Food Protein-Induced Enterocolitis Syndrome Across Lifespan: Focus on Adolescence
Abstract
1. Introduction
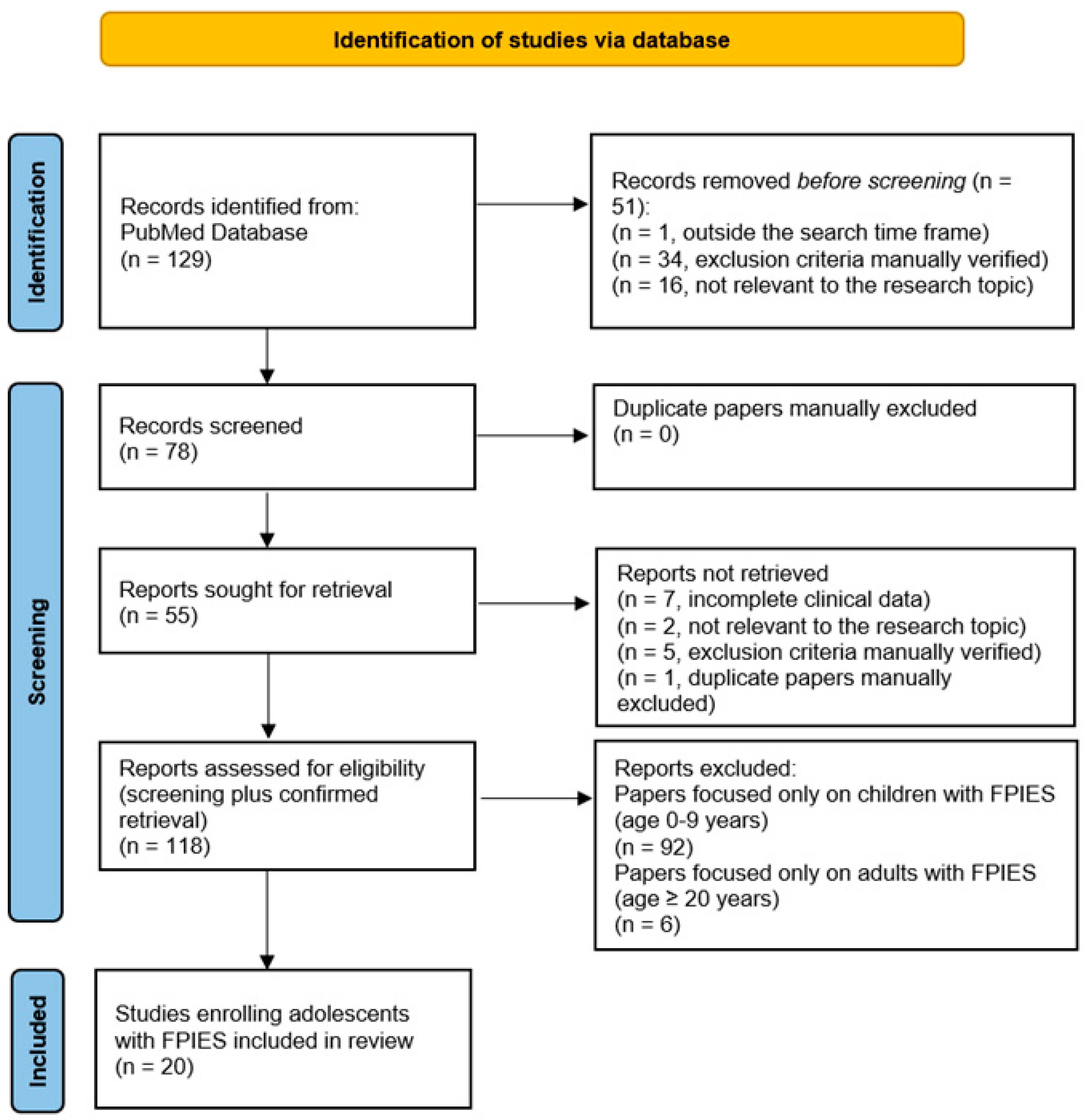
2. Methods
3. Results and Discussion
3.1. FPIES in Infancy/Childhood
3.2. FPIES in Adulthood
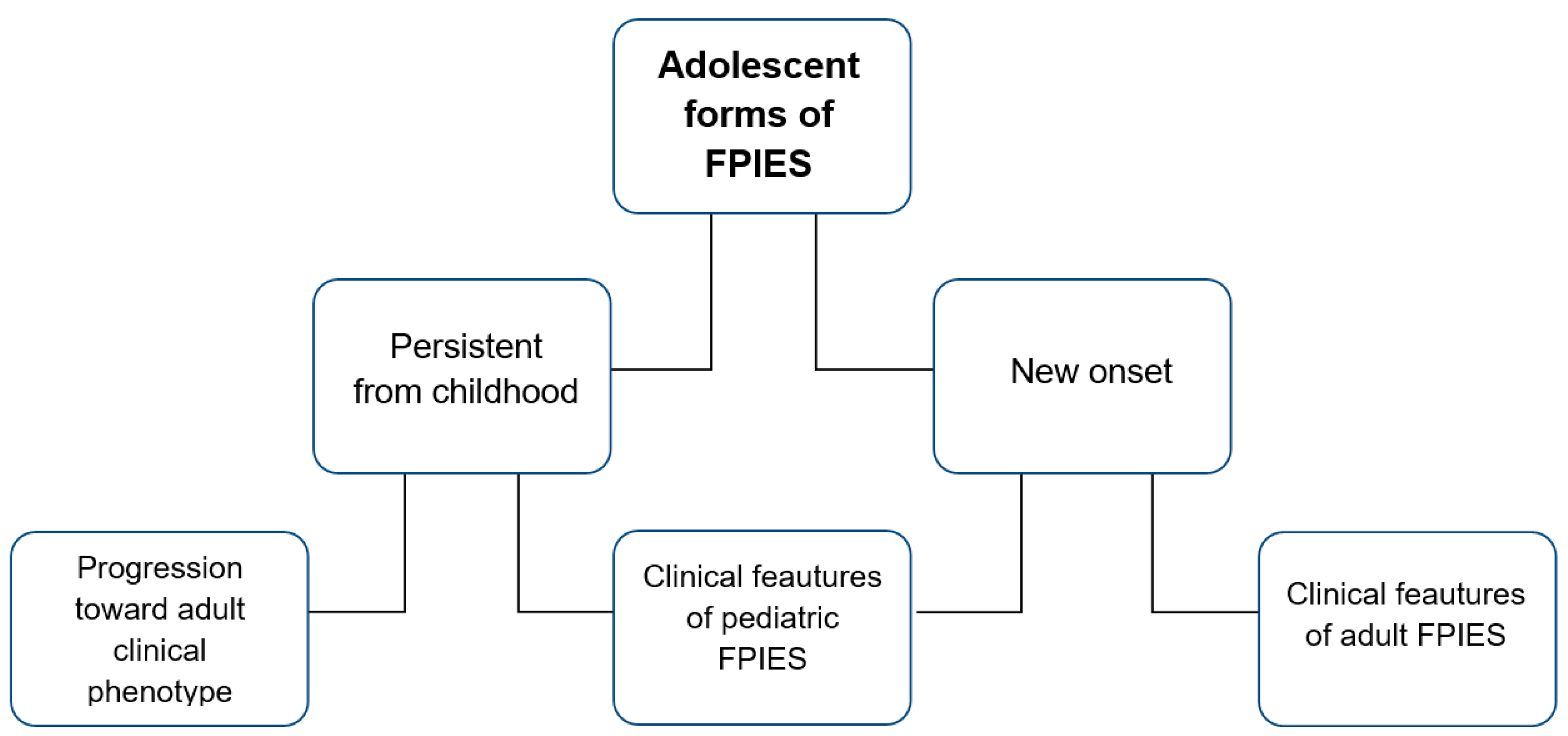
3.3. FPIES in Adolescence
3.3.1. Genetic Biomarkers
3.3.2. Immunologic Biomarkers
3.3.3. Gut Inflammation Biomarkers
3.3.4. Microbiome-Related Biomarkers
- Small sample sizes, due to the rarity of FPIES in this age group, limit statistical power.
