Verhoeven, E.L. Aneurysm Rupture and Mortality During the Waiting Time for a Customised Fenestrated/Branched Stent Graft in Complex Endovascular Aortic Repair. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Kuratani, T.; Shirakawa, Y.; Shimamura, K.; Kin, K.; Sakamoto, T.; Shijo, T.; Watanabe, Y.; Masada, K.; Sakaniwa, R.; et al. Effectiveness of Proximal Landing Zones 0, 1, and 2 Hybrid Thoracic Endovascular Aortic Repair: A Single Centre 12 Year Experience. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 410–420. [Google Scholar] [CrossRef]
- Ozeki, T.; Tokuda, Y.; Narita, Y.; Mutsuga, M. Preemptive Frozen Elephant Trunk Technique for Hemostatic Use inTotal Arch Replacement. Ann. Thorac. Surg. Short Rep. 2025, 3, 398–401. [Google Scholar] [CrossRef]
- Baar, W.; Semmelmann, A.; Anselm, F.; Loop, T.; Heinrich, S.; For The Working Group Of The German Thorax Registry. Risk Factors for Postoperative Pulmonary Complications in Patients Undergoing Thoracotomy for Indications Other than Primary Lung Cancer Resection: A Multicenter Retrospective Cohort Study from the German Thorax Registry. J. Clin. Med. 2025, 14, 1565. [Google Scholar] [CrossRef]
- Etz, C.D.; Zoli, S.; Kari, F.A.; Mueller, C.S.; Bodian, C.A.; Di Luozzo, G.; Plestis, K.A.; Griepp, R.B. Redo lateral thoracotomy for reoperative descending and thoracoabdominal aortic repair: A consecutive series of 60 patients. Ann. Thorac. Surg. 2009, 88, 758–766; discussion 767. [Google Scholar] [CrossRef]
- Afifi, R.O.; Sandhu, H.K.; Trott, A.E.; Nguyen, T.C.; Miller, C.C.; Estrera, A.L.; Safi, H.J. Redo Thoracoabdominal Aortic Aneurysm Repair: A Single-Center Experience Over 25 Years. Ann. Thorac. Surg. 2017, 103, 1421–1428. [Google Scholar] [CrossRef]
- Kawaharada, N.; Morishita, K.; Fukada, J.; Hachiro, Y.; Takahashi, K.; Abe, T. Thoracoabdominal aortic aneurysm repair through redo left-sided thoracotomy. Ann. Thorac. Surg. 2004, 77, 1304–1308. [Google Scholar] [CrossRef]
- Chiesa, R.; Melissano, G.; Civilini, E.; Bertoglio, L.; Setacci, F.; Baccellieri, D. Giant aneurysm 25 years after patch aortoplasty for aortic coarctation. Tex. Heart Inst. J. 2008, 35, 220–221. [Google Scholar]
- Carroll, A.M.; King, R.W.; Ghincea, C.V.; Aftab, M.; Reece, T.B. Spinal cord protection for thoracoabdominal aortic aneurysm repair: From bench to bedside. Ann. Cardiothorac. Surg. 2023, 12, 438–449. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; An, Q. Pseudoaneurysm of the descending aorta two decades after aortic coarctation repair: A case report. J. Cardiothorac. Surg. 2025, 20, 4. [Google Scholar] [CrossRef]
- Sweet, M.P. Anatomic features of the distal aortic arch that influence endovascular aneurysm repair. J. Vasc. Surg. 2016, 64, 891–895. [Google Scholar] [CrossRef]
- Chellasamy, R.T.; Krishnaswami, M. Reinterventions after TEVAR. Indian J. Thorac. Cardiovasc. Surg. 2023, 39 (Suppl. 2), 325–332. [Google Scholar] [CrossRef] [PubMed]
