Total Arch Replacement with Ascyrus Medical Dissection Stent Versus Frozen Elephant Trunk in Acute Type A Aortic Dissection: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Critical Appraisal and Outcomes of Interest
2.6. Statistical Analysis
3. Results
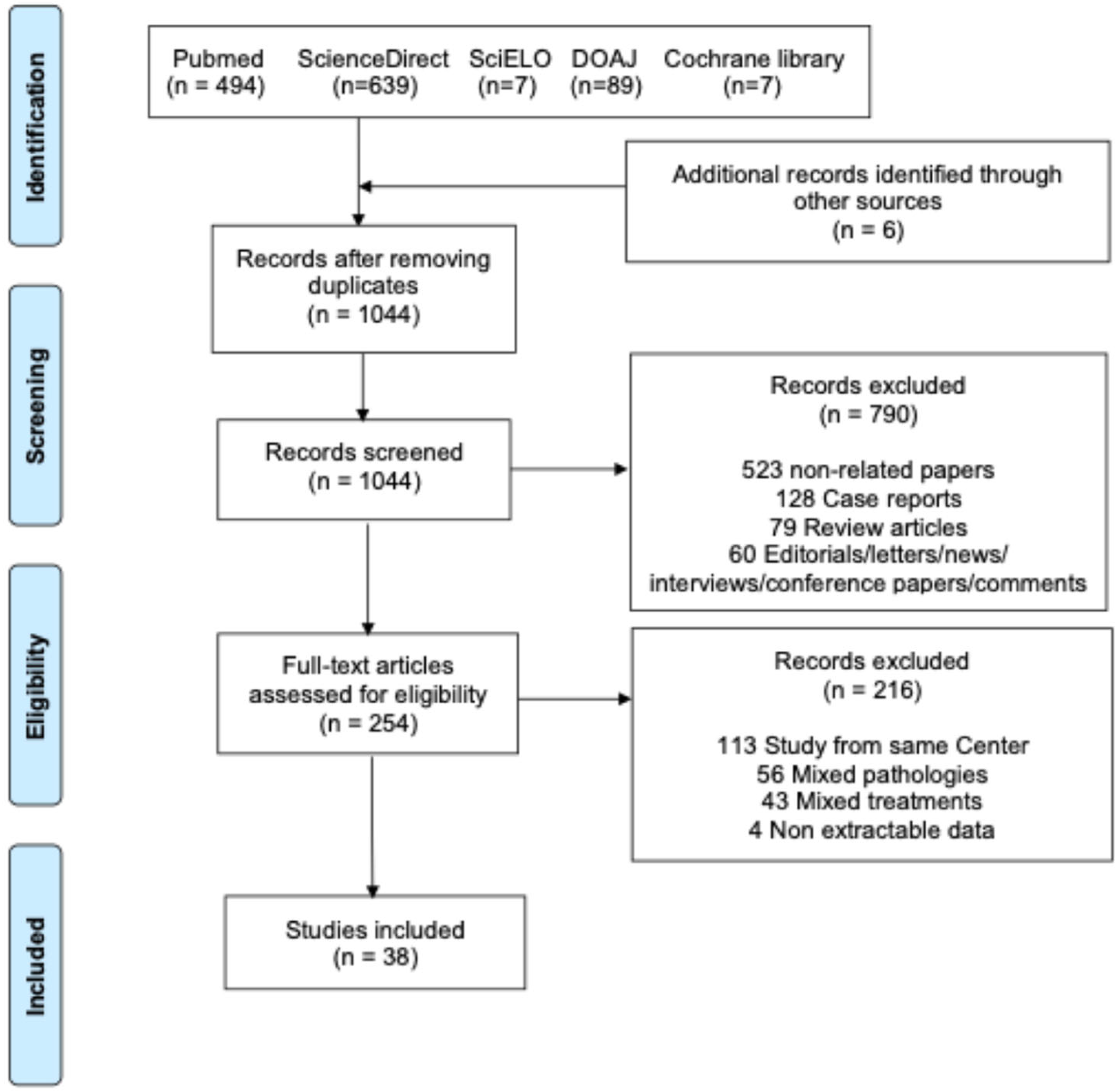
3.1. Study Selection and Characteristics
3.2. Meta-Analysis of the Outcomes
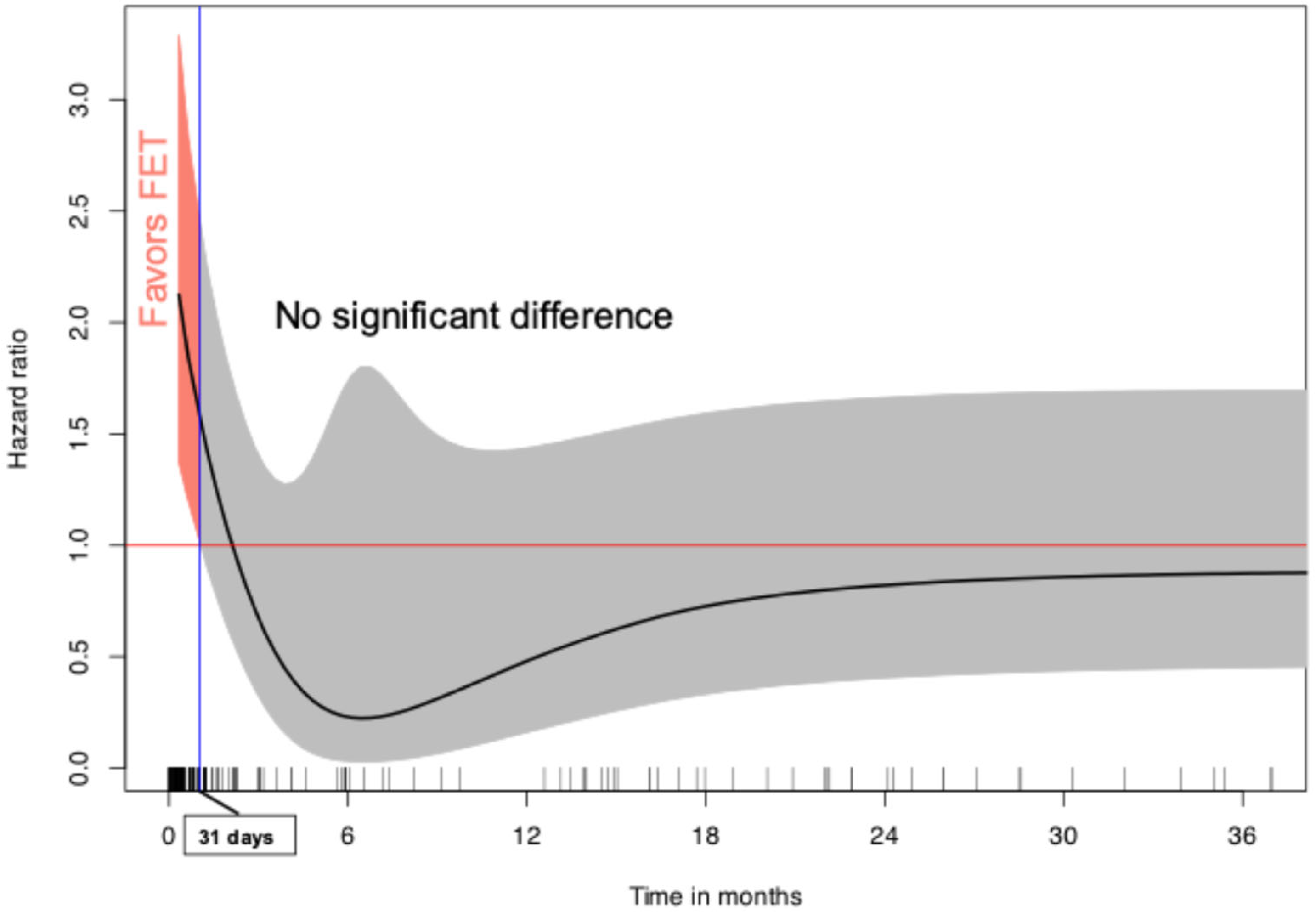
3.3. Individual Patient Data Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMDS | Ascyrus Medical Dissection Stent |
| ATAAD | Acute Type A Aortic Dissection |
| CI | Confidence Interval |
| DANE | Distal Anastomotic New Entry |
| FET | Frozen Elephant Trunk |
| HR | Hazard Ratio |
| IPD | Individual Patient Data |
| IR | Incidence Rate |
| OR | Odds Ratio |
| PEM | Pooled Estimated Mean |
| PER | Pooled Estimated Rate |
| TAR | Total Arch Replacement |
References
- Zhu, Y.; Lingala, B.; Baiocchi, M.; Tao, J.J.; Toro Arana, V.; Khoo, J.W.; Williams, K.M.; Traboulsi, A.A.-R.; Hammond, H.C.; Lee, A.M.; et al. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J. Am. Coll. Cardiol. 2020, 76, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Borst, H.G.; Walterbusch, G.; Schaps, D. Extensive Aortic Replacement Using “Elephant Trunk” Prosthesis. Thorac. Cardiovasc. Surg. 1983, 31, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, R.; Leone, A.; Di Marco, L.; Pacini, D. When and How to Replace the Aortic Arch for Type A Dissection. Ann. Cardiothorac. Surg. 2016, 5, 383–388. [Google Scholar] [CrossRef]
- Di Bartolomeo, R.; Pantaleo, A.; Berretta, P.; Murana, G.; Castrovinci, S.; Cefarelli, M.; Folesani, G.; Di Eusanio, M. Frozen Elephant Trunk Surgery in Acute Aortic Dissection. J. Thorac. Cardiovasc. Surg. 2015, 149, S105–S109. [Google Scholar] [CrossRef]
- Hussain, M.; Jubouri, Y.F.; Hammad, A.; Abubacker, I.; Franchin, M.; Mauri, F.; Piffaretti, G.; Mohammed, I.; Jubouri, M.; Bashir, M. The Frozen Elephant Trunk: An Overview of Hybrid Prostheses. Expert Rev. Med. Devices 2025, 22, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Mehdiani, A.; Sugimura, Y.; Wollgarten, L.; Immohr, M.B.; Bauer, S.; Schelzig, H.; Wagenhäuser, M.U.; Antoch, G.; Lichtenberg, A.; Akhyari, P. Early Results of a Novel Hybrid Prosthesis for Treatment of Acute Aortic Dissection Type A With Distal Anastomosis Line Beyond Aortic Arch Zone Zero. Front. Cardiovasc. Med. 2022, 9, 892516. [Google Scholar] [CrossRef]
- Montagner, M.; Heck, R.; Kofler, M.; Buz, S.; Starck, C.; Sündermann, S.; Kurz, S.; Falk, V. Kempfert Germany Dzhk German Centre For Cardiovascular Research Partner Site Berlin Germany, J. New Hybrid Prosthesis for Acute Type A Aortic Dissection. Surg. Technol. Int. 2020, 36, 95–97. [Google Scholar]
- Bozso, S.J.; Nagendran, J.; MacArthur, R.G.G.; Chu, M.W.A.; Kiaii, B.; El-Hamamsy, I.; Cartier, R.; Shahriari, A.; Moon, M.C. Dissected Aorta Repair Through Stent Implantation Trial: Canadian Results. J. Thorac. Cardiovasc. Surg. 2019, 157, 1763–1771. [Google Scholar] [CrossRef]
