Decoding Sepsis: A 16-Year Retrospective Analysis of Activation Patterns, Mortality Predictors, and Outcomes from a Hospital-Wide Sepsis Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Sepsis Protocol Activation System
- Standardized screening and activation criteria based on systemic inflammatory response syndrome (SIRS) parameters and evidence of organ dysfunction;
- An electronic protocol that could be activated by any physician throughout the hospital upon recognition of suspicious cases;
- A centralized alert system with automated notification of potential sepsis cases during the first 72 h;
- Laboratory and microbiological alert integration;
- An electronic documentation system for protocol activations.
2.3. Patient Selection and Data Collection
- Demographic information: Age and sex;
- Clinical parameters at activation: SIRS criteria met (temperature, heart rate, respiratory rate, white blood cell count);
- Organ dysfunction criteria at activation: Hypotension, hypoxemia, altered mental status, oliguria, elevated creatinine, coagulopathy, hyperbilirubinemia, and hyperlactatemia;
- Sepsis classification: Severe sepsis (sepsis to our protocol) vs. septic shock according to SEPSIS-2 definition;
- Activation details: Time of day, hospital location at activation, and department initiating activation;
- Resource utilization outcomes: ICU admission, length of stay in hospital, and ICU;
- Clinical outcomes: In-hospital mortality and ICU mortality.
2.4. Sepsis Definitions
2.5. Definitions
2.6. Statistical Analysis
2.7. Missing Data Management
2.8. Ethical Approval
2.9. Declaration of Generative AI and AI-Assisted Technologies in the Writing Process:
3. Results
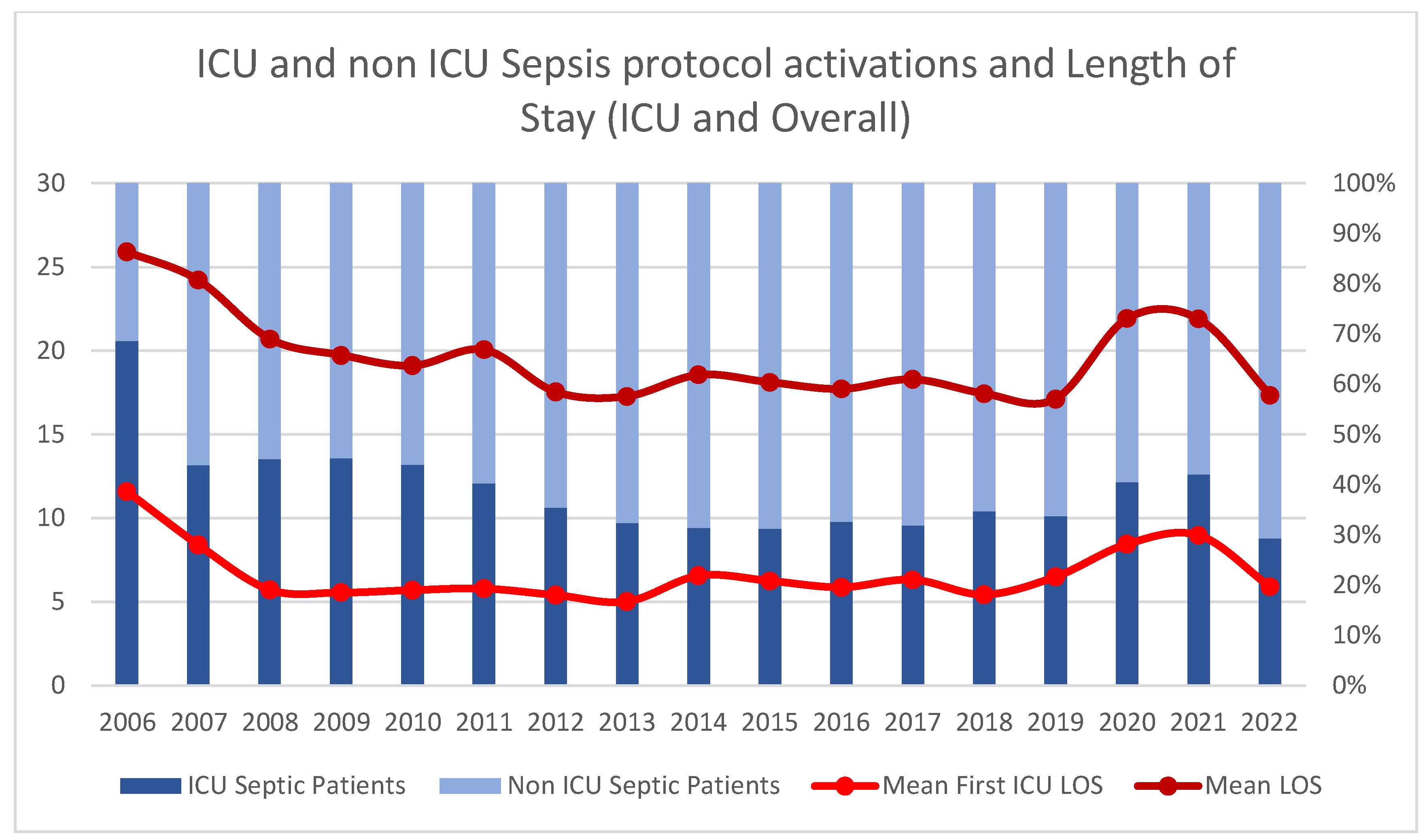
3.1. Patient Characteristics and Protocol Activation Patterns
3.2. SIRS, Organ Dysfunction Criteria and Severity at Activation
3.3. Hospital Location and Timeframe Activation
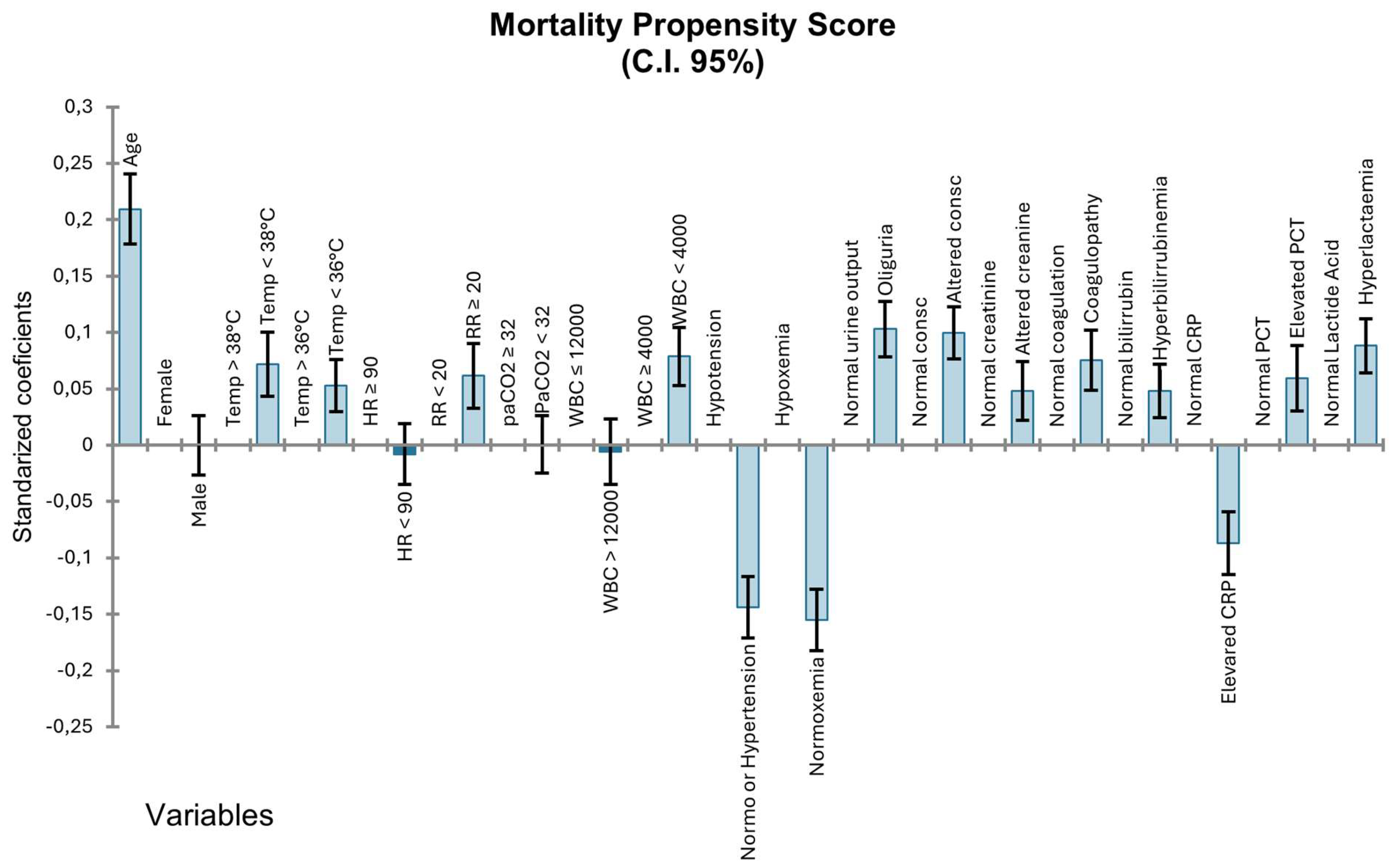
3.4. Mortality Analysis
3.5. Resource Utilization
3.6. Antibiotic Administration Timing
4. Discussion
Recommendations
- What must be done:
- Implement comprehensive hospital-wide sepsis protocols with consistent activation criteria;
- Establish a 24/7 protocol activation capability across all hospital departments;
- Ensure multidisciplinary team involvement, including critical care, infectious diseases, and pharmacy specialists.
- What should be done:
- Achieve antibiotic administration within 1 h of protocol activation in >50% of cases;