- Puberty-related immune modulation complicates the interpretation of mucosal and systemic immune markers.
- Phenotypic heterogeneity: adolescent FPIES can resemble pediatric or adult-onset forms, increasing variability.
- Lack of age-specific normative data impairs the diagnostic precision of existing biomarkers.
- Absence of longitudinal, age-stratified studies leaves key developmental transitions unexplored.
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- González-Delgado, P.; Muriel, J.; Jiménez, T.; Cameo, J.I.; Palazón-Bru, A.; Fernández, J. Food Protein-Induced Enterocolitis Syndrome in Adulthood: Clinical Characteristics, Prognosis, and Risk Factors. J. Allergy Clin. Immunol. Pract. 2022, 10, 2397–2403. [Google Scholar] [CrossRef]
- Powell, G.K. Milk- and Soy-Induced Enterocolitis of Infancy. Clinical Features and Standardization of Challenge. J. Pediatr. 1978, 93, 553–560. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Eigenmann, P.A.; Sampson, H.A. Clinical Features of Food Protein-Induced Enterocolitis Syndrome. J. Pediatr. 1998, 133, 214–219. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.M.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International Consensus Guidelines for the Diagnosis and Management of Food Protein-Induced Enterocolitis Syndrome: Executive Summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126.e4. [Google Scholar] [CrossRef]
- Vazquez-Ortiz, M.; Infante, S. Diagnostic Criteria for Food Protein-Induced Enterocolitis Syndrome: Can We Do Better? Ann. Allergy Asthma Immunol. 2021, 126, 458–459. [Google Scholar] [CrossRef]
- Miceli Sopo, S. Criteria for the Suspicion and Diagnosis of Acute Food Protein-Induced Enterocolitis Syndrome. Allergol. Immunopathol. 2021, 49, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; Mastellone, F.; Bersani, G.; Barbato, M.; Miceli Sopo, B.; Gelsomino, M. Diagnosing Paediatric Mild Acute Food Protein-Induced Enterocolitis Syndrome: Proposal of New Criteria. Clin. Exp. Allergy 2024, 54, 512–514. [Google Scholar] [CrossRef] [PubMed]
- González-Delgado, P.; Entrala, A.; Nuñez-Orjales, R.; Marchan, E.; Fernández, J.; Nowak-Wegrzyn, A. Food Protein-Induced Enterocolitis Syndrome in Adults: Review and Practice Recommendations. Explor. Asthma Allergy 2024, 2, 148–160. [Google Scholar] [CrossRef]
- Anvari, S.; Ruffner, M.A. Adult Food Protein-Induced Enterocolitis Syndrome. Front. Allergy 2022, 3, 889879. [Google Scholar] [CrossRef]
- Barni, S.; Sarti, L.; Mori, F.; Liotti, L.; Pucci, N.; Novembre, E. A Modified Oral Food Challenge in Children with Food Protein-Induced Enterocolitis Syndrome. Clin. Exp. Allergy 2019, 49, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, M.I.; Scaparrotta, A.; Di Filippo, P.; Attanasi, M.; Di Pillo, S.; Chiarelli, F.; Mohn, A. Food Protein-Induced Enterocolitis Syndrome in Children: What’s Known? What’s New? Eur. Ann. Allergy Clin. Immunol. 2018, 50, 99–107. [Google Scholar] [CrossRef]
- Onesimo, R.; Dello Iacono, I.; Giorgio, V.; Limongelli, M.G.; Miceli Sopo, S. Can Food Protein Induced Enterocolitis Syndrome Shift to Immediate Gastrointestinal Hypersensitivity? A Report of Two Cases. Eur. Ann. Allergy Clin. Immunol. 2011, 43, 61–63. [Google Scholar]
- Antunes, J.; Borrego, L.; Romeira, A.; Pinto, P. Skin Prick Tests and Allergy Diagnosis. Allergol. Immunopathol. 2009, 37, 155–164. [Google Scholar] [CrossRef]
- Nomura, I.; Morita, H.; Hosokawa, S.; Hoshina, H.; Fukuie, T.; Watanabe, M.; Ohtsuka, Y.; Shoda, T.; Terada, A.; Takamasu, T.; et al. Four Distinct Subtypes of Non-IgE-Mediated Gastrointestinal Food Allergies in Neonates and Infants, Distinguished by Their Initial Symptoms. J. Allergy Clin. Immunol. 2011, 127, 685–688. [Google Scholar] [CrossRef]
- Katz, Y.; Goldberg, M.R.; Rajuan, N.; Cohen, A.; Leshno, M. The Prevalence and Natural Course of Food Protein-Induced Enterocolitis Syndrome to Cow’s Milk: A Large-Scale, Prospective Population-Based Study. J. Allergy Clin. Immunol. 2011, 127, 647–653.e3. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-B.; Sohn, S.M.; Kim, A.S. Prospective Follow-up Oral Food Challenge in Food Protein-Induced Enterocolitis Syndrome. Arch. Dis. Child. 2009, 94, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; Giorgio, V.; Dello Iacono, I.; Novembre, E.; Mori, F.; Onesimo, R. A Multicentre Retrospective Study of 66 Italian Children with Food Protein-Induced Enterocolitis Syndrome: Different Management for Different Phenotypes. Clin. Exp. Allergy 2012, 42, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; Romano, A.; Bersani, G.; Fantacci, C.; Badina, L.; Longo, G.; Monti, G.; Viola, S.; Tripodi, S.; Barilaro, G.; et al. Cooking Influence in Tolerance Acquisition in Egg-Induced Acute Food Protein Enterocolitis Syndrome. Allergol. Immunopathol. 2019, 47, 221–226. [Google Scholar] [CrossRef]
- Miceli Sopo, S.; Monaco, S.; Badina, L.; Barni, S.; Longo, G.; Novembre, E.; Viola, S.; Monti, G. Food Protein-Induced Enterocolitis Syndrome Caused by Fish and/or Shellfish in Italy. Pediatr. Allergy Immunol. 2015, 26, 731–736. [Google Scholar] [CrossRef]
- Shah, S.; Grohman, R.; Nowak-Wegrzyn, A. Food Protein-Induced Enterocolitis Syndrome (FPIES): Beyond the Guidelines. J. Food Allergy 2023, 5, 55–64. [Google Scholar] [CrossRef]