- Al-Tawil, M.; Jubouri, M.; Tan, S.Z.; Bailey, D.M.; Williams, I.M.; Mariscalco, G.; Piffaretti, G.; Chen, E.P.; Velayudhan, B.; Mohammed, I.; et al. Thoraflex Hybrid vs. AMDS: To Replace the Arch or to Stent It in Type A Aortic Dissection? Asian Cardiovasc. Thorac. Ann. 2023, 31, 596–603. [Google Scholar] [CrossRef]
- Haby, M.M.; Barreto, J.O.M.; Kim, J.Y.H.; Peiris, S.; Mansilla, C.; Torres, M.; Guerrero-Magaña, D.E.; Reveiz, L. What Are the Best Methods for Rapid Reviews of the Research Evidence? A Systematic Review of Reviews and Primary Studies. Res. Synth. Methods 2024, 15, 2–20. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct Individual Patient Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Immohr, M.B.; Mehdiani, A.; Bauer, S.J.; Ise, H.; Sugimura, Y.; Lichtenberg, A.; Akhyari, P. Combining Aortic Arch Dissection Stent Implantation and Root Surgery for Aortic Dissection Type A. J. Cardiothorac. Surg. 2023, 18, 72. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, Y.; Yang, Z.; Ge, Y.; Li, L.; Wang, H. Total Arch Replacement via Single Upper-Hemisternotomy Approach in Patients With Type A Dissection. Ann. Thorac. Surg. 2020, 109, 1394–1399. [Google Scholar] [CrossRef]
- Kong, X.; Ruan, P.; Yu, J.; Jiang, H.; Chu, T.; Ge, J. Innominate Artery Direct Cannulation Provides Brain Protection during Total Arch Replacement for Acute Type A Aortic Dissection. J. Cardiothorac. Surg. 2022, 17, 165. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Wang, B.; Yang, Z.; Xia, L.; Wang, H. Efficacy of Pump-Controlled Selective Antegrade Cerebral Perfusion in Total Arch Replacement: A Propensity-Matched Analysis. Front. Surg. 2022, 9, 918461. [Google Scholar] [CrossRef]
- Luehr, M.; Gaisendrees, C.; Yilmaz, A.K.; Winderl, L.; Schlachtenberger, G.; Van Linden, A.; Wahlers, T.; Walther, T.; Holubec, T. Treatment of Acute Type A Aortic Dissection with the Ascyrus Medical Dissection Stent in a Consecutive Series of 57 Cases. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2023, 63, ezac581. [Google Scholar] [CrossRef]
- Ma, M.; Feng, X.; Wang, J.; Dong, Y.; Chen, T.; Liu, L.; Wei, X. Acute Type I Aortic Dissection: A Propensity-Matched Comparison of Elephant Trunk and Arch Debranching Repairs. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 183–189. [Google Scholar] [CrossRef]
- Mariscalco, G.; Bilal, H.; Catarino, P.; Hadjinikolaou, L.; Kuduvalli, M.; Field, M.; Mascaro, J.; Oo, A.Y.; Quarto, C.; Kuo, J.; et al. Reflection From UK Aortic Group: Frozen Elephant Trunk Technique as Optimal Solution in Type A Acute Aortic Dissection. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Pitts, L.; Montagner, M.; Kofler, M.; Seeber, F.; Heck, R.; Sündermann, S.; Buz, S.; Starck, C.; Falk, V.; Kempfert, J. Classic Hemiarch versus Hemiarch and Hybrid Noncovered Open Stenting for Acute DeBakey Type I Dissection-a Propensity Score-Matched Analysis. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2025, 67, ezaf055. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Tan, L.; Tang, H.; Zhou, X.; Xiao, J.; Xie, D.; Li, J.; Chen, Y. Total Arch Replacement With Frozen Elephant Trunk Using a NEW “Brain-Heart-First” Strategy for Acute DeBakey Type I Aortic Dissection Can Be Performed Under Mild Hypothermia (≥30 °C) With Satisfactory Outcomes. Front. Cardiovasc. Med. 2022, 9, 806822. [Google Scholar] [CrossRef] [PubMed]
