Transoral Robotic Surgery (TORS) in Head and Neck Reconstruction
Abstract
1. Introduction
2. Review of TORS-Based Reconstruction in Head and Neck Oncology
2.1. Expansion of TORS Applications and Indications
2.2. Comparison of Traditional Surgical Approaches with TORS
2.3. Reconstructive Classification and Techniques in TORS
2.4. Oncologic and Functional Outcomes in TORS-Based Reconstruction
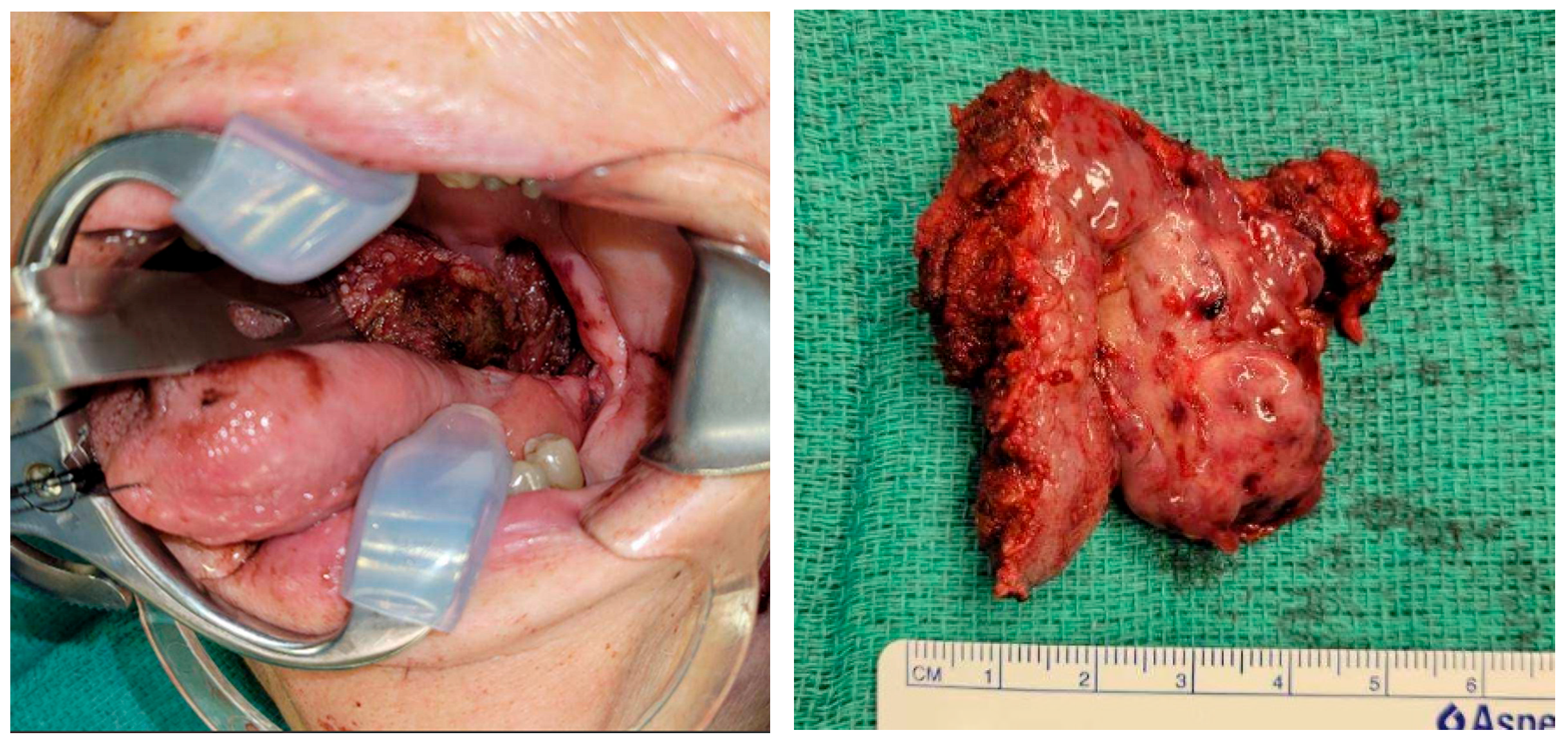
3. Case Report
3.1. Patient Presentation and Surgical Background
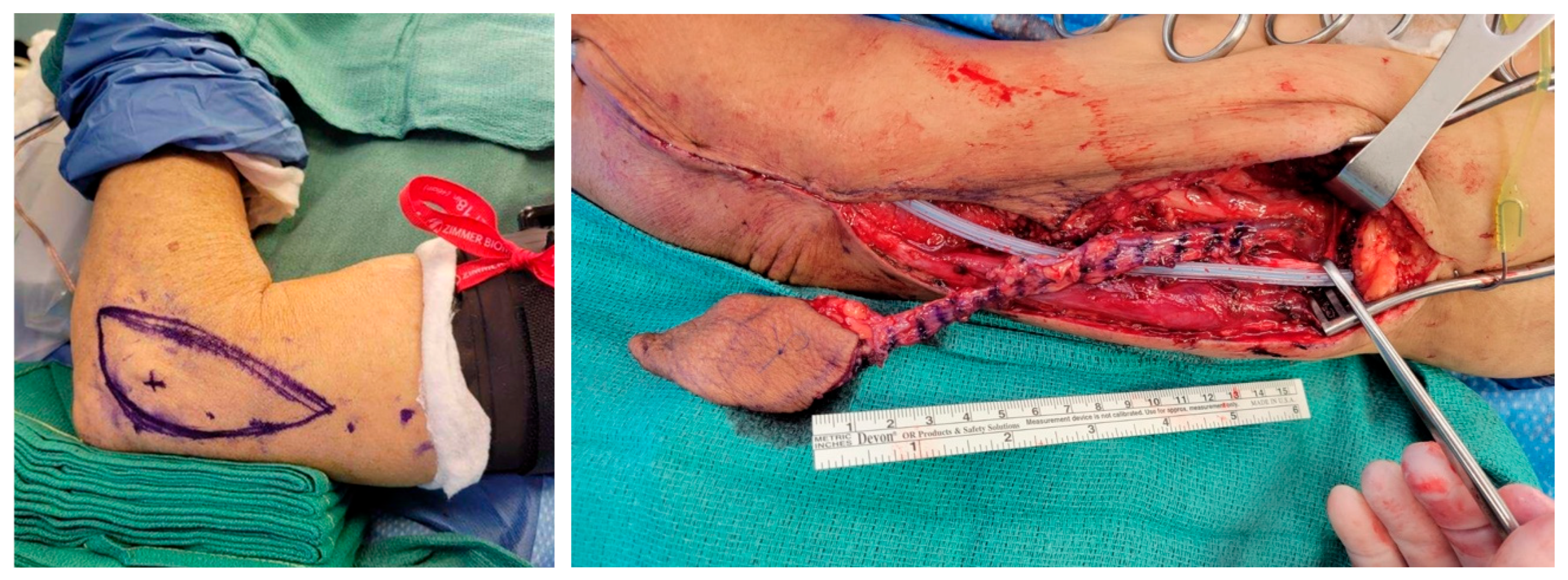
3.2. Flap Design and Harvest
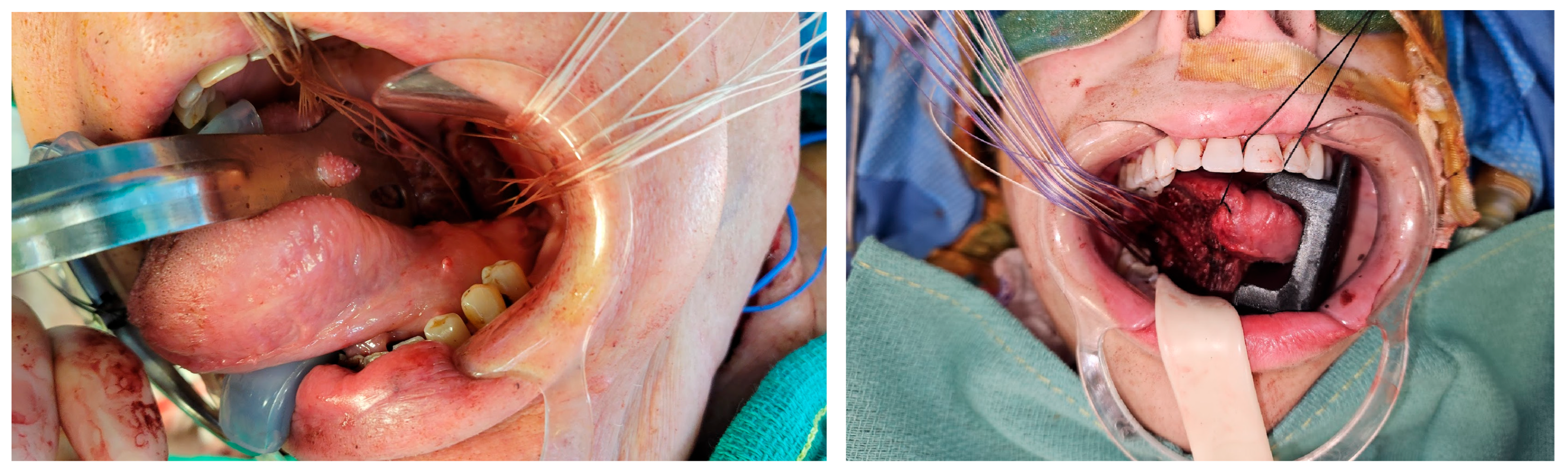
3.3. Flap Inset Technique and Microsurgical Anastomosis
3.4. Postoperative Course
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- de Almeida, J.R.; Park, R.C.; Genden, E.M. Reconstruction of transoral robotic surgery defects: Principles and techniques. J. Reconstr. Microsurg. 2012, 28, 465–472. [Google Scholar] [CrossRef]
- Hajek, M.; Yao, C. Updates in Robotic Head and Neck Reconstructive Surgery. Semin. Plast. Surg. 2023, 37, 184–187. [Google Scholar] [CrossRef]
- Meccariello, G.; Cammaroto, G.; Iannella, G.; De Vito, A.; Ciorba, A.; Bianchini, C.; Corazzi, V.; Pelucchi, S.; Vicini, C.; Capaccio, P. Transoral robotic surgery for oropharyngeal cancer in the era of chemoradiation therapy. Auris Nasus Larynx 2022, 49, 535–546. [Google Scholar] [CrossRef]
- Hedayat, F.; Jerry Htwe, K.K.; Vassiliou, L.V.; Kyzas, P. Morbidity related to the lip-split mandibulotomy approach: A systematic and narrative review. Br. J. Oral Maxillofac. Surg. 2022, 60, 430–436. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, B.W., Jr.; Weinstein, G.S.; Snyder, W.; Hockstein, N.G. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 2006, 116, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Paleri, V.; Fox, H.; Coward, S.; Ragbir, M.; McQueen, A.; Ahmed, O.; Meikle, D.; Saleh, D.; O’Hara, J.; Robinson, M. Transoral robotic surgery for residual and recurrent oropharyngeal cancers: Exploratory study of surgical innovation using the IDEAL framework for early-phase surgical studies. Head Neck 2018, 40, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Verma, V.K.; Mishra, K.S.; Bhoye, H.; Kapoor, R. Reconstruction with free flaps in robotic head-and-neck onco-surgeries. Indian J. Plast. Surg. 2018, 51, 283–289. [Google Scholar] [CrossRef]