- Watanabe, S.; Sato, A.; Uga, M.; Matsukawa, N.; Kusuda, R.; Suzuki, H.; Nagashima, S.; Yauchi, T.; Ohya, Y.; Nomura, I. A Detailed Intake-Status Profiling of Seafoods in Adult Food-Protein-Induced Enterocolitis Syndrome Patients. Allergol. Int. 2024, 73, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Skrabski, F.; Pérez-Pallise, M.E.; De Castro-Martínez, F.J.; Zubeldia, J.M.; Infante, S. Relevant Features of Adult-Onset Food Protein-Induced Enterocolitis Syndrome. J. Allergy Clin. Immunol. Pract. 2021, 9, 1759–1760.e1. [Google Scholar] [CrossRef]
- Crespo, J.; Pérez-Pallise, M.E.; Skrabski, F.; Zambrano, G.; Rojas-Pérez-Ezquerra, P.; Noguerado-Mellado, B.; Zubeldia, J.M.; Infante, S. The Natural Course of Adult-Onset Food Protein-Induced Enterocolitis Syndrome. J. Allergy Clin. Immunol. Pract. 2022, 10, 2986–2992. [Google Scholar] [CrossRef]
- Buckey, T.M.; Patel, P.J.; Milutinovic, P.S. Prevalence and Clinical Presentation of Food Protein-Induced Enterocolitis Syndrome in an Adult Allergy/Immunology Clinic. Ann. Allergy Asthma Immunol. 2024, 133, 222–223. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, P.; Caparrós, E.; Moreno, M.V.; Cueva, B.; Fernández, J. Food Protein-Induced Enterocolitis-like Syndrome in a Population of Adolescents and Adults Caused by Seafood. J. Allergy Clin. Immunol. Pract. 2019, 7, 670–672. [Google Scholar] [CrossRef]
- Adolescent Health. Available online: https://www.who.int/health-topics/adolescent-health (accessed on 28 June 2025).
- Su, K.-W.; Patil, S.U.; Stockbridge, J.L.; Martin, V.M.; Virkud, Y.V.; Huang, J.-L.; Shreffler, W.G.; Yuan, Q. Food Aversion and Poor Weight Gain in Food Protein-Induced Enterocolitis Syndrome: A Retrospective Study. J. Allergy Clin. Immunol. 2020, 145, 1430–1437.e11. [Google Scholar] [CrossRef]
- Coppola, S.; Carucci, L.; Agizza, A.; Nocerino, R.; Carandente, R.; Catalano, M.F.; Berni Canani, R. The Impact of Dietary Counseling on the Nutritional Status of Pediatric Patients with Non-IgE-Mediated Gastrointestinal Food Allergies: A Non-Randomized, Prospective Intervention Study. Nutrients 2025, 17, 1080. [Google Scholar] [CrossRef]
- Huang, J.; White, A.A. The Heterogeneity of Food Protein-Induced Enterocolitis Syndrome in Adults. J. Allergy Clin. Immunol. Pract. 2025, 13, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Infante, S.; Marco-Martín, G.; Sánchez-Domínguez, M.; Rodríguez-Fernández, A.; Fuentes-Aparicio, V.; Alvarez-Perea, A.; Cabrera-Freitag, P.; Morales-Cabeza, C.; Zubeldia, J.M.; Zapatero, L. Food Protein-Induced Enterocolitis Syndrome by Fish: Not Necessarily a Restricted Diet. Allergy 2018, 73, 728–732. [Google Scholar] [CrossRef]
- Camino-Mera, A.; Pardo-Seco, J.; Bello, X.; Argiz, L.; Boyle, R.J.; Custovic, A.; Herberg, J.; Kaforou, M.; Arasi, S.; Fiocchi, A.; et al. Whole Exome Sequencing Identifies Epithelial and Immune Dysfunction-Related Biomarkers in Food Protein-Induced Enterocolitis Syndrome. Clin. Exp. Allergy 2024, 54, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Inage, E.; Yamada, H.; Kudo, T.; Toriumi, S.; Sakaguchi, K.; Tanaka, Y.; Jimbo, K.; Ohtsuka, Y.; Shimizu, T. Efficacy of Sequential Fecal-Marker Examination for Evaluating Gastrointestinal Inflammation in Solid Food Protein-Induced Enterocolitis Syndrome. Allergol. Int. 2024, 73, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, A.; Kapel, N.; Nicolis, I.; Tounian, P.; Bruneau, A.; Kapandji, N.; Adel-Patient, K.; Thomas, M. Identification of Gut Biomarkers of FPIES in a Longitudinal Comparative Pediatric Study. Allergy 2025, 80, 1389–1399. [Google Scholar] [CrossRef]
- Rizzi, A.; Lo Presti, E.; Chini, R.; Gammeri, L.; Inchingolo, R.; Lohmeyer, F.M.; Nucera, E.; Gangemi, S. Emerging Role of Alarmins in Food Allergy: An Update on Pathophysiological Insights, Potential Use as Disease Biomarkers, and Therapeutic Implications. J. Clin. Med. 2023, 12, 2699. [Google Scholar] [CrossRef]
- Wada, T.; Toma, T.; Muraoka, M.; Matsuda, Y.; Yachie, A. Elevation of Fecal Eosinophil-Derived Neurotoxin in Infants with Food Protein-Induced Enterocolitis Syndrome. Pediatr. Allergy Immunol. 2014, 25, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Matsuda, Y.; Toma, T.; Koizumi, E.; Okamoto, H.; Yachie, A. Increased CD69 Expression on Peripheral Eosinophils from Patients with Food Protein-Induced Enterocolitis Syndrome. Int. Arch. Allergy Immunol. 2016, 170, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, G.N.; Bencharitiwong, R.; Grishin, A.; Caubet, J.-C.; Bardina, L.; Sicherer, S.H.; Sampson, H.A.; Nowak-Węgrzyn, A. The Role of Casein-Specific IgA and TGF-β in Children with Food Protein-Induced Enterocolitis Syndrome to Milk. Pediatr. Allergy Immunol. 2014, 25, 651–656. [Google Scholar] [CrossRef]
- Adel-Patient, K.; Lezmi, G.; Castelli, F.A.; Blanc, S.; Bernard, H.; Soulaines, P.; Dumond, P.; Ah-Leung, S.; Lageix, F.; de Boissieu, D.; et al. Deep Analysis of Immune Response and Metabolic Signature in Children with Food Protein Induced Enterocolitis to Cow’s Milk. Clin. Transl. Allergy 2018, 8, 38. [Google Scholar] [CrossRef]
- Galliano, I.; Montanari, P.; Monti, G.; Dini, M.; Calvi, C.; Clemente, A.; Pau, A.; Gambarino, S.; Bergallo, M. IL10 and CXCL10 mRNA Expression in Food Protein-Induced Enterocolitis Syndrome. Cytokine 2024, 182, 156720. [Google Scholar] [CrossRef]