- Shi, E.; Gu, T.; Yu, Y.; Yu, L.; Wang, C.; Fang, Q.; Zhang, Y. Early and Midterm Outcomes of Hemiarch Replacement Combined with Stented Elephant Trunk in the Management of Acute DeBakey Type I Aortic Dissection: Comparison with Total Arch Replacement. J. Thorac. Cardiovasc. Surg. 2014, 148, 2125–2131. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Z. Acute Aortic Dissection Surgery: Hybrid Debranching Versus Total Arch Replacement. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1487–1493. [Google Scholar] [CrossRef]
- Shrestha, M.; Beckmann, E.; Krueger, H.; Fleissner, F.; Kaufeld, T.; Koigeldiyev, N.; Umminger, J.; Ius, F.; Haverich, A.; Martens, A. The Elephant Trunk Is Freezing: The Hannover Experience. J. Thorac. Cardiovasc. Surg. 2015, 149, 1286–1293. [Google Scholar] [CrossRef]
- Szeto, W.Y.; Fukuhara, S.; Fleischman, F.; Sultan, I.; Brinkman, W.; Arnaoutakis, G.; Takayama, H.; Eudailey, K.; Brinster, D.; Jassar, A.; et al. A Novel Hybrid Prosthesis for Open Repair of Acute DeBakey Type I Dissection with Malperfusion: Early Results from the PERSEVERE Trial. J. Thorac. Cardiovasc. Surg. 2025, 170, 114–123.e3. [Google Scholar] [CrossRef]
- Takagi, S.; Goto, Y.; Yanagisawa, J.; Ogihara, Y.; Okawa, Y. Strategy for Acute DeBakey Type I Aortic Dissection Considering Midterm Results: A Retrospective Cohort Study Comparing Ascending Aortic Replacement and Total Arch Replacement with Frozen Elephant Trunk Technique. J. Cardiothorac. Surg. 2024, 19, 15. [Google Scholar] [CrossRef]
- Tong, G.; Sun, Z.; Wu, J.; Zhao, S.; Chen, Z.; Zhuang, D.; Liu, Y.; Yang, Y.; Liang, Z.; Fan, R.; et al. Aortic Balloon Occlusion Technique Does Not Improve Peri-Operative Outcomes for Acute Type A Acute Aortic Dissection Patients With Lower Body Malperfusion. Front. Cardiovasc. Med. 2022, 9, 835896. [Google Scholar] [CrossRef]
- Wei, J.; Hu, Z.; Wang, W.; Ding, R.; Chen, Z.; Yuan, X.; Xu, F. Posterior False Lumen and Paraplegia After FET Procedure in Acute Type A Aortic Dissection. Ann. Thorac. Surg. 2024, 117, 1136–1143. [Google Scholar] [CrossRef]
- King, W.R.; Carroll, A.M.; Higa, K.C.; Cleveland, J.C.; Rove, J.Y.; Aftab, M.; Reece, B.T. Frozen Elephant Trunk for Acute Type A Dissection: Is Risk from Procedure or Patient Characteristics? Aorta 2023, 11, 112–115. [Google Scholar] [CrossRef]
- Wisniewski, K.; Motekallemi, A.; Dell’Aquila, A.M.; Oberhuber, A.; Schaefers, J.F.; Ibrahim, A.; Martens, S.; Rukosujew, A. Single-Center Experience With the ThoraflexTM Hybrid Prosthesis: Indications, Implantation Technique and Results. Front. Cardiovasc. Med. 2022, 9, 924838. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, Y.; Wang, C.; Cheng, Y.; Rong, R. Intraoperative Platelet Transfusion Is Associated with Increased Postoperative Sternal Wound Infections among Type A Aortic Dissection Patients after Total Arch Replacement. Transfus. Med. 2014, 24, 400–405. [Google Scholar] [CrossRef]