- Implement systematic staff education programs with periodic reinforcement;
- Establish continuous quality monitoring with feedback mechanisms.
- What may be done:
- Consider integration of advanced biomarkers (PCT, PSP, MDW) for enhanced early detection;
- Explore the implementation of machine learning algorithms for automated sepsis screening;
- Develop post-sepsis syndrome management protocols.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aOR | Adjusted odds ratio |
| CEIC | Clinical Research Ethics Committee |
| CI | Confidence interval |
| COVID-19 | Coronavirus disease 2019 |
| ED | Emergency department |
| ER | Emergency room |
| ICU | Intensive care unit |
| IdISBa | Health Research Institute of the Balearic Islands |
| IQR | Interquartile range |
| LOS | Length of stay |
| MSU | Multidisciplinary sepsis unit |
| OR | Odds ratio |
| PaCO2 | Partial pressure of carbon dioxide |
| PaO2/FiO2 | Ratio of arterial oxygen partial pressure to fractional inspired oxygen |
| PIMIS | Computerized Multidisciplinary and Integral Sepsis Protocol |
| SD | Standard deviation |
| SIRS | Systemic inflammatory response syndrome |
| WBC | White blood cell count |
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Rhee, C.; Jones, T.M.; Hamad, Y.; Pande, A.; Varon, J.; O’Brien, C.; Anderson, D.J.; Warren, D.K.; Dantes, R.B.; Epstein, L.; et al. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw. Open 2019, 2, e187571. [Google Scholar] [CrossRef]
- Paoli, C.J.; Reynolds, M.A.; Sinha, M.; Gitlin, M.; Crouser, E. Epidemiology and Costs of Sepsis in the United States-An Analysis Based on Timing of Diagnosis and Severity Level. Crit. Care Med. 2018, 46, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.; González Del Castillo, J.; Martínez-Martínez, M.; Plata-Menchaca, E.P.; Larrosa, M.N. Time to Decision in Sepsis. Rev. Esp. Quim. Quimioter. 2023, 36, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Ruppe, E.; Ferrer, R. Focus on Sepsis: New Concepts and Findings in Sepsis Care. Intensive Care Med. 2018, 44, 1997–1999. [Google Scholar] [CrossRef]
- Giglio, A.; Aranda, M.; Ferre, A.; Borges-Sá, M.M. Adult Code Sepsis: A Narrative Review of Its Implementation and Impact. J. Intensive Care Med. 2024. [Google Scholar] [CrossRef]
- Chuang, C.L.; Yeh, H.T.; Niu, K.Y.; Chen, C.B.; Seak, C.J.; Yen, C.C. Diagnostic Performances of Procalcitonin and C-Reactive Protein for Sepsis: A Systematic Review and Meta-Analysis. Eur. J. Emerg. Med. 2025, 32, 248–258. [Google Scholar] [CrossRef]