- Kimura, M.; Ito, Y.; Shimomura, M.; Morishita, H.; Meguro, T.; Adachi, Y.; Seto, S. Cytokine Profile after Oral Food Challenge in Infants with Food Protein-Induced Enterocolitis Syndrome. Allergol. Int. 2017, 66, 452–457. [Google Scholar] [CrossRef]
- Lee, E.; Barnes, E.H.; Mehr, S.; Campbell, D.E. Differentiating Acute Food Protein-Induced Enterocolitis Syndrome from Its Mimics: A Comparison of Clinical Features and Routine Laboratory Biomarkers. J. Allergy Clin. Immunol. Pract. 2019, 7, 471–478.e3. [Google Scholar] [CrossRef]
- Caubet, J.C.; Bencharitiwong, R.; Ross, A.; Sampson, H.A.; Berin, M.C.; Nowak-Węgrzyn, A. Humoral and Cellular Responses to Casein in Patients with Food Protein-Induced Enterocolitis to Cow’s Milk. J. Allergy Clin. Immunol. 2017, 139, 572–583. [Google Scholar] [CrossRef]
- Xiong, J.; Ma, Y.-J.; Liao, X.-S.; Li, L.-Q.; Bao, L. Gut Microbiota in Infants with Food Protein Enterocolitis. Pediatr. Res. 2025, 97, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.M.; Sabater, C.; Gutiérrez-Díaz, I.; Navarro, S.; Rodriguez, S.; Molinos, C.; Jiménez, S.; Claver, A.; Espin, B.; Domínguez, G.; et al. The Intestinal Microbiome of Infants with Cow’s Milk-Induced FPIES Is Enriched in Taxa and Genes of Enterobacteria. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Caparrós, E.; Cenit, M.C.; Muriel, J.; Benítez-Páez, A.; Moreno, M.V.; González-Delgado, P.; Rubio, G.; Sanz, Y.; Fernández, J. Intestinal Microbiota Is Modified in Pediatric Food Protein-Induced Enterocolitis Syndrome. J. Allergy Clin. Immunol. Glob. 2022, 1, 217–224. [Google Scholar] [CrossRef]
- Miceli Sopo, S.; Sinatti, D.; Gelsomino, M. Oral Desensitization in Egg Acute Food Protein-Induced Enterocolitis Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5766–5768. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, M.; Barni, S.; Mastellone, F.; Bersani, G.; Barbato, M.; Condemi, C.; Mori, F.; Vazquez-Ortiz, M.; Indirli, G.C.; Miceli Sopo, B.; et al. Severity Trend of Recurrence in Pediatric Food Protein-Induced Enterocolitis Syndrome. J. Allergy Clin. Immunol. Pract. 2025, 13, 842–850. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, P.; Anvari, S.; Entrala, A.; Infante, S. Medical Algorithm: Diagnosis and Management of Adult Food Protein-Induced Enterocolitis Syndrome. Allergy 2024, 79, 2881–2884. [Google Scholar] [CrossRef]
- Beaudoin, M.; Mehra, A.; Wong, L.S.Y.; Vazquez-Ortiz, M.; González-Delgado, P.; Nowak-Wegrzyn, A. An Algorithm for the Diagnosis and Treatment of Food Protein-Induced Enterocolitis Syndrome (FPIES), 2024 Update. Allergy 2025, 80, 362–365. [Google Scholar] [CrossRef]
- Ye, L.; Wong, T.; Lavine, E.; Cook, V.E.; Erdle, S.C. Using the Canadian Egg Ladder in Children with Food Protein-Induced Enterocolitis Syndrome: A Case Series. Allergy Asthma Clin. Immunol. 2023, 19, 87. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Azzopardi, P.S.; Wickremarathne, D.; Patton, G.C. The Age of Adolescence. Lancet Child Adolesc. Health 2018, 2, 223–228. [Google Scholar] [CrossRef]
- Christie, D.; Viner, R. Adolescent Development. BMJ 2005, 330, 301–304. [Google Scholar] [CrossRef]
- Ferro, M.A.; Van Lieshout, R.J.; Scott, J.G.; Alati, R.; Mamun, A.A.; Dingle, K. Condition-Specific Associations of Symptoms of Depression and Anxiety in Adolescents and Young Adults with Asthma and Food Allergy. J. Asthma 2016, 53, 282–288. [Google Scholar] [CrossRef]
- Ferro, M.A.; Van Lieshout, R.J.; Ohayon, J.; Scott, J.G. Emotional and Behavioral Problems in Adolescents and Young Adults with Food Allergy. Allergy 2016, 71, 532–540. [Google Scholar] [CrossRef]
- Withers, A.L. Management Issues for Adolescents with Cystic Fibrosis. Pulm. Med. 2012, 2012, 134132. [Google Scholar] [CrossRef]
- Vazquez-Ortiz, M.; Angier, E.; Blumchen, K.; Comberiati, P.; Duca, B.; DunnGalvin, A.; Gore, C.; Hox, V.; Jensen, B.; Pite, H.; et al. Understanding the Challenges Faced by Adolescents and Young Adults with Allergic Conditions: A Systematic Review. Allergy 2020, 75, 1850–1880. [Google Scholar] [CrossRef]
- Egmose, B.; Huniche, L.; Bindslev-Jensen, C.; Nielsen, D.S.; Mørtz, C.G. Exploring Young Adults’ Experiences with Food Allergy during Their Teenage Years: A Practice Research Study. Scand. J. Caring Sci. 2024, 38, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.S.; Ratliff, E.L.; Cosgrove, K.T.; Steinberg, L. We Know Even More Things: A Decade Review of Parenting Research. J. Res. Adolesc. 2021, 31, 870–888. [Google Scholar] [CrossRef] [PubMed]
- Spolidoro, G.C.I.; Amera, Y.T.; Ali, M.M.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Frequency of Food Allergy in Europe: An Updated Systematic Review and Meta-Analysis. Allergy 2023, 78, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Spolidoro, G.C.I.; Ali, M.M.; Amera, Y.T.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Prevalence Estimates of Eight Big Food Allergies in Europe: Updated Systematic Review and Meta-Analysis. Allergy 2023, 78, 2361–2417. [Google Scholar] [CrossRef]
- Atzert, N.; Gore, C.; Knibb, R.C.; Alviani, C.; Angier, E.; Blumchen, K.; Comberiati, P.; Duca, B.; DunnGalvin, A.; Garriga-Baraut, T.; et al. Improved Transition Management of Adolescents and Young Adults with Allergy and/or Asthma: An EAACI Task Force Report on a Follow-Up European Survey. Allergy 2025, 80, 1592–1612. [Google Scholar] [CrossRef]