- Awad, L.; Reed, B.; Bollen, E.; Langridge, B.J.; Jasionowska, S.; Butler, P.E.M.; Ponniah, A. The emerging role of robotics in plastic and reconstructive surgery: A systematic review and meta-analysis. J. Robot. Surg. 2024, 18, 254. [Google Scholar] [CrossRef]
- Chalmers, R.; Schlabe, J.; Yeung, E.; Kerawala, C.; Cascarini, L.; Paleri, V. Robot-Assisted Reconstruction in Head and Neck Surgical Oncology: The Evolving Role of the Reconstructive Microsurgeon. ORL 2018, 80, 178–185. [Google Scholar] [CrossRef]
- Gorphe, P.; Temam, S.; Moya-Plana, A.; Leymarie, N.; Kolb, F.; Bout-Roumazeilles, A.; Qassemyar, Q.; Benmoussa, N.; Honart, J.F. Indications and Clinical Outcomes of Transoral Robotic Surgery and Free Flap Reconstruction. Cancers 2021, 13, 2831. [Google Scholar] [CrossRef]
- Riva, F.M.G.; Kerawala, C. Perforator Flaps and Robotic Surgery for Head and Neck Reconstruction. Oral Maxillofac. Surg. Clin. N. Am. 2024, 36, 557–566. [Google Scholar] [CrossRef]
- Longfield, E.A.; Holsinger, F.C.; Selber, J.C. Reconstruction after robotic head and neck surgery: When and why. J. Reconstr. Microsurg. 2012, 28, 445–450. [Google Scholar] [CrossRef]
- Selber, J.C.; Sarhane, K.A.; Ibrahim, A.E.; Holsinger, F.C. Transoral robotic reconstructive surgery. Semin. Plast. Surg. 2014, 28, 35–38. [Google Scholar] [CrossRef]
- Chan, J.Y.W.; Chan, R.C.L.; Chow, V.L.Y.; Tsang, R.K.Y.; Wong, S.T.S.; Wei, W.I. Transoral robotic total laryngopharyngectomy and free jejunal flap reconstruction for hypopharyngeal cancer. Oral Oncol. 2017, 72, 194–196. [Google Scholar] [CrossRef]
- White, H.; Ford, S.; Bush, B.; Holsinger, F.C.; Moore, E.; Ghanem, T.; Carroll, W.; Rosenthal, E.; Sweeny, L.; Magnuson, J.S. Salvage surgery for recurrent cancers of the oropharynx: Comparing TORS with standard open surgical approaches. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 773–778. [Google Scholar] [CrossRef]