- Paraskevas, T.; Chourpiliadi, C.; Demiri, S.; Micahilides, C.; Karanikolas, E.; Lagadinou, M.; Velissaris, D. Presepsin in the Diagnosis of Sepsis. Clin. Chim. Acta 2023, 550, 117588. [Google Scholar] [CrossRef]
- Martínez, S.L.; Del Castillo, J.G. Usefulness of Monocyte Distribution Width (MDW) as a Sepsis Biomarker. Rev. Española Quimioter. 2022, 35, 2. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Corbett, S.W.; Steele, R.; Banta, J.; Clark, R.T.; Hayes, S.R.; Edwards, J.; Cho, T.W.; Wittlake, W.A. Implementation of a Bundle of Quality Indicators for the Early Management of Severe Sepsis and Septic Shock Is Associated with Decreased Mortality. Crit. Care Med. 2007, 35, 1105–1112. [Google Scholar] [CrossRef]
- Damiani, E.; Donati, A.; Serafini, G.; Rinaldi, L.; Adrario, E.; Pelaia, P.; Busani, S.; Girardis, M. Effect of Performance Improvement Programs on Compliance with Sepsis Bundles and Mortality: A Systematic Review and Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0125827. [Google Scholar] [CrossRef] [PubMed]
- de Dios, B.; Borges, M.; Smith, T.D.; del Castillo, A.; Socias, A.; Gutiérrez, L.; Nicolás, J.; Lladó, B.; Roche, J.A.; Díaz, M.P.; et al. Computerised Sepsis Protocol Management. Description of an Early Warning System. Enfermedades Infecc. Microbiol. Clin. 2018, 36, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef]
- Levy, M.M.; Gesten, F.C.; Phillips, G.S.; Terry, K.M.; Seymour, C.W.; Prescott, H.C.; Friedrich, M.; Iwashyna, T.J.; Osborn, T.; Lemeshow, S. Mortality Changes Associated with Mandated Public Reporting for Sepsis the Results of the New York State Initiative. Am. J. Respir. Crit. Care Med. 2018, 198, 1406–1412. [Google Scholar] [CrossRef]
- Ferreras Amez, J.; Arribas Entrala, B.; Sarrat Torres, M.; García Noaín, A.; Claudevilla Martínez, A.; Colás Oros, C.; Aldrén Pérez, B.; Rodero Álvarez, F. Evaluación de los resultados antes y después de la implantación del Código Sepsis en Aragón. Emergencias 2017, 29, 154–160. [Google Scholar]
- Méndez, R.; Figuerola, A.; Chicot, M.; Barrios, A.; Pascual, N.; Ramasco, F.; Rodríguez, D.; García, Í.; Von Wernitz, A.; Zurita, N.; et al. Código Sepsis: Esquivando La Mortalidad En Un Hospital Terciario. Rev. Española Quimioter. 2021, 35, 43. [Google Scholar] [CrossRef]
