Abstract
Background/Objectives: The aim of this study is to describe fertility preservation (FP) techniques performed over the last 10 years at a tertiary hospital in northern Spain in patients under 18 diagnosed with cancer. Methods: A retrospective medical record review was conducted for patients aged 0 to 18 years diagnosed between January 2014 and December 2023 in the Pediatric Oncology Unit at a university hospital. We evaluated patient characteristics, the timing of FP procedures, and potential risk factors for ovarian insufficiency and early azoospermia. Additionally, we assessed the agreement between two gonadotoxicity risk classifications. Results: In our center, FP is more frequently offered to pubertal patients (12 to 16 years old), prior to treatment in those at high risk of subsequent gonadotoxicity (>80%), and after treatment in those at low risk (<20%). Additionally, the increased provision of FP over the last five years of the study suggests improved clinician uptake of this long-term effect of cancer treatment. Our study found weak agreement between available gonadotoxicity risk classifications, complicating the identification of FP candidates. Long-term follow-up of survivors allowed for the detection of ovarian insufficiency (1.2%) and early azoospermia (0.7%), enabling hormone replacement therapy when necessary. Hematopoietic stem cell transplantation (HSCT) emerged as a predictor of early infertility. Conclusions: Our study highlights the prevalence of gonadotoxicity in pediatric cancer patients at our center and the increasing access to FP techniques. The findings emphasize the importance of personalized medicine, tailored FP strategies based on individual risk, and long-term follow-up to assess fertility status.
1. Introduction
Over recent decades, advances in the treatment of childhood cancer have significantly improved survival rates. In Spain, the 5 year overall survival rate for pediatric cancer was 54.8% in the 1980s, rising to 84% by 2017, according to the most recent report from the National Registry of Childhood Tumors (RETI-SEHOP) [1]. The quality of life and long-term effects of antineoplastic therapy have become increasingly relevant, underscoring the importance of ongoing follow-up care for childhood cancer survivors.
Among these late effects, gonadotoxicity represents a major concern [2,3]. In males, infertility is primarily characterized by early azoospermia due to the damage to spermatogonia, the cells responsible for gamete production [4,5]. In females, it manifests as primary ovarian insufficiency (POI), resulting from both qualitative and quantitative depletion of the ovarian follicular reserve. The cumulative incidence of POI among long-term childhood cancer survivors has been reported to reach approximately 8% by the age of 40, which is significantly higher than that observed in their unaffected siblings [6].
Therefore, assessing the risk of future infertility at the time of cancer diagnosis is crucial to determine whether fertility preservation (FP) strategies should be considered [3].
Risk stratification tools have been developed to evaluate the likelihood of treatment-induced gonadotoxicity based on tumor type and therapeutic regimen [4,7,8]. The most gonadotoxic interventions include bilateral gonadal surgery, myeloablative conditioning prior to hematopoietic stem cell transplantation (HSCT), pelvic or gonadal radiotherapy (RT), and the administration of high-dose alkylating agents [3,4,8].
Fertility preservation should also be considered for patients with relapsed or secondary malignancies who require intensified, and potentially more gonadotoxic, treatments [9]. Furthermore, the risk of central hypogonadism must be evaluated in patients with brain tumors undergoing cranial or craniospinal RT or surgery involving the pituitary gland. In particular, a maximum dose (Dmax) ≥ 40 Gy to the hypothalamus or pituitary gland has been associated with impaired gonadotropin production and consequent gonadal dysfunction [3,10].
Current guidelines recommend offering FP to patients with an estimated infertility risk greater than 50% and a realistic probability of surviving at least five years [8,11]. The selection of FP techniques depends primarily on the patient’s age and pubertal status. In prepubertal girls, ovarian tissue cryopreservation (OTC) is considered an acceptable and established option [12], whereas testicular tissue cryopreservation (TTC) remains an emerging option for prepubertal boys [3]. OTC is considered a safe and effective procedure, associated with low complication rates and minimal delays in cancer treatment [9]. Although data on children under five years of age are limited [4], successful live births have been reported following prepubertal OTC, with more than 130 live births documented to date [13]. In contrast, TTC remains experimental, and no live births have been documented to date [9,14]. Due to its surgical nature and the limited amount of tissue that can be harvested, TTC is typically reserved for prepubertal boys at high risk of gonadotoxicity [15,16].
In adolescents and young adults, established FP options include sperm cryopreservation and oocyte cryopreservation. In females, oocyte cryopreservation (OC) becomes feasible after the onset of puberty and involves hormonal stimulation to retrieve mature oocytes, typically requiring a 2–3 weeks delay in treatment initiation. Therefore, OC is generally recommended for clinically stable patients or for those at high risk of relapse who have completed first-line therapy with a low or moderate risk of gonadotoxicity [3,11]. Conversely, sperm cryopreservation is a non-invasive and widely accessible technique that should be offered to all pubertal or postpubertal males who are concerned about future fertility [3].