- Xiang, J.; He, L.; Pen, T.; Li, D.; Wei, S. Outcomes of Two-Stage Type II Hybrid Aortic Arch Repair in Elderly Patients with Acute Type A Aortic Dissection. Sci. Rep. 2024, 14, 1522. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, W.; Zhu, D.; Guo, Y.; Zhang, E. Treatment Strategies for Left Subclavian Artery during Total Arch Replacement Combined with Stented Elephant Trunk Implantation. J. Thorac. Cardiovasc. Surg. 2014, 147, 639–643. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Yao, Y.; Evidence in Cardiovascular Anesthesia (EICA) Group. Postoperative Pulmonary Complications in Patients Undergoing Aortic Surgery: A Single-Center Retrospective Study. Medicine 2023, 102, e34668. [Google Scholar] [CrossRef]
- Yang, C.; Hou, P.; Wang, D.; Wang, Z.; Duan, W.; Liu, J.; Yu, S.; Fu, F.; Jin, Z. Serum Myoglobin Is Associated With Postoperative Acute Kidney Injury in Stanford Type A Aortic Dissection. Front. Med. 2022, 9, 821418. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, X.; Lu, F.; Song, Z.; Wang, J.; Han, L.; Xu, Z. Acute Type A Dissection without Intimal Tear in Arch: Proximal or Extensive Repair? J. Thorac. Cardiovasc. Surg. 2014, 147, 1251–1255. [Google Scholar] [CrossRef]
- Azuma, S.; Shimada, R.; Motohashi, Y.; Yoshii, Y. Postoperative Results of the in Situ Fenestrated Open Stent Technique for Acute Aortic Dissection Type A. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 331–338. [Google Scholar] [CrossRef]
- Bozso, S.J.; Nagendran, J.; Chu, M.W.A.; Kiaii, B.; El-Hamamsy, I.; Ouzounian, M.; Forcillo, J.; Kempfert, J.; Starck, C.; Moon, M.C. Three-Year Outcomes of the Dissected Aorta Repair Through Stent Implantation Trial. J. Thorac. Cardiovasc. Surg. 2022, 167, 1661–1669. [Google Scholar] [CrossRef]
- Chabry, Y.; Porterie, J.; Gautier, C.-H.; Nader, J.; Chaufour, X.; Alsac, J.M.; Reix, T.; Marcheix, B.; Koskas, F.; Ruggieri, V.G.; et al. The Frozen Elephant Trunk Technique in an Emergency: THORAFLEX French National Registry Offers New Insights. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 59, 458–466. [Google Scholar] [CrossRef]
- Chen, X.; Huang, F.; Xu, M.; Wang, L.; Jiang, Y.; Xiao, L.; Chen, X.; Qiu, Z. The Stented Elephant Trunk Procedure Combined Total Arch Replacement for Debakey I Aortic Dissection: Operative Result and Follow-Up. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 594–598. [Google Scholar] [CrossRef]
- Chivasso, P.; Mastrogiovanni, G.; Bruno, V.D.; Miele, M.; Colombino, M.; Triggiani, D.; Cafarelli, F.; Leone, R.; Rosapepe, F.; De Martino, M.; et al. Systematic Total Arch Replacement with Thoraflex Hybrid Graft in Acute Type A Aortic Dissection: A Single Centre Experience. Front. Cardiovasc. Med. 2022, 9, 997961. [Google Scholar] [CrossRef] [PubMed]