- Méndez, R.; Figuerola, A.; Ramasco, F.; Chicot, M.; Pascual, N.F.; García, Í.; von Wernitz, A.; Zurita, N.D.; Semiglia, A.; Pizarro, A.; et al. Decrease in Mortality after the Implementation of a Hospital Model to Improve Performance in Sepsis Care: Princess Sepsis Code. J. Pers. Med. 2024, 14, 149. [Google Scholar] [CrossRef]
- Churpek, M.M.; Snyder, A.; Han, X.; Sokol, S.; Pettit, N.; Howell, M.D.; Edelson, D.P. Quick Sepsis-Related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients Outside the Intensive Care Unit. Am. J. Respir. Crit. Care Med. 2017, 195, 906–911. [Google Scholar] [CrossRef]
- Norman, B.C.; Cooke, C.R.; Wes Ely, E.; Graves, J.A. Sepsis-Associated 30-Day Risk-Standardized Readmissions: Analysis of a Nationwide Medicare Sample. Crit. Care Med. 2017, 45, 1130–1137. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and Clinical Management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef]
- Filbin, M.R.; Lynch, J.; Gillingham, T.D.; Thorsen, J.E.; Pasakarnis, C.L.; Nepal, S.; Matsushima, M.; Rhee, C.; Heldt, T.; Reisner, A.T. Presenting Symptoms Independently Predict Mortality in Septic Shock: Importance of a Previously Unmeasured Confounder. Crit. Care Med. 2018, 46, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Ribeiro, O.; Aragão, I.; Costa-Pereira, A.; Cardoso, T. Differences in Compliance with Surviving Sepsis Campaign Recommendations According to Hospital Entrance Time: Day versus Night. Crit. Care 2013, 17, R79. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Rumbus, Z.; Matics, R.; Hegyi, P.; Zsiboras, C.; Szabo, I.; Illes, A.; Petervari, E.; Balasko, M.; Marta, K.; Miko, A.; et al. Fever Is Associated with Reduced, Hypothermia with Increased Mortality in Septic Patients: A Meta-Analysis of Clinical Trials. PLoS ONE 2017, 12, e0170152. [Google Scholar] [CrossRef]
- Drewry, A.M.; Fuller, B.M.; Bailey, T.C.; Hotchkiss, R.S. Body Temperature Patterns as a Predictor of Hospital-Acquired Sepsis in Afebrile Adult Intensive Care Unit Patients: A Case-Control Study. Crit. Care 2013, 17, R200. [Google Scholar] [CrossRef]
- Motzkus, C.A.; Chrysanthopoulou, S.A.; Luckmann, R.; Rincon, T.A.; Lapane, K.L.; Lilly, C.M. ICU Admission Source as a Predictor of Mortality for Patients With Sepsis. J. Intensive Care Med. 2018, 33, 510–516. [Google Scholar] [CrossRef]
- Powell, E.S.; Khare, R.K.; Courtney, D.M.; Feinglass, J. Lower Mortality in Sepsis Patients Admitted through the ED vs Direct Admission. Am. J. Emerg. Med. 2012, 30, 432–439. [Google Scholar] [CrossRef]