- Smaill, B.H.; McGiffin, D.C.; Legrice, I.J.; Young, A.A.; Hunter, P.J.; Galbraith, A.J. The effect of synthetic patch repair of coarctation on regional deformation of the aortic wall. J. Thorac. Cardiovasc. Surg. 2000, 120, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Preventza, O.; Livesay, J.J.; Cooley, D.A.; Krajcer, Z.; Cheong, B.Y.; Coselli, J.S. Coarctation-associated aneurysms: A localized disease or diffuse aortopathy. Ann. Thorac. Surg. 2013, 95, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.Q.; Barros, A.; Leite-Moreira, A.; Miranda, J.O. Risk Factors for Recoarctation and Mortality in Infants Submitted to Aortic Coarctation Repair: A Systematic Review. Pediatr. Cardiol. 2020, 41, 561–575. [Google Scholar] [CrossRef]
- Meidell Blylod, V.; Rinnström, D.; Pennlert, J.; Ostenfeld, E.; Dellborg, M.; Sörensson, P.; Christersson, P.; Thilén, U.; Johansson, B. Interventions in Adults With Repaired Coarctation of the Aorta. J. Am. Heart Assoc. 2022, 11, e023954. [Google Scholar] [CrossRef]
- Andrade, L.; Hoskoppal, A.; Hunt Martin, M.; Whitehead, K.; Ou, Z.; Kuang, J.; Cox, D. Intracranial aneurysm and coarctation of the aorta: Prevalence in the current era. Cardiol. Young 2021, 31, 229–232. [Google Scholar] [CrossRef]
- Lim, M.S.; Cordina, R.; Kotchetkova, I.; Celermajer, D.S. Late complication rates after aortic coarctation repair in patients with or without a bicuspid aortic valve. Heart 2022, 108, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Weismann, C.G.; Grell, B.S.; Odermarsky, M.; Mellander, M.; Liuba, P. Echocardiographic Predictors of Recoarctation After Surgical Repair: A Swedish National Study. Ann. Thorac. Surg. 2021, 111, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Taksøe-Vester, C.A.; Mikolaj, K.; Petersen, O.B.B.; Vejlstrup, N.G.; Christensen, A.N.; Feragen, A.; Nielsen, M.; Svendsen, M.B.S.; Tolsgaard, M.G. Role of artificial-intelligence-assisted automated cardiac biometrics in prenatal screening for coarctation of aorta. Ultrasound Obstet Gynecol. 2024, 64, 36–43. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutolo, S.A.; Soto, D.; Loschi, D.; Santoro, A.; Moldovan, H.; Broasca, M.; Melissano, G. Post-Coarctectomy Pseudoaneurysm with Recurrent Coarctation Treated with Open Surgery: A Comprehensive Literature Review and Case Report. J. Clin. Med. 2025, 14, 5800. https://doi.org/10.3390/jcm14165800
Cutolo SA, Soto D, Loschi D, Santoro A, Moldovan H, Broasca M, Melissano G. Post-Coarctectomy Pseudoaneurysm with Recurrent Coarctation Treated with Open Surgery: A Comprehensive Literature Review and Case Report. Journal of Clinical Medicine. 2025; 14(16):5800. https://doi.org/10.3390/jcm14165800
Chicago/Turabian StyleCutolo, Serena Arianna, Diego Soto, Diletta Loschi, Annarita Santoro, Horatius Moldovan, Marian Broasca, and Germano Melissano. 2025. "Post-Coarctectomy Pseudoaneurysm with Recurrent Coarctation Treated with Open Surgery: A Comprehensive Literature Review and Case Report" Journal of Clinical Medicine 14, no. 16: 5800. https://doi.org/10.3390/jcm14165800
APA StyleCutolo, S. A., Soto, D., Loschi, D., Santoro, A., Moldovan, H., Broasca, M., & Melissano, G. (2025). Post-Coarctectomy Pseudoaneurysm with Recurrent Coarctation Treated with Open Surgery: A Comprehensive Literature Review and Case Report. Journal of Clinical Medicine, 14(16), 5800. https://doi.org/10.3390/jcm14165800