- Cuko, B.; Pernot, M.; Busuttil, O.; Baudo, M.; Rosati, F.; Taymoor, S.; Modine, T.; Labrousse, L. Frozen Elephant Trunk Technique for Aortic Arch Surgery: The Bordeaux University Hospital Experience with Thoraflex Hybrid Prosthesis. J. Cardiovasc. Surg. 2023, 64, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Qiu, J.; Zhao, R.; Cao, F.; Qiu, J.; Wang, D.; Fan, S.; Xie, E.; Song, J.; Yu, C. A Novel Sutureless Integrated Stented (SIS) Graft Prosthesis for Type A Aortic Dissection: A Pilot Study for a Prospective, Multicenter Clinical Trial. Front. Cardiovasc. Med. 2021, 8, 806104. [Google Scholar] [CrossRef]
- Dohle, D.-S.; Mattern, L.; Pfeiffer, P.; Probst, C.; Ghazy, A.; Treede, H. Island Remodelling in Acute and Chronic Aortic Dissection Treated with Frozen Elephant Trunk. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2023, 63, ezad061. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, H.; Kim, J.B.; Gu, J.; Li, M.; Li, G.; Du, J.; Gu, W.; Shao, Y.; Ni, B. False Lumen-Dependent Segmental Arteries Are Associated with Spinal Cord Injury in Frozen Elephant Trunk Procedure for Acute Type I Aortic Dissection. JTCVS Open 2023, 15, 16–24. [Google Scholar] [CrossRef]
- Gao, J.; Yan, J.; Duan, Y.; Yu, J.; Li, W.; Luo, Z.; Yu, W.; Xie, D.; Liu, Z.; Xiong, J. Aortic Arch Branch-Prioritized Reconstruction for Type A Aortic Dissection Surgery. Front. Cardiovasc. Med. 2023, 10, 1321700. [Google Scholar] [CrossRef]
- Hoffman, A.; Damberg, A.L.M.; Schälte, G.; Mahnken, A.H.; Raweh, A.; Autschbach, R. Thoracic Stent Graft Sizing for Frozen Elephant Trunk Repair in Acute Type A Dissection. J. Thorac. Cardiovasc. Surg. 2013, 145, 964–969.e1. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Z.; Ren, Z.; Hu, R.; Wu, H. Simplified Total Aortic Arch Replacement with an in Situ Stent Graft Fenestration Technique for Acute Type A Aortic Dissection. J. Vasc. Surg. 2017, 66, 711–717. [Google Scholar] [CrossRef]
- Huang, F.; Li, X.; Zhang, Z.; Li, C.; Ren, F. Comparison of Two Surgical Approaches for Acute Type A Aortic Dissection: Hybrid Debranching versus Total Arch Replacement. J. Cardiothorac. Surg. 2022, 17, 166. [Google Scholar] [CrossRef]
- Ok, Y.J.; Kang, S.R.; Kim, H.J.; Kim, J.B.; Choo, S.J. Comparative Outcomes of Total Arch versus Hemiarch Repair in Acute DeBakey Type I Aortic Dissection: The Impact of 21 Years of Experience. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 60, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, I.; Piani, D.; Lechiancole, A.; Sponga, S.; Di Nora, C.; Londero, F.; Muser, D.; Onorati, F.; Bortolotti, U.; Livi, U. Hemiarch Versus Arch Replacement in Acute Type A Aortic Dissection: Is the Occam’s Razor Principle Applicable? J. Clin. Med. 2021, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.W.A.; Losenno, K.L.; Dubois, L.A.; Jones, P.M.; Ouzounian, M.; Whitlock, R.; Dagenais, F.; Boodhwani, M.; Bhatnagar, G.; Poostizadeh, A.; et al. Early Clinical Outcomes of Hybrid Arch Frozen Elephant Trunk Repair With the Thoraflex Hybrid Graft. Ann. Thorac. Surg. 2019, 107, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Z.C.P.; Jubouri, M.; Mohammed, I.; Bashir, M. What Is the Long-Term Clinical Efficacy of the ThoraflexTM Hybrid Prosthesis for Aortic Arch Repair? Front. Cardiovasc. Med. 2022, 9, 842165. [Google Scholar] [CrossRef]