The objective of this study is to describe the fertility preservation strategies implemented over the past 10 years in a Spanish tertiary hospital, which treats a median of 55 new pediatric cancer cases annually in patients aged 0–17 years. Specifically, we aimed to evaluate the characteristics of patients offered FP, the timing of these interventions during oncologic treatment, and potential risk factors for ovarian failure and early azoospermia in our cohort. Additionally, we analyzed the level of agreement between two widely used gonadotoxicity risk classification systems in routine clinical practice [7,8].
2. Materials and Methods
2.1. Participants and Ethics
A retrospective observational study was conducted at a tertiary hospital in northern Spain (Bilbao). The study protocol was approved by the Research Ethics Committee of the affiliated university hospital (protocol code E24/08).
Inclusion criteria: patients aged 0–17 years who were diagnosed with cancer between January 2014 and December 2023 at the Pediatric Hematology and Oncology Unit of the hospital.
Exclusion criteria: patients diagnosed with non-malignant hematologic conditions or benign tumors; those with a very low 5-year survival probability at diagnosis; patients with a known genetic syndrome prior to cancer diagnosis; patients referred from other institutions for follow-up care after receiving treatment elsewhere; and patients who were diagnosed at our center but subsequently referred to another hospital for treatment and follow-up.
2.2. Data Collection
A retrospective review of electronic medical records was conducted for all eligible patients. Demographic, clinical, and fertility-related data—including information on fertility preservation (FP) interventions—were systematically extracted.
Patients were stratified into three age groups according to pubertal status: prepubertal (<12 years), peripubertal (12–16 years), and postpubertal (>16 years) [16].
The risk of gonadal damage was assessed using two validated classification systems: the 2005 classification by Wallace et al. [7] and the 2021 Spanish consensus classification by Santaballa et al. [8]. For malignancies not explicitly addressed in these systems, exposure to alkylating agents was quantified using the validated Cyclophosphamide Equivalent Dose (CED) metric [17] and categorized according to the 2024 risk classification proposed by Talbot et al. [4].
Patients who had undergone pituitary surgery, had a diagnosis of hypopituitarism, or had received cranial or craniospinal radiotherapy with a maximum dose (Dmax) ≥ 40 Gy to the hypothalamus or pituitary gland were classified as high risk for hypogonadotropic hypogonadism [9,18].
Radiotherapy-related gonadal risk was defined as follows: a Dmax ≥ 4 Gy to the testes in males; a Dmax ≥ 15 Gy to the ovaries in prepubertal females; and a Dmax ≥ 10 Gy to the ovaries in postpubertal females [4,9].
Semen analysis results were categorized as follows: normospermic if sperm count was >15 million/mL with >40% motility; oligo-/asthenospermic if spermatozoa were present but outside these parameters; and azoospermic if no spermatozoa were detected [5].
Early primary ovarian insufficiency (POI) in females was diagnosed based on the presence of amenorrhea for at least four months and follicle-stimulating hormone (FSH) levels > 30 IU/L [19,20,21]. In these patients, the serum level of anti-Müllerian hormone (AMH), when available, was considered to assess its potential relationship with ovarian reserve [22,23,24]. In cases where FSH data were unavailable, POI was diagnosed based on an anti-Müllerian hormone (AMH) level < 0.1 ng/mL and ultrasonographic evidence of ovarian atrophy [22,23,24,25,26]. Although AMH and ultrasound are not currently included in the formal diagnostic criteria for POI, they are reliable indicators of ovarian reserve.
In males, early infertility was defined as either azoospermia or severe oligospermia on semen analysis [4,5].
2.3. Statistical Analysis
All data were anonymized and compiled using IBM SPSS Statistics (version 21, Chicago, EEUU). Statistical analyses were conducted using R software (version 4.3.3; R Core Team, 2024, New Zealand).
For qualitative variables, absolute and relative frequencies were reported. For quantitative variables, the mean and standard deviation (SD) were calculated when data followed a normal distribution; otherwise, the median and interquartile range (IQR) were reported. The Shapiro–Wilk test was used to assess the normality of quantitative variables.
Group comparisons for qualitative variables were performed using the Chi-square test or Fisher’s exact test, as appropriate. For quantitative variables, Student’s t-test was applied when distributions were normal, while the Kruskal–Wallis test was used for non-normally distributed variables.
Agreement between the two gonadotoxicity risk classification systems was assessed using Cohen’s kappa coefficient with quadratic weighting [27]. To identify potential risk factors associated with azoospermia or early POI, univariate logistic regression analyses were conducted, and model discrimination was evaluated using the area under the receiver operating characteristic (ROC) curve [28].
3. Results
3.1. Characteristics of Patients
Between January 2014 and December 2023, a total of 532 patients aged 0 to 17 years were diagnosed in the Pediatric Hematology and Oncology Unit of our university hospital (Figure 1).