- Khaleva, E.; Vazquez-Ortiz, M.; Comberiati, P.; DunnGalvin, A.; Pite, H.; Blumchen, K.; Garriga-Baraut, T.; Hox, V.; Santos, A.F.; Gore, C.; et al. Current Transition Management of Adolescents and Young Adults with Allergy and Asthma: A European Survey. Clin. Transl. Allergy 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.N.; Boyle, R.J.; Gore, C.; Simpson, A.; Custovic, A. Food Protein-Induced Enterocolitis Syndrome Can Occur in Adults. J. Allergy Clin. Immunol. 2012, 130, 1199–1200. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Hayashi, D.; Kajita, N.; Kinoshita, M.; Ishii, T.; Tsumura, Y.; Horimukai, K.; Yoshida, K.; Takahashi, T.; Morita, H. Recent dramatic increase in patients with food protein-induced enterocolitis syndrome (FPIES) provoked by hen’s egg in Japan. J. Allergy Clin. Immunol. Pract. 2022, 10, 1110–1112.e2. [Google Scholar] [CrossRef]
- Mehr, S.; Frith, K.; Barnes, E.H.; Campbell, D.E.; FPIES Study Group. Food Protein-Induced Enterocolitis Syndrome in Australia: A Population-Based Study, 2012–2014. J. Allergy Clin. Immunol. 2017, 140, 1323–1330. [Google Scholar] [CrossRef]
- Michelet, M.; Schluckebier, D.; Petit, L.-M.; Caubet, J.-C. Food Protein-Induced Enterocolitis Syndrome—A Review of the Literature with Focus on Clinical Management. J. Asthma Allergy 2017, 10, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; Sinatti, D.; Gelsomino, M. Retrospective Analysis of 222 Oral Food Challenges with a Single Dose in Acute Food Protein-Induced Enterocolitis Syndrome. Pediatr. Allergy Immunol. 2021, 32, 1066–1072. [Google Scholar] [CrossRef]
- Miceli Sopo, S.; Monaco, S.; Cerchiara, G.; Bersani, G. A Very Unusual Case of Food Allergy, between FPIES and IgE-Mediated Food Allergy. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 42–44. [Google Scholar]
- Banzato, C.; Piacentini, G.L.; Comberiati, P.; Mazzei, F.; Boner, A.L.; Peroni, D.G. Unusual Shift from IgE-Mediated Milk Allergy to Food Protein-Induced Enterocolitis Syndrome. Eur. Ann. Allergy Clin. Immunol. 2013, 45, 209–211. [Google Scholar]
- Akashi, M. Food Protein-Induced Enterocolitis Syndrome. Keio J. Med. 2023, 72, 1–10. [Google Scholar] [CrossRef]
- Ibrahim, T.; Argiz, L.; Infante, S.; Arasi, S.; Nurmatov, U.; Vazquez-Ortiz, M. Oral Food Challenge Protocols in Food Protein-Induced Enterocolitis Syndrome: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2025, 13, 814–832. [Google Scholar] [CrossRef] [PubMed]
- Haddad, C.; Banerjee, A.; Eubanks, J.; Rana, R.; Rider, N.L.; Pompeii, L.; Anvari, S. A Second Slice of FPIES: A Single-Center Reappraisal of Pediatric FPIES. J. Allergy Clin. Immunol. Pract. 2024, 12, 2118–2126. [Google Scholar] [CrossRef]
- Caubet, J.C.; Ford, L.S.; Sickles, L.; Järvinen, K.M.; Sicherer, S.H.; Sampson, H.A.; Nowak-Węgrzyn, A. Clinical Features and Resolution of Food Protein-Induced Enterocolitis Syndrome: 10-Year Experience. J. Allergy Clin. Immunol. 2014, 134, 382–389. [Google Scholar] [CrossRef]
- Banerjee, A.; Nobleza, K.; Haddad, C.; Eubanks, J.; Rana, R.; Rider, N.L.; Pompeii, L.; Nguyen, D.; Anvari, S. Applying Market Basket Analysis to Determine Complex Coassociations Among Food Allergens in Children with Food Protein-Induced Enterocolitis Syndrome (FPIES). Health Serv. Res. Manag. Epidemiol. 2024, 11, 23333928241264020. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; D’Antuono, A.; Morganti, A.; Bianchi, A. Food Protein-Induced Enterocolitis Syndrome Due to Oysters Ingestion. Isr. Med. Assoc. J. 2015, 17, 188–189. [Google Scholar]
- Ruiz-García, M.; Díez, C.E.; García, S.S.; del Río, P.R.; Ibáñez, M.D. Diagnosis and Natural History of Food Protein-Induced Enterocolitis Syndrome in Children from a Tertiary Hospital in Central Spain. J. Investig. Allergol. Clin. Immunol. 2014, 24, 354–356. [Google Scholar]
- Goswami, R.; Blazquez, A.B.; Kosoy, R.; Rahman, A.; Nowak-Węgrzyn, A.; Berin, M.C. Systemic Innate Immune Activation in Food Protein-Induced Enterocolitis Syndrome. J. Allergy Clin. Immunol. 2017, 139, 1885–1896.e9. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Chen, X.; Dunkin, D.; Agashe, C.; Baker, M.G.; Bird, J.A.; Molina, E.; Nowak-Wegrzyn, A.; Berin, M.C. Untargeted Serum Metabolomic Analysis Reveals a Role for Purinergic Signaling in FPIES. J. Allergy Clin. Immunol. 2023, 151, 797–802. [Google Scholar] [CrossRef]
- Carucci, L.; Biancardi, L.; Nocerino, R.; Ciliberti, L.; Caldaria, E.; Bedogni, G.; Palmese, F.; Calabrò, F.; Berni Canani, R. The Naples Pediatric Food Allergy (NAPFA) Score: A Multivariable Model for the Prediction of Food Allergy in Children. Pediatr. Allergy Immunol. 2025, 36, e70071. [Google Scholar] [CrossRef]
- Argiz, L.; Valsami-Fokianos, M.; Arasi, S.; Barni, S.; Boscia, S.; Bracaglia, G.; Bracamonte, T.; Carballeira, I.; Dinardo, G.; Echeverria, L.; et al. Clinical-Hematological Changes and Predictors of Severity in Acute Food Protein-Induced Enterocolitis Syndrome Reactions at Oral Food Challenge: A Multicenter Observational Study. J. Allergy Clin. Immunol. Pract. 2024, 12, 2454–2467.e8. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C.; Lozano-Ojalvo, D.; Agashe, C.; Baker, M.G.; Bird, J.A.; Nowak-Wegrzyn, A. Acute FPIES Reactions Are Associated with an IL-17 Inflammatory Signature. J. Allergy Clin. Immunol. 2021, 148, 895–901.e6. [Google Scholar] [CrossRef] [PubMed]