- Rahmel, T.; Schmitz, S.; Nowak, H.; Schepanek, K.; Bergmann, L.; Halberstadt, P.; Hörter, S.; Peters, J.; Adamzik, M. Long-Term Mortality and Outcome in Hospital Survivors of Septic Shock, Sepsis, and Severe Infections: The Importance of Aftercare. PLoS ONE 2020, 15, e0228952. [Google Scholar] [CrossRef]
- Prescott, H.C.; Angus, D.C. Enhancing Recovery From Sepsis: A Review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Al Rahmoun, M.; Sabaté-Elabbadi, A.; Guillemot, D.; Brun-Buisson, C.; Watier, L. Impacts of the COVID-19 Pandemic on Sepsis Incidence, Etiology and Hospitalization Costs in France: A Retrospective Observational Study. BMC Infect. Dis. 2025, 25, 627. [Google Scholar] [CrossRef] [PubMed]
- Ramasco, F.; Figuerola, A.; Mendez, R.; Serrano, D.R.; von Wernitz, A.; Hernández-Aceituno, A.; Sáez, C.; Cardeñoso, L.; Martin, E.; García-Vázquez, N.; et al. Initial Clinical Outcomes and Prognostic Variables in the Implementation of a Code Sepsis in a High Complexity University Hospital. Rev. Española Quimioter. 2019, 32, 238. [Google Scholar]
- Reshetnikov, A.; Abaeva, O.; Prisyazhnaya, N.; Romanova, T.; Romanov, S.; Sobolev, K.; Manukyan, A. The Impact of the COVID-19 Pandemic on Burnout Levels among Healthcare Workers: A Comparative Analysis of the Pandemic Period and Post-Pandemic Period. Heliyon 2024, 10, e36769. [Google Scholar] [CrossRef]
- Evans, R.A.; McAuley, H.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, Cognitive, and Mental Health Impacts of COVID-19 after Hospitalisation (PHOSP-COVID): A UK Multicentre, Prospective Cohort Study. Lancet Respir. Med. 2021, 9, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Deutschman, C.S.; Legrand, M. Facing COVID-19 in the ICU: Vascular Dysfunction, Thrombosis, and Dysregulated Inflammation. Intensive Care Med. 2020, 46, 1105–1108. [Google Scholar] [CrossRef]
- Oladunjoye, O.; Gallagher, M.; Wasser, T.; Oladunjoye, A.; Paladugu, S.; Donato, A. Mortality Due to COVID-19 Infection: A Comparison of First and Second Waves. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 747. [Google Scholar] [CrossRef]
- Gasch-Illescas, A.; Calle-Serrano, M.; Vallejo-Vaz, A.J.; Praena-Fernández, J.M.; Guerrero, J.A.; Calderón, E.J.; Pollán, M.; Medrano, F.J. Impact of the First Wave of the COVID-19 Pandemic on Non-COVID Inpatient Care in Southern Spain. Sci. Rep. 2023, 13, 1634. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]

| Concept | Operational Definition |
|---|---|