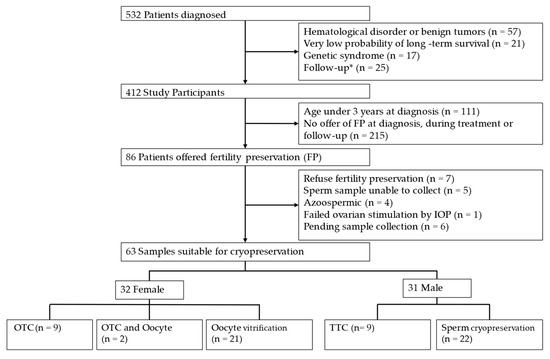
Figure 1.
Flow diagram. * Patients treated elsewhere who start follow-up in our center or diagnosed here but referred elsewhere for treatment and follow-up. OTC: Ovarian tissue cryopreservation. TTC: Testicular tissue cryopreservation.
The lower rate of fertility preservation offers could be due to factors such as limited availability of techniques early in the study period, patients’ clinical condition at diagnosis, poor prognosis in some cases, and tumor types associated with a low risk of treatment-related infertility.
The median age at diagnosis was 6 years (interquartile range [IQR]: 2–12 years). A male predominance was observed, with 230 of 412 patients being male (56%). The most frequent tumor group was extracranial solid tumors (n = 163/412, 39.6%), followed by hematologic malignancies (n = 160/412, 38.8%) and central nervous system (CNS) and intracranial tumors (n = 89/412, 21.6%). The baseline characteristics of the patients at diagnosis are summarized in Table 1 and depicted in Figure 2.

Table 1.
Descriptive characteristics of patients at diagnosis (n = 412).
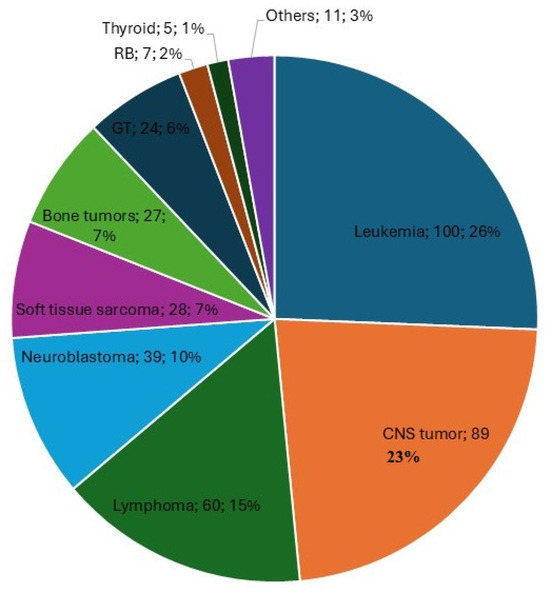
Figure 2.
Primary diseases of children and adolescents included in the study. This figure includes the tumor type, the total number of patients diagnosed with that tumor, and the corresponding percentage relative to the total number of patients. CNS: Central nervous system and intracranial tumors, GT: Gonadal tumors. RB: retinoblastoma.
3.2. Patient Characteristics at Study
Table 2 shows the characteristics of the patients as of December 2023, at the conclusion of the patient inclusion period in the study.

Table 2.
Descriptive characteristics of patients at study (n = 412).
3.3. Risk of Infertility Associated with Antineoplastic Treatment
Figure 3 illustrates the risk of infertility in patients according to the 2005 classification by Wallace et al. [7] and the 2021 Spanish consensus by Santaballa et al. [8].
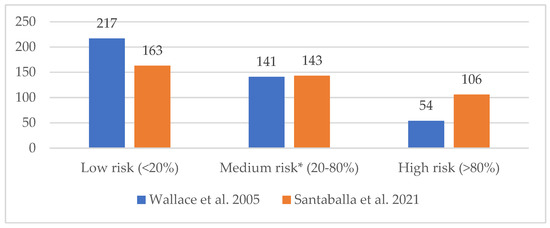
Figure 3.
Risk of infertility associated with antineoplastic treatment (n = 412), according to the classification by Wallace et al. [7] and Santaballa et al. [8]. * In Santaballa et al. consensus [8], the medium-risk category is subdivided into two subcategories: low–intermediate risk (20–50% decreased probability of pregnancy or increased risk of infertility), with 123 patients in our population; intermediate–high risk (50–80% decreased probability of pregnancy or increased risk of infertility), with 20 patients in our population.
We assessed the degree of agreement between both risk classifications [7,8] using a Cohen’s kappa coefficient with quadratic error weighting, which revealed a weak agreement (0.40 ≤ κCohen ≤ 0.59) [27]. Consequently, we opted to establish the gonadotoxicity risk for our patients based on the 2021 Spanish consensus classification [8], as it aligns with the treatment protocols at our institution and is more current than the 2005 classification by Wallace et al. [7].
Only a small proportion of patients were categorized at high risk for hypogonadotropic hypogonadism (n = 18/412, 4.4%). This was mainly due to hypopituitarism (n = 15/18, 83%), pituitary gland surgery (n = 13/18, 66.6%), or cranial/pineal RT with a Dmax ≥ 40 Gy to the pituitary gland (n = 2/18, 11%).