- Carucci, L.; Nocerino, R.; Coppola, S.; Bedogni, G.; Capasso, P.; Giglio, V.; Berni Canani, R. Factors Influencing the Natural History of Non-IgE-Mediated Gastrointestinal Food Allergies in Paediatric Age: A Prospective Multicentre Cohort Study. BMJ Paediatr. Open 2025, 9, e003203. [Google Scholar] [CrossRef] [PubMed]
- Ullberg, J.; Ullberg, D.; Fech-Bormann, M.; Fagerberg, U.L. Resolution of Food Protein-Induced Enterocolitis Syndrome-A Long-Term Follow-Up Study of 113 Swedish Children. J. Allergy Clin. Immunol. Pract. 2024, 12, 2127–2134.e1. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; Bersani, G.; Monaco, S.; Cerchiara, G.; Lee, E.; Campbell, D.; Mehr, S. Ondansetron in Acute Food Protein-Induced Enterocolitis Syndrome, a Retrospective Case-Control Study. Allergy 2017, 72, 545–551. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Warren, C.M.; Brown-Whitehorn, T.; Cianferoni, A.; Schultz-Matney, F.; Gupta, R.S. Food Protein-Induced Enterocolitis Syndrome in the US Population-Based Study. J. Allergy Clin. Immunol. 2019, 144, 1128–1130. [Google Scholar] [CrossRef]
- Noimark, L.; Cox, H.E. Nutritional Problems Related to Food Allergy in Childhood. Pediatr. Allergy Immunol. 2008, 19, 188–195. [Google Scholar] [CrossRef]
- Doggui, R.; Ward, S.; Johnson, C.; Bélanger, M. Trajectories of Eating Behaviour Changes during Adolescence. Nutrients 2021, 13, 1313. [Google Scholar] [CrossRef]
- Kimura, M.; Shimomura, M.; Morishita, H.; Meguro, T. Prognosis of Infantile Food Protein-Induced Enterocolitis Syndrome in Japan. Pediatr. Int. 2017, 59, 855–860. [Google Scholar] [CrossRef]
- Pecora, V.; Prencipe, G.; Valluzzi, R.; Dahdah, L.; Insalaco, A.; Cianferoni, A.; De Benedetti, F.; Fiocchi, A. Inflammatory Events during Food Protein-Induced Enterocolitis Syndrome Reactions. Pediatr. Allergy Immunol. 2017, 28, 464–470. [Google Scholar] [CrossRef]
- Kunigami, C.; Imai, T.; Yamashita, K.; Takagi, T.; Okawa, M.; Honda, A.; Okada, Y.; Maeda, M.; Kamiya, T. Procalcitonin Level after Positive Food Protein-Induced Enterocolitis Syndrome (FPIES) Oral Food Challenge Predicts Short-Term Tolerance. J. Allergy Clin. Immunol. Pract. 2024, 12, 1937–1939.e1. [Google Scholar] [CrossRef] [PubMed]
- Kono, I.; Okamoto, M.; Inoue, S.; Tanaka, Y. Markedly Elevated Procalcitonin in Food Protein Induced Enterocolitis Syndrome. Kobe J. Med. Sci. 2021, 67, E7–E9. [Google Scholar]
- Umezawa, K.; Nagata, N.; Kabashima, S.; Inuzuka, Y.; Ogasawara, H.; Shimada, M.; Hamaguchi, S.; Natsume, O.; Fukuie, T.; Shimosawa, T.; et al. Urinary Prostaglandin Metabolites as Potential Biomarkers for Differentiating IgE-Mediated Food Allergy and Food Protein-Induced Enterocolitis Syndrome. Allergy, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Harris, R.A.; Bush, A.H.; Eagar, T.N.; Qian, J.; Greenwood, M.P.; Opekun, A.R.; Baldassano, R.; Guthery, S.L.; Noe, J.D.; Otley, A.; et al. Exome Sequencing Implicates DGKZ, ESRRA, and GXYLT1 for Modulating Granuloma Formation in Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 354–357. [Google Scholar] [CrossRef]
- Suenaert, P.; Maerten, P.; Van Assche, G.; Van Driessche, W.; Geboes, K.; Bulteel, V.; Simaels, J.; Augustijns, P.; Ceuppens, J.L.; Rutgeerts, P.; et al. Effects of T Cell-Induced Colonic Inflammation on Epithelial Barrier Function. Inflamm. Bowel Dis. 2010, 16, 1322–1331. [Google Scholar] [CrossRef]
- Shamji, M.H.; Durham, S.R. Mechanisms of Allergen Immunotherapy for Inhaled Allergens and Predictive Biomarkers. J. Allergy Clin. Immunol. 2017, 140, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Kabat, A.M.; Harrison, O.J.; Riffelmacher, T.; Moghaddam, A.E.; Pearson, C.F.; Laing, A.; Abeler-Dörner, L.; Forman, S.P.; Grencis, R.K.; Sattentau, Q.; et al. The Autophagy Gene Atg16l1 Differentially Regulates Treg and TH2 Cells to Control Intestinal Inflammation. Elife 2016, 5, e12444. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-W.; Cetinbas, M.; Martin, V.M.; Virkud, Y.V.; Seay, H.; Ndahayo, R.; Rosow, R.; Elkort, M.; Gupta, B.; Kramer, E.; et al. Early Infancy Dysbiosis in Food Protein-Induced Enterocolitis Syndrome: A Prospective Cohort Study. Allergy 2023, 78, 1595–1604. [Google Scholar] [CrossRef]
- McDonald, P.J.; Goldblum, R.M.; Van Sickle, G.J.; Powell, G.K. Food Protein-Induced Enterocolitis: Altered Antibody Response to Ingested Antigen. Pediatr. Res. 1984, 18, 751–755. [Google Scholar] [CrossRef]
- Shek, L.P.C.; Bardina, L.; Castro, R.; Sampson, H.A.; Beyer, K. Humoral and Cellular Responses to Cow Milk Proteins in Patients with Milk-Induced IgE-Mediated and Non-IgE-Mediated Disorders. Allergy 2005, 60, 912–919. [Google Scholar] [CrossRef]
- Su, K.-W.; Shreffler, W.G.; Yuan, Q. Gastrointestinal Immunopathology of Food Protein-Induced Enterocolitis Syndrome and Other Non-Immunoglobulin E-Mediated Food Allergic Diseases. Ann. Allergy Asthma Immunol. 2021, 126, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Kalach, N.; Kapel, N.; Waligora-Dupriet, A.-J.; Castelain, M.-C.; Cousin, M.O.; Sauvage, C.; Ba, F.; Nicolis, I.; Campeotto, F.; Butel, M.J.; et al. Intestinal Permeability and Fecal Eosinophil-Derived Neurotoxin Are the Best Diagnosis Tools for Digestive Non-IgE-Mediated Cow’s Milk Allergy in Toddlers. Clin. Chem. Lab Med. 2013, 51, 351–361. [Google Scholar] [CrossRef]