- Pitts, L.; Moon, M.C.; Luehr, M.; Kofler, M.; Montagner, M.; Sündermann, S.; Buz, S.; Starck, C.; Falk, V.; Kempfert, J. The Ascyrus Medical Dissection Stent: A One-Fits-All Strategy for the Treatment of Acute Type A Aortic Dissection? J. Clin. Med. 2024, 13, 2593. [Google Scholar] [CrossRef]
- EL-Andari, R.; Bozso, S.J.; Nagendran, J.; Chung, J.; Ouzounian, M.; Moon, M.C. Aortic Remodelling Based on False Lumen Communications in Patients Undergoing Acute Type I Dissection Repair with AMDS Hybrid Prosthesis: A Substudy of the DARTS Trial. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2024, 65, ezae194. [Google Scholar] [CrossRef]
- Rylski, B.; Hahn, N.; Beyersdorf, F.; Kondov, S.; Wolkewitz, M.; Blanke, P.; Plonek, T.; Czerny, M.; Siepe, M. Fate of the Dissected Aortic Arch after Ascending Replacement in Type A Aortic Dissection. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2017, 51, 1127–1134. [Google Scholar] [CrossRef]
- Lawrence, K.M.; Desai, N. The AMDS Stent Reduces Postoperative Distal Anastomotic New Re-Entry (DANE) Tears, But Will It Reduce the Need for Late Aortic Reintervention? Can. J. Cardiol. 2024, 40, 476–477. [Google Scholar] [CrossRef]
- White, A.; Elfaki, L.; O’Brien, D.; Manikala, V.; Bozso, S.; Ouzounian, M.; Moon, M.C. The Use of the Ascyrus Medical Dissection Stent in Acute Type A Aortic Dissection Repair Reduces Distal Anastomotic New Entry Tear. Can. J. Cardiol. 2024, 40, 470–475. [Google Scholar] [CrossRef]
- Diaz-Castrillon, C.E.; Serna-Gallegos, D.; Arnaoutakis, G.; Grimm, J.; Szeto, W.Y.; Chu, D.; Sezer, A.; Sultan, I. Volume-Failure-to-Rescue Relationship in Acute Type A Aortic Dissections: An Analysis of The Society of Thoracic Surgeons Database. J. Thorac. Cardiovasc. Surg. 2024, 168, 1416–1425.e7. [Google Scholar] [CrossRef]
- Rahouma, M.; Baudo, M.; Mynard, N.; Kamel, M.; Khan, F.M.; Shmushkevich, S.; Mehta, K.; Hosny, M.; Dabsha, A.; Khairallah, S.; et al. Volume Outcome Relationship in Postesophagectomy Leak: A Systematic Review and Meta-Analysis. Int. J. Surg. Lond. Engl. 2024, 110, 2349–2354. [Google Scholar] [CrossRef]
- Badhwar, V.; Vemulapalli, S.; Mack, M.A.; Gillinov, A.M.; Chikwe, J.; Dearani, J.A.; Grau-Sepulveda, M.V.; Habib, R.; Rankin, J.S.; Jacobs, J.P.; et al. Volume-Outcome Association of Mitral Valve Surgery in the United States. JAMA Cardiol. 2020, 5, 1092–1101. [Google Scholar] [CrossRef]

| Variable | AMDS (N = 319) | FET (N = 4129) | p-Value |
|---|---|---|---|
| Mean age, years | 60.8 ± 2.9 | 52.0 ± 5.2 | <0.001 |
| Male | 70.5% (225/319) | 77.6% (3205/4129) | 0.005 |
| Mean BMI | 28.5 ± 1.2 | 26.0 ± 1.4 | 0.023 |
| Connective tissue disease | 0% (0/148) | 7.1% (202/2832) | 0.001 |
| Diabetes | 8.8% (24/273) | 6.7% (244/3637) | 0.234 |
| COPD | 7.1% (16/226) | 5.6% (86/1544) | 0.449 |
| Hypertension | 69.0% (220/319) | 75.4% (2761/3663) | 0.014 |
| CKD | 17.2% (55/319) | 3.8% (79/2096) | <0.001 |
| CAD | 11.0% (30/273) | 7.7% (123/1605) | 0.082 |
| Reintervention | 3.7% (8/218) | 3.5% (89/2528) | >0.999 |
| Acute neurological deficit | 29.4% (53/180) | 5.9% (128/2186) | <0.001 |
| Hemopericardium | 12.3% (21/171) | 17.1% (151/882) | 0.146 |