Gonadal RT dose was identified as a high-risk factor for gonadotoxicity in most of the patients (n = 13/16, 81%). Gonadal surgery was performed in 26 patients (n = 26/412, 6.3%), though only one required bilateral gonadal removal (n = 1/412, 0.24%). Additionally, 9.5% of patients (n = 39/412) required HSCT, and three patients (n = 3/412, 0.7%) underwent chimeric antigen receptor T-cell (CART) therapy.
3.4. Fertility Preservation
Fertility preservation options were offered to 20.8% of patients diagnosed with tumors in childhood at our center (n = 86). The majority of these patients were able to provide a sample suitable for cryopreservation (n = 63/86, 73.3%). Fertility preservation was offered equally to males (n = 44/86, 51.2%) and females (n = 42/86, 48.8%).
Among those who visited the reproductive medicine specialist (n = 79), serology for CMV, HIV, and hepatitis B/C was negative prior to sample collection in most patients (n = 66/79, 83.5%). In cases where a positive result was obtained, samples were stored separately to prevent contamination. Hormonal studies were performed at diagnosis in 30 patients (n = 30/79, 38%), with AMH levels measured in half of the female adolescents (n = 10/20, 50%). The median AMH level at diagnosis was 1.94 ng/mL (SD = 1.06).
We analyzed the association between the offer of fertility preservation and various clinical variables (Table 3). A statistically significant relationship was found between the gonadotoxicity risk group and the offer of fertility preservation (p < 0.001), with a higher frequency of offers in high-risk patients (n = 33/86, 38.4%). No significant differences were observed in the offer of fertility preservation based on tumor group (p = 0.328) or with regard to gonadal RT as part of treatment (p = 0.08).

Table 3.
Bivariate table relating fertility preservation offer to variables of interest.
3.4.1. Patient Age
A statistically significant association was found between the offer of fertility preservation and the age group at diagnosis (p < 0.001) (see Table 3), with offers being more frequent in patients older than 12 years old (n = 60/86, 70%).
A significant association was also found between the age group at fertility preservation and the technique used (p < 0.001) (see Table 4). In the prepubertal age group (<12 years), the predominant technique was OTC (57.1%, n = 8/14). In the peripubertal (12–16 years) group, sperm cryopreservation was the most common technique in men (35.6%, n = 16/45), whereas in the postpubertal (>16 years) group, oocyte cryopreservation following ovarian hormonal stimulation predominated in women (35%, n = 7/20).

Table 4.
Bivariate table relating age group at fertility preservation to fertility preservation options.
3.4.2. Time of Fertility Preservation
FP strategies at our center evolved over time: In 2017, a formal protocol for semen cryopreservation in pubertal males was implemented; in 2018, testicular tissue cryopreservation was introduced as part of a research initiative; oocyte cryopreservation became available on-site in 2015 through multidisciplinary coordination; and ovarian tissue cryopreservation, authorized since 2010, was first performed in a prepubertal girl in 2017. Prior to these dates, FP techniques could be offered to selected patients, but procedures were carried out at a referral hospital located outside our autonomous community
FP was most frequently offered prior to the initiation of antineoplastic treatment (59.3%, n = 51/86). The remaining patients were offered FP after the completion of antineoplastic treatment (23.3%, n = 20/86), during treatment (9.3%, n = 8/86), both before and after treatment (2.3%, n = 2/86), or at the time of diagnosis of relapsed disease or second malignancies (5.8%, n = 5/86). Some patients underwent FP more than four years after their cancer diagnosis (16.4%, n = 13/86); all of them had been diagnosed during the first five years of the study period (2014–2018).
A statistically significant association was found between the timing of FP and the risk of gonadotoxicity [8] (p 0.007), as detailed in Table 5. Among those who underwent FP prior to the initiation of antineoplastic treatment, the high-risk gonadotoxicity group predominated (41.2%, n= 21/51). In contrast, among those who underwent FP after treatment, most patients had a low risk of gonadotoxicity (55%, n= 11/20). All patients who underwent FP at the time of diagnosis of relapsed disease or second malignancies had a gonadotoxicity risk greater than 50% (100%, n= 5/5).

Table 5.
Bivariate table relating the timing of preservation to the risk of gonadotoxicity.
To evaluate trends in the offer of FP, patients were categorized into two subgroups based on the diagnostic period: the first five years of the study (2014–2018) and the last five years (2019–2023). A significant association was observed between the diagnosis period and the offer of FP (p 0.02), as well as between the diagnosis period and the timing of FP (p < 0.001). No significant differences were found between the type of FP technique performed across the two periods (p 0.11), as shown in Table 6.

Table 6.
Evolution of fertility preservation offer according to diagnosis period.
During the second period, physicians offered FP more frequently (55.8%, n = 48/86), and samples suitable for cryopreservation were obtained more often (68.3%, n = 43/63). Regarding the timing of FP, among patients diagnosed in the first period, FP predominantly occurred after the completion of antineoplastic treatment (42.1%, n = 16/38), whereas in the second period, FP was more commonly offered prior to the initiation of treatment (75%, n = 36/48).