- Roca, M.; Rodriguez Varela, A.; Donat, E.; Cano, F.; Hervas, D.; Armisen, A.; Vaya, M.J.; Sjölander, A.; Ribes-Koninckx, C. Fecal Calprotectin and Eosinophil-Derived Neurotoxin in Healthy Children Between 0 and 12 Years. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 394–398. [Google Scholar] [CrossRef]
- Sipponen, T.; Kolho, K.-L. Fecal Calprotectin in Diagnosis and Clinical Assessment of Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2015, 50, 74–80. [Google Scholar] [CrossRef]
- Boyer, J.; Sgambelluri, L.; Yuan, Q. Association of Antibiotic Usage with Food Protein-Induced Enterocolitis Syndrome Development from a Caregiver’s Survey. JPGN Rep. 2021, 2, e132. [Google Scholar] [CrossRef]
- Entrala, A.; Lluch-Bernal, M.M.; Domínguez-Ortega, J.; Molero-Luis, M.; Crespo Sánchez, G.; Quirce, S.; Rodríguez-Perez, R. Measurement of Fecal Calprotectin and Zonulin as Biomarkers in Adults with Fish-Induced Food Protein-Induced Enterocolitis Syndrome. J. Investig. Allergol. Clin. Immunol. 2025, 35, 230–232. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, P.; Anvari, S.; Jimenez, A.I.; Gallego-Velez, P.; Sanchez-Fortun, C.; Jimenez-Rodriguez, T.-W.; de la Calle, F.M.; Fernandez, J.; Barrachina, J. Cytokine Variations After Positive Oral Food Challenges in Adult-Onset Food Protein-Induced Enterocolitis. Allergy, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Bursle, C.; Brown, D.; Cardinal, J.; Connor, F.; Calvert, S.; Coman, D. DMP1-CDG (CDG1e) with Significant Gastrointestinal Manifestations; Phenotype and Genotype Expansion. JIMD Rep. 2017, 34, 27–32. [Google Scholar] [CrossRef]
- Hua, A.; Slack, I.F.; O’Shea, K.; Schuler, C.F. Adults with FPIES May Face Delayed Diagnoses. J. Allergy Clin. Immunol. Glob. 2024, 3, 100304. [Google Scholar] [CrossRef]
- Banerjee, A.; Bird, J.A.; Scurlock, A.M.; Varshney, P.; Brunner, E.; Bhagwath, A.; Daines, B.; Gupta, M.; Hood, T.; Lee, M.; et al. Multicenter Food Protein-Induced Enterocolitis Syndrome (FPIES) Data Collection: Leveraging a REDCap FPIES Registry for Improved Clinical Outcomes. J. Allergy Clin. Immunol. Glob. 2025, 4, 100434. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, G.; Chapa-Rodriguez, A.; Katzka, D.A.; Spergel, J.M.; Gold, B.; Bredenoord, A.J.; Dellon, E.S.; Huang, J.; Gupta, S.K. Transition of Care of Patients with Eosinophilic Gastrointestinal Diseases: Challenges and Opportunities. Transl. Sci. Rare Dis. 2022, 6, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Angelino, G.; Romeo, E.F.; Lopetuso, L.R.; Ricca, O.; Filoni, S.; Borrelli, E.; Torroni, F.; Faraci, S.; Rea, F.; et al. A Transition Clinic Model for Inflammatory Bowel Disease between Two Tertiary Care Centers: Outcomes and Predictive Factors. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8469–8476. [Google Scholar] [CrossRef]
- Leung, Y.; Heyman, M.B.; Mahadevan, U. Transitioning the Adolescent Inflammatory Bowel Disease Patient: Guidelines for the Adult and Pediatric Gastroenterologist. Inflamm. Bowel Dis. 2011, 17, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Goodhand, J.; Hedin, C.R.; Croft, N.M.; Lindsay, J.O. Adolescents with IBD: The Importance of Structured Transition Care. J. Crohns Colitis 2011, 5, 509–519. [Google Scholar] [CrossRef]
- Rosen, D.S.; Blum, R.W.; Britto, M.; Sawyer, S.M.; Siegel, D.M. Society for Adolescent Medicine Transition to Adult Health Care for Adolescents and Young Adults with Chronic Conditions: Position Paper of the Society for Adolescent Medicine. J. Adolesc. Health 2003, 33, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Hommel, K.A.; Davis, C.M.; Baldassano, R.N. Medication Adherence and Quality of Life in Pediatric Inflammatory Bowel Disease. J. Pediatr. Psychol. 2008, 33, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Baldassano, R.; Ferry, G.; Griffiths, A.; Mack, D.; Markowitz, J.; Winter, H. Transition of the Patient with Inflammatory Bowel Disease from Pediatric to Adult Care: Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Bollegala, N.; Nguyen, G.C. Transitioning the Adolescent with IBD from Pediatric to Adult Care: A Review of the Literature. Gastroenterol. Res. Pract. 2015, 2015, 853530. [Google Scholar] [CrossRef]
- Mazur, A.; Dembinski, L.; Schrier, L.; Hadjipanayis, A.; Michaud, P.-A. European Academy of Paediatric Consensus Statement on Successful Transition from Paediatric to Adult Care for Adolescents with Chronic Conditions. Acta Paediatr. 2017, 106, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
| Adolescent FPIES | Study Country Reference no.° | Type of Study | Age Range [Years] | No. of Participants Enrolled | Relevant Findings |
|---|---|---|---|---|---|
| Clinical Features | Spain [25] | Prospective follow-up study | ≥14 | 25 | Clinical features of FPIES related to seafood |
| Spain [30] | Retrospective study | 4–12 | 80 | Clinical features of FPIES related to fish | |
| US [72] | Retrospective chart review | 0–18 | 210 | Comparative study of 2 FPIES cohorts diagnosed during different guidelines and recommendations | |
| US [73] | Ambispective study | 0.5–45 | 160 | Clinical features in a large cohort of patients | |
| US [74] | Retrospective analysis of electronic medical records | 0–17 | 210 | Identification of patterns and associations in FPIES through Market Basket Analysis | |
| Italy [75] | Case report | 14 | 1 | The first case of mollusk (oyster) FPIES in an adolescent | |