- Biron, V.L.; O’Connell, D.A.; Barber, B.; Clark, J.M.; Andrews, C.; Jeffery, C.C.; Cote, D.W.; Harris, J.; Seikaly, H. Transoral robotic surgery with radial forearm free flap reconstruction: Case control analysis. J. Otolaryngol. Head Neck Surg. 2017, 46, 20. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Costantino, A.; Rizzo, D.; Crescio, C.; Gallus, R.; Spriano, G.; Mercante, G.; Festa, B.M.; Accorona, R.; Pignataro, L.; et al. Do We Have Enough Evidence to Specifically Recommend Transoral Robotic Surgery in HPV-Driven Oropharyngeal Cancer? A Systematic Review. Pathogens 2023, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Viros Porcuna, D.; Vina Soria, C.; Vila Poyatos, J.; Palau Viarnes, M.; Malagon Lopez, P.; Gonzalez Lluch, C.; Higueras Sune, C.; Pollan Guisasola, C.M.; Carrasco Lopez, C. Oropharyngeal free flap reconstruction: Transoral robotic surgery versus open approach. Laryngoscope Investig. Otolaryngol. 2023, 8, 1564–1570. [Google Scholar] [CrossRef]
- Monroe, D.; Pyne, J.M.; McLennan, S.; Kimmis, R.; Yoon, J.; Biron, V.L. Characteristics and outcomes of transoral robotic surgery with free-flap reconstruction for oropharyngeal cancer: A systematic review. J. Robot. Surg. 2023, 17, 1287–1297. [Google Scholar] [CrossRef]
- de Almeida, J.R.; Park, R.C.; Villanueva, N.L.; Miles, B.A.; Teng, M.S.; Genden, E.M. Reconstructive algorithm and classification system for transoral oropharyngeal defects. Head Neck 2014, 36, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Barrette, L.X.; De Ravin, E.; Carey, R.M.; Mady, L.J.; Cannady, S.B.; Brody, R.M. Reconstruction following transoral robotic surgery for head and neck cancer: Systematic review. Head Neck 2022, 44, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Ruccia, F.; Mavilakandy, A.; Imtiaz, H.; Erskine, J.; Liew, Y.Y.; Ali, M.; Khajuria, A. The application of robotics in plastic and reconstructive surgery: A systematic review. Int. J. Med. Robot. 2024, 20, e2661. [Google Scholar] [CrossRef]
- Haymerle, G.; Charters, E.K.; Froggatt, C.; Wykes, J.; Palme, C.E.; Clark, J.R. Transoral robotic free flap inset in oropharyngeal cancer. Clin. Otolaryngol. 2021, 46, 642–644. [Google Scholar] [CrossRef]
- Asairinachan, A.; O’Duffy, F.; Li, M.P.; Fua, T.; Chauhan, A.; Magarey, M.J.R.; Dixon, B.J. Facial artery musculomucosal flaps in oropharyngeal reconstruction following salvage transoral robotic surgery: A review of outcomes. J. Laryngol. Otol. 2019, 133, 884–888. [Google Scholar] [CrossRef]