| SIRS Criteria | Temperature > 38 °C or <36 °C heart rate > 90 beats/minute respiratory rate > 20 breaths/minute or PaCO2 < 32 mmHg white blood cell count > 12,000/mm3 or <4000/mm3 or >10% immature bands |
| Organ dysfunction criteria | Systolic blood pressure < 90 mmHg or mean arterial pressure < 70 mmHg or decrease > 40 mmHg from baseline PaO2/FiO2 < 300 urine output < 0.5 mL/kg/h for at least 2 h creatinine increase > 0.5 mg/dL from baseline international normalized ratio > 1.5 or activated partial thromboplastin time > 60 s platelet count < 100,000/mm3 total bilirubin > 4 mg/dL lactate > 3 mmol/L altered mental status |
| Sepsis | Presence of at least two SIRS criteria plus at least one organ dysfunction criterion |
| Septic shock | Severe sepsis with hypotension requiring vasopressor therapy despite adequate fluid resuscitation |
| Item | Overall | Sepsis | Septic Shock | p-Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Frequency | 100% | 89% | 11% | - |
| Male index | 60.97% | 61.05% | 60.23% | 0.561 |
| Age | 65.75 years | 65.42 years | 68.99 years | <0.0001 |
| SIRS | ||||
| Temperature > 38 °C | 48.55% | 50.05% | 33.8% | <0.0001 |
| Temperature < 36 °C | 7.86% | 7.07% | 15.56% | <0.0001 |
| Heart Ratio > 90 | 75.57% | 75.04% | 80.71% | <0.0001 |
| Respiratory rate > 20 | 50.37% | 49.42% | 59.79% | <0.0001 |
| pCO2 < 32 | 15.04% | 13.7% | 28.15% | <0.0001 |
| WBC > 12.000 | 61.09% | 61.77% | 54.43% | <0.0001 |
| WBC < 4.000 | 8.84% | 8.15% | 15.71% | <0.0001 |
| C-Reactive Protein 2x NUL | 72.6% | 72.58% | 72.89% | 0.804 |
| Procalcitonin 2x NUL | 35.83% | 34.15% | 52.34% | <0.0001 |
| Disfunctions | ||||
| Hypotension | 40.52% | 34.47% | 100% | <0.0001 |
| Hypoxemia | 36.47% | 36.36% | 37.52% | 0.396 |
| Oliguria | 19.0% | 16.13% | 48.4% | <0.0001 |
| Altered mental status | 15.32% | 14.02% | 28.15% | <0.0001 |
| Elevated creatinine | 25.45% | 23.31% | 46.54% | <0.0001 |
| Coagulopathy | 20.53% | 21.59% | 31.79% | <0.0001 |
| Hyperbilirubinemia | 4.83% | 4.42% | 8.86% | <0.0001 |
| Hyperlacticaemia | 17.02% | 8.57% | 100% | <0.0001 |
| Activation criteria | ||||
| Mean activation criteria | 5.57 | 5.3 | 8.15 | <0.0001 |
| Disfunctions | 1.81 | 1.59 | 4.01 | <0.0001 |
| Item | Ward | ICU | ER | p-Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Male index | 59.4% | 63% | 59.8% | ICU-ER = 0.001 ICU-Ward < 0.001 ER-Ward = 0.732 |
| Age (years) | 65.92 | 64.43 | 67.19 | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| SIRS | ||||
| Temperature > 38 °C | 50.3% | 48.9% | 46.7% | ICU-ER = 0.02 ICU-Ward = 0.168 ER-Ward = 0.001 |