3.4.3. Quality of the Samples Obtained and Complications
A total of 75 patients (87%, n = 75/86) underwent fertility preservation (FP) techniques. The majority experienced no complications related to the procedure (92%, n = 69/75). Only minor complications associated with oocyte cryopreservation (8%, n = 6/75) were recorded, which did not require additional measures, except analgesia. Among these complications, one patient experienced pain after the procedure, another had pain following multiple cycles of ovarian stimulation and repeated punctures, while four patients underwent several ovarian punctures without reporting pain associated with the technique. Table 7 outlines the quality of the samples obtained for fertility preservation.

Table 7.
Quality of the samples obtained.
3.5. Follow-Up After Treatment
Follow-up of childhood cancer survivors in the pediatric oncology department lasted between 4 and 10 years in most cases (58%, n = 239/412). During the follow-up period, 38 patients who had reached puberty were referred to a reproductive medicine specialist for fertility evaluation (9.2%, n = 38/412). Referrals were most common between 4 and 10 years after tumor diagnosis (52.6%, n = 20/38). The majority of those referred were adolescent or young adult females (65.8%, n = 25/38). The primary reason for referral was the consideration of fertility preservation (65.8%, n = 25/38), while the remainder were referred to assess their fertility status following oncological treatment (34.2%, n = 13/38). No patient requested the use of the preserved samples to have offspring during the study period.
A significant association was found between gonadotoxicity risk [8] and the assessment of reproductive status after cancer treatment (p < 0.05), as presented in Table 8.

Table 8.
Bivariate table relating post-treatment fertility assessment to gonadotoxic risk.
In the gynecological assessment following antineoplastic treatment, AMH were evaluated in the majority of women (80%, n = 20/25), with a median of 1.1 ng/mL (IQR; 0.18–2.3). AMH levels were significantly lower in adolescents with primary ovarian insufficiency (POI) (p = 0.02).
Early infertility was diagnosed in eight patients (1.9%, n = 8/412). The mean age at diagnosis was 16.38 years (SD = 2.92). The majority of these patients were women with POI (63%, n = 5/8). Of the five women diagnosed with POI, four were receiving sex hormone replacement therapy (80%). In univariate logistic regression analysis to identify factors associated with POI or early azoospermia, hematopoietic stem cell transplantation (HSCT) was statistically significant (OR 20.83, 95% CI 3.13–205.8, p = 0.003). These results suggest that a patient who underwent HSCT is 20.83 times more likely to experience ovarian failure or early azoospermia compared to a patient who did not undergo HSCT. The area under curve ROC (AUCROC) was 0.77, indicating that the diagnostic accuracy of the logistic regression model is acceptable (0.7 < AUCROC < 0.8) [28], as shown in Figure 4.
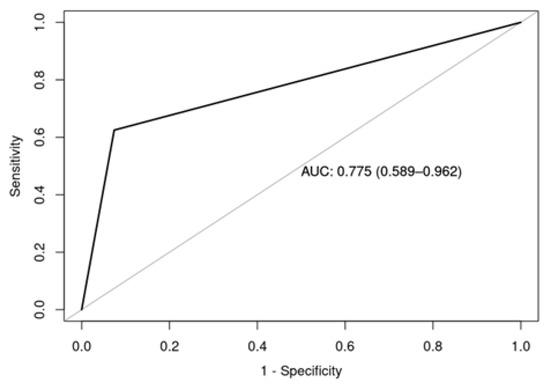
Figure 4.
Area under the curve ROC of the univariate logistic regression model to identify factors present in patients with POI or early azoospermia.
4. Discussion
Gonadotoxicity, a long-term effect of oncological treatments, has gained increasing attention in pediatric oncology due to its potential risks to fertility [4,8].
This study provides an overview of fertility preservation (FP) practices over the last decade in pediatric oncology patients at a tertiary hospital in northern Spain. The primary limitations of this study are its single-center design and the relatively smaller sample size compared to larger reference centers. Additionally, at the start of the study period, experimental techniques for prepubertal patients were introduced in our center, which limited experience with these methods in the early years of the study. Early in the study period, variability in clinical practice among physicians may have influenced FP decision-making, highlighting the importance of establishing standardized protocols and interdepartmental coordination. A further limitation is that our study did not evaluate institutional or systemic factors—such as guideline implementation or team training—that may have influenced the observed increase in FP uptake. Although follow-up visits were temporarily reduced during the COVID-19 pandemic, fertility preservation procedures remained available, and no significant disruptions in access or timing were observed for eligible patients. Despite these limitations, the results reflect a growing uptake among the healthcare team about the long-term effects of oncological treatments, as well as significant improvements in the provision of fertility preservation throughout the study period.
4.1. Characteristics of the Cohort
The findings of our study align with previous reports on the age and sex distribution of childhood cancer patients [1,29]. Although hematological tumors are the most common in national cancer registries [1,29], our cohort showed a predominance of extracranial solid tumors, followed by hematological malignancies. The presence of metastatic disease (13.6%) and relapses or second malignancies (18.4%) highlights the complexity of this patient population, which in turn influences fertility preservation decisions.