- Holcomb, A.J.; Richmon, J.D. Transoral robotic salvage oropharyngectomy with submental artery island flap reconstruction. Head Neck 2021, 43, E13–E19. [Google Scholar] [CrossRef] [PubMed]
- Viros Porcuna, D.; Pollan Guisasola, C.; Vina Soria, C.; Cirauqui Cirauqui, B.; Pardo Munoz, L.; Collura, F.; Mesia Nin, R. Transoral robotic surgery for squamous cell carcinoma of the oropharynx in a primarily human papillomavirus-negative patient population. Clin. Transl. Oncol. 2020, 22, 1303–1311. [Google Scholar] [CrossRef]
- Parhar, H.S.; Brody, R.M.; Shimunov, D.; Rajasekaran, K.; Rassekh, C.H.; Basu, D.; O’Malley, B.W., Jr.; Chalian, A.A.; Newman, J.G.; Loevner, L.; et al. Retropharyngeal Internal Carotid Artery Management in TORS Using Microvascular Reconstruction. Laryngoscope 2021, 131, E821–E827. [Google Scholar] [CrossRef] [PubMed]
- Charters, E.; Bogaardt, H.; Freeman-Sanderson, A.; Ballard, K.; Davies, S.; Oates, J.; Clark, J. Swallowing and communication outcomes following primary transoral robotic surgery for advanced or recurrent oropharyngeal cancer: Case series. Int. J. Speech-Lang. Pathol. 2022, 24, 407–416. [Google Scholar] [CrossRef]
- D’Andrea, G.; Bordenave, L.; Nguyen, F.; Tao, Y.; Paleri, V.; Temam, S.; Moya-Plana, A.; Gorphe, P. A prospective longitudinal study of quality of life in robotic-assisted salvage surgery for oropharyngeal cancer. Eur. J. Surg. Oncol. 2022, 48, 1243–1250. [Google Scholar] [CrossRef]
- Williamson, A.; Haywood, M.; Awad, Z. Feasibility of Free Flap Reconstruction Following Salvage Robotic-Assisted Resection of Recurrent and Residual Oropharyngeal Cancer in 3 Patients. Ear Nose Throat J. 2021, 100, 1113S–1118S. [Google Scholar] [CrossRef]
- Holsinger, F.C.; Ismaila, N.; Adkins, D.R.; Barber, B.R.; Burnette, G.; Fakhry, C.; Galloway, T.J.; Goepfert, R.P.; Miles, B.A.; Paleri, V.; et al. Transoral Robotic Surgery in the Multidisciplinary Care of Patients with Oropharyngeal Squamous Cell Carcinoma: ASCO Guideline. J. Clin. Oncol. 2025, 43, 1369–1392. [Google Scholar] [CrossRef]