| Outcome | Group | No. of Studies | No. of Patients | Estimate [95%CI] | Heterogeneity: I2, p-Value | Group Difference |
|---|---|---|---|---|---|---|
| Bilateral SACP | AMDS | 5 | 273 | 54.0% [24.6–80.9] | 92.2%, p < 0.0001 | p = 0.587 |
| FET | 20 | 2122 | 65.2% [38.5–84.9] | 93.1%, p < 0.0001 | ||
| Hypothermic temperature | AMDS | 4 | 180 | 27.2 °C [26.6–27.8] | 91.4%, p < 0.0001 | p < 0.001 |
| FET | 27 | 3454 | 23.9 °C [22.9–25.0] | 99.8%, p < 0.0001 | ||
| Root replacement | AMDS | 5 | 226 | 33.2% [22.0–46.7] | 65.3%, p = 0.0213 | p = 0.199 |
| FET | 28 | 3474 | 26.8% [21.5–33.0] | 89.6%, p < 0.0001 | ||
| VSRR | AMDS | 4 | 180 | 31.8% [9.7–66.7] | 84.1%, p = 0.0003 | p = 0.001 |
| FET | 26 | 3321 | 3.2% [1.8–5.7] | 85.5%, p < 0.0001 | ||
| Bentall | AMDS | 4 | 180 | 5.4% [1.0–24.7] | 62.1%, p = 0.0479 | p = 0.171 |
| FET | 26 | 3321 | 16.6% [12.5–21.6] | 88.7%, p < 0.0001 | ||
| Root repair | AMDS | 5 | 226 | 3.6% [0.3–35.8] | 83.9%, p < 0.0001 | p = 0.049 |
| FET | 27 | 3408 | 0.9% [0.3–2.5] | 78.0%, p < 0.0001 | ||
| Wheat | AMDS | 6 | 319 | 0.0% [0.0–0.0] | - | p = 0.920 |
| FET | 28 | 3474 | 1.4% [0.9–2.3] | 40.7%, p = 0.0142 | ||
| CABG | AMDS | 6 | 319 | 7.2% [3.0–16.0] | 66.0%, p = 0.0118 | p = 0.930 |
| FET | 28 | 3474 | 6.9% [4.3–10.8] | 92.8%, p < 0.0001 | ||
| AV repair | AMDS | 6 | 319 | 3.0% [0.4–18.3] | 79.4%, p = 0.0002 | p = 0.803 |
| FET | 28 | 3474 | 3.9% [2.1–7.2] | 91.2%, p < 0.0001 | ||
| AV replacement | AMDS | 6 | 319 | 2.5% [0.3–18.1] | 79.1%, p = 0.0002 | p = 0.935 |
| FET | 28 | 3474 | 2.7% [1.6–4.7] | 81.4%, p < 0.0001 | ||
| MV surgery | AMDS | 6 | 319 | 1.6% [0.5–4.3] | 0.0%, p = 0.7141 | p = 0.782 |
| FET | 28 | 3474 | 1.3% [0.7–2.3] | 56.7%, p = 0.0001 | ||
| CPB time | AMDS | 4 | 180 | 238 min [189–299] | 96.3%, p < 0.0001 | p = 0.262 |
| FET | 32 | 4129 | 204 min [192–217] | 99.2%, p < 0.0001 | ||
| CXC time | AMDS | 4 | 180 | 134 min [102–176] | 96.0%; p < 0.0001 | p = 0.394 |
| FET | 30 | 3997 | 119 min [112–126] | 98.5%, p < 0.0001 | ||
| CA time | AMDS | 6 | 319 | 54 min [30–98] | 99.6%, p < 0.0001 | p = 0.047 |
| FET | 27 | 3645 | 29 min [24–35] | 99.8%, p < 0.0001 | ||
| SACP time | AMDS | 3 | 63 | 57 min [22–144] | 98.3%, p < 0.0001 | p = 0.869 |
| FET | 14 | 1281 | 52 min [37–74] | 99.9%, p < 0.0001 |
| Outcome | Group | No. of Studies | No. of Patients | Estimate [95%CI] | Heterogeneity: I2, p-Value | Group Difference |
|---|---|---|---|---|---|---|
| Surgical bleeding | AMDS | 4 | 180 | 21.2% [14.5–29.9] | 17.7%, p= 0.3024 | p < 0.001 |
| FET | 23 | 2867 | 6.4% [4.1–10.1] | 87.7%, p < 0.0001 | ||
| Dialysis | AMDS | 4 | 120 | 16.4% [10.6–24.3] | 0.0%, p = 0.4049 | p = 0.999 |
| FET | 17 | 2096 | 16.4% [11.8–22.3] | 88.0%, p < 0.0001 | ||
| CVA | AMDS | 6 | 319 | 8.8% [4.9–15.4] | 47.3%, p = 0.0909 | p = 0.717 |
| FET | 19 | 2100 | 7.7% [5.1–11.7] | 80.5%, p < 0.0001 | ||
| SCI | AMDS | 3 | 111 | 0.0% [0.0–0.0] | - | p = 0.178 |
| FET | 19 | 2207 | 5.2% [3.6–7.3] | 56.6%, p = 0.0013 | ||
| ICU time | AMDS | 6 | 319 | 7.8 days [6.1–10.0] | 74.4%, p = 0.0015 | p = 0.599 |