4.2. Risk of Gonadotoxicity and Fertility Preservation Techniques
The agreement between the gonadotoxicity risk classification proposed by Wallace et al. (2005) [7] and the Spanish consensus by Santaballa et al. (2021) [8] was weak. The classification by Talbot et al. (2024) [4], stratifies gonadotoxicity risk based on the treatment received, but it lacks a table correlating the most common tumor types with their associated gonadotoxicity risks based on typical therapeutic protocols. This omission limits the practical applicability of such classifications in clinical settings, as it necessitates a more time-consuming evaluation of the cumulative doses each patient will receive. Moreover, there is an ongoing challenge in that cancer treatments are continuously evolving, while FP techniques are advancing at a slower pace. As new therapeutic agents and protocols are developed, it becomes essential to assess their gonadotoxicity through longitudinal data to better understand their reproductive risks. Consequently, risk classifications may always carry inherent limitations, as gonadotoxicity data tend to lag behind information on long-term fertility outcomes. This underscores the need for future studies to develop a consensus and standardized classification system for identifying patients who are suitable candidates for fertility preservation.
In accordance with the existing literature [3,8], our study observed that fertility preservation was more frequently offered to pubertal patients, prior to treatment in those at high risk of gonadotoxicity (>80%) and at follow-up for patients at lower risk of infertility (<20%). In adolescents, the most commonly employed techniques were oocyte and sperm cryopreservation, whereas in prepubertal children, ovarian and testicular tissue cryopreservation were the only methods. These techniques were introduced in our center in 2017 and 2018, respectively, as part of a research initiative conducted by our hospital’s affiliated health research institute.
Regarding quality criteria for fertility preservation, preoperative infectious serology and hormonal assessments prior to FP procedures are recommended [4]. Additionally, the majority of patients in our study (60%) adhered to the recommendation of cryopreserving at least 10 to 15 oocytes [8].
Notably, fertility preservation was most commonly offered before the initiation of antineoplastic treatment (59%). This finding highlights the importance of early detection of gonadotoxicity risk at the time of diagnosis. Furthermore, a higher proportion of patients diagnosed in the latter half of the study period were offered fertility preservation, indicating increased uptake among the medical team at our center about the long-term reproductive risks of oncological treatments.
4.3. Long-Term Follow-Up and Outcomes
Although other studies have reported live births following fertility preservation techniques in the pediatric population [9,14,30], not enough time has elapsed to report the rate of utilization of preserved samples or live births in our cohort.
Referral to a reproductive medicine specialist during follow-up allowed us to identify cases of ovarian failure and early azoospermia, particularly in pubertal and young adult women. These patients exhibited significantly lower levels of AMH, which is consistent with early ovarian failure and reinforces its role as a valuable marker of ovarian reserve [22,23,24,25,26].
POI is often linked to early infertility, but it also results in hormonal deficits, such as estrogen deficiency, leading to long-term sequelae like osteoporosis and cardiovascular disorders [31]. Estrogen replacement therapy until the average age of menopause has been shown to reduce cardiovascular disease mortality in women with POI in the general population [21,32]. In our cohort, most women with POI (80%) received sex hormone replacement therapy in line with current recommendations.
4.4. Predictors of POI and Azoospermia
The conditioning treatment associated with hematopoietic stem cell transplantation (HSCT) was found to be a significant predictor of POI and early azoospermia, with an odds ratio of 20.83 according to the logistic regression model. This finding highlights the critical need for tailored fertility preservation strategies for patients in this high-risk subgroup.
5. Conclusions
The findings of this study underscore the importance of a multidisciplinary approach that integrates pediatric oncology and reproductive medicine to optimize long-term quality of life outcomes for childhood cancer survivors. Continued research into safe and effective fertility preservation techniques for prepubertal children and the development of predictive fertility markers remain essential.
In conclusion, our study highlights the need for individualized FP strategies based on each patient’s risk profile and emphasizes the importance of long-term fertility follow-up. Additionally, emerging national and international FP registries—such as UKSTORE in the UK—represent valuable tools for tracking FP uptake and outcomes, and can help improve risk classification through large-scale, collaborative datasets beyond the limitations of single-center studies.
This study provides a detailed overview of FP techniques applied over the past decade in a tertiary pediatric oncology center, demonstrating how FP practices have evolved and become more tailored according to individual patient risk factors. Our findings support the implementation of personalized FP strategies and highlight the critical role of systematic, long-term follow-up to monitor reproductive outcomes and inform future clinical decision-making.
Author Contributions
Conceptualization, A.C.-N. and R.L.-A.; methodology, A.C.-N.; software, A.C.-N.; validation, A.C.-N.; formal analysis, A.C.-N.; investigation, A.C.-N.; resources, A.C.-N.; data curation, A.C.-N.; writing—original draft preparation, A.C.-N.; writing—review and editing, all authors; visualization, A.C.-N.; supervision, all authors; project administration, A.C.-N.; funding acquisition, A.C.-N. and I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Integrated Health Organization of Ezkerraldea-Enkarterri-Cruces (protocol code E24/08, date of approval 30 April 2024).