- Konofaos, P.; Hammond, S.; Ver Halen, J.P.; Samant, S. Reconstructive Techniques in Transoral Robotic Surgery for Head and Neck Cancer: A North American Survey. Plast. Reconstr. Surg. 2013, 131, 188e–197e. [Google Scholar] [CrossRef]
- Hans, S.; Jouffroy, T.; Veivers, D.; Hoffman, C.; Girod, A.; Badoual, C.; Rodriguez, J.; Brasnu, D. Transoral robotic-assisted free flap reconstruction after radiation therapy in hypopharyngeal carcinoma: Report of two cases. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2359–2364. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Liu, S.A.; Lai, C.S.; Chen, Y.W.; Lu, C.T.; Yen, J.H.; Chen, I.C. Functional Outcomes and Complications of Robot-Assisted Free Flap Oropharyngeal Reconstruction. Ann. Plast. Surg. 2017, 78, S76–S82. [Google Scholar] [CrossRef]
- Sethi, R.K.V.; Chen, M.M.; Malloy, K.M. Complications of Transoral Robotic Surgery. Otolaryngol. Clin. N. Am. 2020, 53, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Holsinger, F.C.; Orosco, R.K. Hybrid supracricoid partial laryngectomy with cricohyoidoepiglottopexy via transoral robotic surgery. Laryngoscope 2019, 129, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Dobbs, T.; Swan, M.C.; Weinstein, G.S.; Goodacre, T.E. Trans-oral robotic cleft surgery (TORCS) for palate and posterior pharyngeal wall reconstruction: A feasibility study. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 97–100. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.S.; Lee, Z.-H.; Yu, J.Z. Transoral Robotic Surgery (TORS) in Head and Neck Reconstruction. J. Clin. Med. 2025, 14, 5775. https://doi.org/10.3390/jcm14165775
Song SS, Lee Z-H, Yu JZ. Transoral Robotic Surgery (TORS) in Head and Neck Reconstruction. Journal of Clinical Medicine. 2025; 14(16):5775. https://doi.org/10.3390/jcm14165775
Chicago/Turabian StyleSong, Sophia Sijia, Z-Hye Lee, and Jessie Zexi Yu. 2025. "Transoral Robotic Surgery (TORS) in Head and Neck Reconstruction" Journal of Clinical Medicine 14, no. 16: 5775. https://doi.org/10.3390/jcm14165775
APA StyleSong, S. S., Lee, Z.-H., & Yu, J. Z. (2025). Transoral Robotic Surgery (TORS) in Head and Neck Reconstruction. Journal of Clinical Medicine, 14(16), 5775. https://doi.org/10.3390/jcm14165775