| FET | 23 | 3446 | 7.0 days [5.2–9.5] | 99.5%, p < 0.0001 | ||
| LOS time | AMDS | 5 | 213 | 14.8 days [13.3–16.4] | 14.3%, p = 0.3232 | p < 0.001 |
| FET | 20 | 2420 | 19.2 days [17.3–21.4] | 98.0%, p < 0.0001 | ||
| Hospital mortality | AMDS | 6 | 319 | 14.5% [11.0–18.9] | 0.0%, p = 0.9751 | p = 0.037 |
| FET | 31 | 3917 | 10.0% [8.1–12.3] | 73.9%, p = 0.0012 | ||
| Follow-up time | AMDS | 3 | 196 | 0.8 yr [0.2–3.6] | 99.7%, p < 0.0001 | p = 0.218 |
| FET | 18 | 1520 | 2.1 yr [1.6–2.8] | 99.8%, p < 0.0001 | ||
| Late death | AMDS | 3 | 196 | 7.8%/yr [1.6–36.8] | 84.7%, p = 0.0014 | p = 0.285 |
| FET | 17 | 1472 | 3.3%/yr [2.4–4.4] | 43.0%, p = 0.0311 |
| AMDS | FET | |
|---|---|---|
| Variable | OR (95%CI), p-Value | OR (95%CI), p-Value |
| Mean age, years | 1.06 (0.92–1.22), p = 0.408 | 1.01 (0.98–1.05), p = 0.484 |
| Male % | 0.98 (0.93–1.02), p = 0.314 | 1.01 (0.98–1.03), p = 0.586 |
| Mean BMI | 0.75 (0.53–1.05), p = 0.095 | 1.05 (0.76–1.44), p = 0.779 |
| Connective tissue disease % | No patient | 0.98 (0.95–1.02), p = 0.284 |
| Diabetes % | 0.92 (0.83–1.03), p = 0.144 | 0.99 (0.96–1.02), p = 0.565 |
| COPD % | 0.95 (0.85–1.07), p = 0.424 | 1.01 (0.93–1.09), p = 0.824 |
| Hypertension % | 1.02 (0.99–1.04), p = 0.142 | 0.98 (0.96–1.01), p = 0.309 |
| CKD % | 0.97 (0.93–1.01), p = 0.150 | 1.04 (0.93–1.16), p = 0.522 |
| CAD % | 0.96 (0.83–1.11), p = 0.606 | 0.95 (0.90–1.01), p = 0.129 |
| Reintervention % | 0.72 (0.19–2.69), p = 0.629 | 1.05 (0.92–1.20), p = 0.434 |
| Acute neurological deficit % | 1.03 (0.94–1.13), p = 0.582 | 0.97 (0.91–1.04), p = 0.449 |
| Hemopericardium % | 0.98 (0.92–1.05), p = 0.647 | 1.01 (0.98–1.04), p = 0.571 |
| BSACP % | 0.99 (0.98–1.01), p = 0.321 | 1.00 (0.99–1.01), p = 0.731 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudo, M.; Rosati, F.; D’Alonzo, M.; Fiore, A.; Muneretto, C.; Benussi, S.; Di Bacco, L. Total Arch Replacement with Ascyrus Medical Dissection Stent Versus Frozen Elephant Trunk in Acute Type A Aortic Dissection: A Meta-Analysis. J. Clin. Med. 2025, 14, 5170. https://doi.org/10.3390/jcm14145170
Baudo M, Rosati F, D’Alonzo M, Fiore A, Muneretto C, Benussi S, Di Bacco L. Total Arch Replacement with Ascyrus Medical Dissection Stent Versus Frozen Elephant Trunk in Acute Type A Aortic Dissection: A Meta-Analysis. Journal of Clinical Medicine. 2025; 14(14):5170. https://doi.org/10.3390/jcm14145170
Chicago/Turabian StyleBaudo, Massimo, Fabrizio Rosati, Michele D’Alonzo, Antonio Fiore, Claudio Muneretto, Stefano Benussi, and Lorenzo Di Bacco. 2025. "Total Arch Replacement with Ascyrus Medical Dissection Stent Versus Frozen Elephant Trunk in Acute Type A Aortic Dissection: A Meta-Analysis" Journal of Clinical Medicine 14, no. 14: 5170. https://doi.org/10.3390/jcm14145170
APA StyleBaudo, M., Rosati, F., D’Alonzo, M., Fiore, A., Muneretto, C., Benussi, S., & Di Bacco, L. (2025). Total Arch Replacement with Ascyrus Medical Dissection Stent Versus Frozen Elephant Trunk in Acute Type A Aortic Dissection: A Meta-Analysis. Journal of Clinical Medicine, 14(14), 5170. https://doi.org/10.3390/jcm14145170