Informed Consent Statement
Patient consent was waived with the consent of the Ethics Committee of our hospital. Patients diagnosed with pediatric tumors in our hospital and their families are informed and sign an informed consent form in order to include the data on the evolution of each patient in a national database called the Spanish Registry of Pediatric Tumors (RETI-SEHOP) [1]. Since this was a retrospective study with a large number of patients over 10 years and the patients or legal representatives had signed the national database registry, the ethics committee of our center accepted the waiver of informed consent to carry out the present study.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Acknowledgments
Paula Gonzalez Urdiales, Jimena de Pedro Olabarri, and Borja Santos Zorrozua.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cañete Nieto, A.; Romaguera, E.; Alfonso Comos, P.; Valero Poveda, S.; Porta Cebolla, S.; Valderrama Zurián, J.; Peris Bonet, R. Cáncer Infantil En España. Estadísticas 1980–2023; Registro Español de Tumores Infantiles (RETI-SEHOP): Valencia, Spain, 2024. [Google Scholar]
- Armstrong, G.T.; Chen, Y.; Yasui, Y.; Leisenring, W.; Gibson, T.M.; Mertens, A.C.; Stoval, M. Reduction in Late Mortality among Five-Year Survivors of Childhood Cancer. N. Engl. J. Med. 2016, 374, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.L.; Font-Gonzalez, A.; Hudson, M.M.; van Santen, H.M.; Loeffen, E.A.H.; Burns, K.C.; Quinn, G.P.; van Dulmen-den Broeder, E.; Byrne, J.; Haupt, R.; et al. Fertility Preservation for Female Patients with Childhood, Adolescent, and Young Adult Cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e45–e56. [Google Scholar] [CrossRef]
- Talbot, L.; Corkum, K.S.; McCracken, K.; Cost, N.G.; Aldrink, J.H. Oncofertility Care for Children, Adolescents, and Young Adults at Risk for Treatment-Related Fertility Loss. Pediatr. Blood Cancer 2024, 72, 1–11. [Google Scholar] [CrossRef]
- Lehmann, V.; Kutteh, W.H.; Sparrow, C.K.; Bjornard, K.L.; Klosky, J.L. Fertility-Related Services in Pediatric Oncology across the Cancer Continuum: A Clinic Overview. Support. Care Cancer 2020, 28, 3955–3964. [Google Scholar] [CrossRef]
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature Menopause in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2006, 98, 890–896. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Anderson, R.A.; Irvine, D.S. Fertility Preservation for Young Patients with Cancer: Who Is at Risk and What Can Be Offered? Lancet Oncol. 2005, 6, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Santaballa, A.; Márquez-Vega, C.; Rodríguez-Lescure, A.; Rovirosa, A.; Vázquez, L.; Zeberio-Etxetxipia, I.; Andrés, M.; Bassas, L.; Ceballos-Garcia, E.; Domingo, J.; et al. Multidisciplinary Consensus on the Criteria for Fertility Preservation in Cancer Patients. Clin. Transl. Oncol. 2022, 24, 227–243. [Google Scholar] [CrossRef]
- Burns, K.C.; Hoefgen, H.; Strine, A.; Dasgupta, R. Fertility Preservation Options in Pediatric and Adolescent Patients with Cancer. Cancer 2018, 124, 1867–1876. [Google Scholar] [CrossRef]
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of Female Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2677–2685. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Smith, A.G.; Kelsey, T.W.; Edgar, A.E.; Anderson, R.A. Fertility Preservation for Girls and Young Women with Cancer: Population-Based Validation of Criteria for Ovarian Tissue Cryopreservation. Lancet Oncol. 2014, 15, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Talbot, L.; Corkum, K.S.; McCracken, K.; Cost, N.G.; Aldrink, J.H. Fertility Preservation in Patients Undergoing Gonadotoxic Therapy or Gonadectomy: A Committee Opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Donnez, J. Fertility Preservation in Women for Medical and Social Reasons: Oocytes vs Ovarian Tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 63–80. [Google Scholar] [CrossRef]
- Duffin, K.; Neuhaus, N.; Andersen, C.Y.; Barraud-Lange, V.; Feraille, A.; Braye, A.; Eguizabal, C.; Ginsberg, J.P.; Gook, D.; Goossens, E.; et al. A 20-Year Overview of Fertility Preservation in Boys: New Insights Gained through a Comprehensive International Survey. Hum. Reprod. Open 2024, 2024, hoae010. [Google Scholar] [CrossRef]