| Spain [76] | Retrospective Study | 0.11–14 | 16 | Clinical and developmental characteristics of a Spanish case series | |
| Nutritional status | Italy [28] | Non-randomized, prospective intervention study | 0–14 | 100 | Deleterious impact on nutritional status and utility of dietary counseling |
| US [27] | Retrospective study | All | 203 | Multiple sensitizations to food as risk factors for food aversion. Persistent FPIES in twenty-one percent of patients between 6 and 17 years of age | |
| Pathophysiology | US [77] | Cross-sectional study | 1–21 | 30 | Systemic innate activation and redistribution of lymphocytes related to reactions to foods |
| US, Spain and Poland [78] | Cross-sectional study | 2–16 | 20 | Adenosine and serotonin pathways in gastrointestinal inflammation | |
| Diagnosis | Italy [79] | Cross-sectional study | 0–14 | 627 | Multivariable regression model to predict suspected FA |
| Genetic Biomarkers | Spain and Italy [31] | Multicenter retrospective study | 1–12 | 38 | Identification of single-nucleotide polymorphisms and genes capable of revealing susceptibility to FPIES |
| Biomarkers | Japan [32] | Observational study | 0–15 | 27 | Potential use of fecal hemoglobin, fecal lactoferrin and fecal calprotectin to assess intestinal inflammatory status |
| Spain and Italy [80] | Observational multicenter prospective study | 0–18 | 81 | Potential diagnostic role of hematocrit, hemoglobin, platelets, and leukocytes | |
| US and Poland [81] | Cross-sectional study | 1.5–16 | 11 | Acute reactions associated with IL-17 inflammatory signature | |
| OFC safety | Italy [47] | Retrospective study | 0–17 | 202 | OFC is not safe enough for acute FPIES at home |
| Tolerance | Italy [82] | Multicenter retrospective comparative cohort study | 0–13 | 123 total (21 FPIES) | Identification of non-modifiable and modifiable factors influencing the time of immune tolerance acquisition and the occurrence of allergic march. |
| Sweden [83] | Prospective follow-up study | 0–16.5 | 113 | Achievement of tolerance | |
| Therapy | Italy and Australia [84] | Retrospective case series | 0.4–14 | 66 | Potential use of ondansentron |
| Type | Gene/SNP/Microbiota | Function/Role | Reference |
|---|---|---|---|
| Gene | DGKZ | Promotes TGF-β signaling by regulating epithelial barrier function and IgA production | [31,93,94,95] |
| Gene | SIRPA | Immunoinhibitory receptor, associated with innate immune dysfunction, upregulated in acute FPIES reactions | [31,77] |
| Gene | FLG | Functions in the epithelial barrier of skin and intestine; a crucial risk factor for food allergy | [31] |
| Gene | ↑ATG16L1 | Involved in autophagy, associated with inflammatory bowel disease (IBD). Higher expression levels in the transverse colon are associated with FPIES | [31,96] |
| Gene | ↑RBM8A | Involved in food allergies and thrombocytopenia with absent radius (TAR) syndrome. Higher expression levels in stomach and pancreas are associated with FPIES | [31] |
| Gene | ↓PIAS3 | Regulates immune response; potentially associated with FPIES pathogenesis in the pancreas | [31] |
| Gene | ↓RPIA | Regulates cellular metabolism via the pentose phosphate pathway; potentially associated with FPIES in the esophageal tract | [31] |
| Microbiota | ↓Bifidobacterium, ↓Clostridium | Modulates intestinal immune response and contributes to epithelial barrier regulation | [97] |
| ↓short-chain fatty acids (SCFAs) | Gut immune tolerance | [45] |
| Childhood | Adulthood | Adolescence | |
|---|---|---|---|
| Predominance | Slight male or equal sex distribution | Female | Female |
| Main trigger foods | Cow’s milk, fish, egg, rice, soy | Shellfish, fish | Shellfish, fish |
| Prevalent symptoms (in order of frequency) | Vomiting (100%) pallor, hypotonia, lethargy | Abdominal pain (100%) diarrhea, vomiting | Abdominal pain, diarrhea |
| Diagnosis | Diagnostic criteria panels | Clinical history and OFC | Clinical history and OFC |
| Natural history | Favorable | Less favorable | Less favorable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbato, M.; Gelsomino, M.; Bersani, G.; Mastellone, F.; Giorgio, V.; Iezzi, L.; Buonagura, R.; Caruso, C.; Miceli Sopo, S.; Rizzi, A. Food Protein-Induced Enterocolitis Syndrome Across Lifespan: Focus on Adolescence. J. Clin. Med. 2025, 14, 5799. https://doi.org/10.3390/jcm14165799
Barbato M, Gelsomino M, Bersani G, Mastellone F, Giorgio V, Iezzi L, Buonagura R, Caruso C, Miceli Sopo S, Rizzi A. Food Protein-Induced Enterocolitis Syndrome Across Lifespan: Focus on Adolescence. Journal of Clinical Medicine. 2025; 14(16):5799. https://doi.org/10.3390/jcm14165799
Chicago/Turabian StyleBarbato, Marta, Mariannita Gelsomino, Giulia Bersani, Francesco Mastellone, Valentina Giorgio, Ludovica Iezzi, Rosa Buonagura, Cristiano Caruso, Stefano Miceli Sopo, and Angela Rizzi. 2025. "Food Protein-Induced Enterocolitis Syndrome Across Lifespan: Focus on Adolescence" Journal of Clinical Medicine 14, no. 16: 5799. https://doi.org/10.3390/jcm14165799
APA StyleBarbato, M., Gelsomino, M., Bersani, G., Mastellone, F., Giorgio, V., Iezzi, L., Buonagura, R., Caruso, C., Miceli Sopo, S., & Rizzi, A. (2025). Food Protein-Induced Enterocolitis Syndrome Across Lifespan: Focus on Adolescence. Journal of Clinical Medicine, 14(16), 5799. https://doi.org/10.3390/jcm14165799