- Mulder, R.L.; Font-Gonzalez, A.; Green, D.M.; Loeffen, E.A.H.; Hudson, M.M.; Loonen, J.; Yu, R.; Ginsberg, J.P.; Mitchell, R.T.; Byrne, J.; et al. Fertility Preservation for Male Patients with Childhood, Adolescent, and Young Adult Cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e57–e67. [Google Scholar] [CrossRef]
- Braye, A.; Tournaye, H.; Goossens, E. Setting Up a Cryopreservation Programme for Immature Testicular Tissue: Lessons Learned After More Than 15 Years of Experience. Clin. Med. Insights Reprod. Health 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Green, D.M.; Nolan, V.G.; Goodman, P.J.; Whitton, J.A. The Cyclophosphamide Equivalent Dose as an Approach for Quantifying Alkylating Agent Exposure. A Report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 2014, 61, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Fraser, O.; Crowne, E.; Tacey, M.; Cramer, R.; Cameron, A. Correlating Measured Radiotherapy Dose with Patterns of Endocrinopathy: The Importance of Minimizing Pituitary Dose. Pediatr. Blood Cancer 2022, 69, e29847. [Google Scholar] [CrossRef] [PubMed]
- Takae, S.; Furuta, S.; Iwahataa, H.; Iwahata, Y.; Keino, D.; Kanamori, R.; Oyama, K.; Tanaka, K.; Shiraishi, E.; Suzuki, Y.; et al. Cryopreservation of Paediatric Ovarian Tissue with an Updated Version of the Edinburgh Criteria for Appropriate Patient Selection. Reprod. Biomed. Online 2022, 44, 667–676. [Google Scholar] [CrossRef]
- Grellet-Grün, M.; Delepine, B.; Le Van Quyen, P.; Avérous, G.; Durlach, A.; Greze, C.; Ladureau-Fritsch, L.; Lichtblau, I.; Canepa, A.S.; Liné, A.; et al. A 16-Year Bicentric Retrospective Analysis of Ovarian Tissue Cryopreservation in Pediatric Units: Indications, Results, and Outcome. Front. Endocrinol. 2023, 14, 1–7. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Li, Z.; Krasin, M.J.; Brooke, R.J.; Wilson, C.L.; Green, D.M.; Klosky, J.L.; Barnes, N.; Clark, K.L.; Farr, J.B.; et al. Premature Ovarian Insufficiency in Childhood Cancer Survivors: A Report From the St. Jude Lifetime Cohort. J. Clin. Endocrinol. Metab. 2017, 102, 2242–2250. [Google Scholar] [CrossRef]
- Lie Fong, S.; Visser, J.A.; Welt, C.K.; de Rijke, Y.B.; Eijkemans, M.J.C.; Broekmans, F.J.; Roes, E.M.; Peters, W.H.M.; Hokken-Koelega, A.C.S.; Fauser, B.C.J.M.; et al. Serum Anti-Müllerian Hormone Levels in Healthy Females: A Nomogram Ranging from Infancy to Adulthood. J. Clin. Endocrinol. Metab. 2012, 97, 4650–4655. [Google Scholar] [CrossRef]
- Visser, J.A.; De Jong, F.H.; Laven, J.S.E.; Themmen, A.P.N. Anti-Müllerian Hormone: A New Marker for Ovarian Function. Reproduction 2006, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Vet, A.; Laven, J.S.E.; De Jong, F.H.; Themmen, A.P.N.; Fauser, B.C.J.M. Antimüllerian Hormone Serum Levels: A Putative Marker for Ovarian Aging. Fertil. Steril. 2002, 77, 357–362. [Google Scholar] [CrossRef]
- Anderson, R.A.; Mitchell, R.T.; Kelsey, T.W.; Spears, N.; Telfer, E.E.; Wallace, W.H.B. Cancer Treatment and Gonadal Function: Experimental and Established Strategies for Fertility Preservation in Children and Young Adults. Lancet Diabetes Endocrinol. 2015, 3, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian Aging: Mechanisms and Clinical Consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- de la Cruz Ortega, M.; Sainz de Aja Gallastegui, L.; de Castro Laiz, V. Cáncer Infantil en la Comunidad Autónoma Vasca 1990–2018; Unidad de Vigilancia Epidemiológica de la Comunidad Autónoma de Euskadi: Euskadi, Spain, 2023; Available online: https://www.euskadi.eus/contenidos/informacion/registros_cancer/es_def/adjuntos/Cancer_infantil_2023.pdf (accessed on 12 March 2025).
- Demeestere, I.; Simon, P.; Dedeken, L.; Moffa, F.; Tsépélidis, S.; Brachet, C.; Delbaere, A.; Devreker, F.; Ferster, A. Live Birth after Autograft of Ovarian Tissue Cryopreserved during Childhood. Hum. Reprod. 2015, 30, 2107–2109. [Google Scholar] [CrossRef]
- Van Dorp, W.; Mulder, R.L.; Kremer, L.C.M.; Hudson, M.M.; Van Den Heuvel-Eibrink, M.M.; Van Den Berg, M.H.; Levine, J.M.; Van Dulmen-den Broeder, E.; Di Iorgi, N.; Albanese, A.; et al. Recommendations for Premature Ovarian Insufficiency Surveillance for Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Report From the International Late Effects of Childhood Cancer Guideline Harmonization Group in Collaboration With the PanCareSurFup Consortium. J. Clin. Oncol. 2016, 34, 3440–3450. [Google Scholar] [CrossRef]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).