| Temperature < 36 °C | 5.5% | 11.5% | 5.5% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward = 0.91 |
| Heart Ratio > 90 | 72.1% | 78.4% | 74.9% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward = 0.003 |
| Respiratory rate > 20 | 44.9% | 56.2% | 47.9% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward = 0.005 |
| pCO2 < 32 | 15.4% | 8.7% | 22.2% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| WBC > 12.000 | 63.1% | 61% | 59.6% | ICU-ER = 0.136 ICU-Ward = 0.034 ER-Ward = 0.001 |
| WBC < 4.000 | 9.7% | 10.3% | 6.5% | ICU-ER < 0.0001 ICU-Ward = 0.313 ER-Ward < 0.0001 |
| C-Reactive Protein | 75.3% | 76.9% | 65.4% | ICU-ER < 0.0001 ICU-Ward = 0.071 ER-Ward < 0.0001 |
| Procalcitonin | 31.2% | 47.1% | 26.1% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| Disfunctions | ||||
| Hypotension | 31.9% | 50.1% | 36% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| Hypoxemia | 32.3% | 41.2% | 34.2% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward = 0.061 |
| Oliguria | 13.4% | 29.9% | 10.6% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| Altered mental status | 14.1% | 16.3% | 15.1% | ICU-ER = 0.08 ICU-Ward = 0.003 ER-Ward = 0.211 |
| Elevated creatinine | 24.3% | 30.2% | 20.7% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| Coagulopathy | 18.6% | 34.3% | 11.8% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| Hyperbilirubinemia | 4% | 6.9% | 3.1% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward = 0.027 |
| Hyperlacticaemia | 13% | 17.8% | 19.3% | ICU-ER = 0.043 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| Activation criteria | ||||
| Mean activation criteria | 5.192 | 6.257 | 5.055 | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| Disfunctions | 1.516 | 2.267 | 1.508 | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward = 0.498 |
| Outcomes | ||||
| Shock | 6.1% | 12.9% | 7.4% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward = 0.014 |
| LOS | 15.3447 | 28.331 | 11.060 | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward < 0.0001 |
| Mortality | 11% | 23% | 10.9% | ICU-ER < 0.0001 ICU-Ward < 0.0001 ER-Ward = 0.845 |
| Activation Criteria | Odds Ratio (CI 95%) |
|---|---|
| Temperature > 38 °C | 0.595 (0.543–0.652) |
| Temperature < 36 °C | 2.267 (1.976–2.602) |
| Heart Ratio > 90 | 1.082 (9.742–1.202) |
| Respiratory Ratio > 20 | 1.769 (1.614–1.939) |
| PaCO2 < 32 | 1.335 (1.187–1.501) |
| WBC > 12.000 | 0.839 (0.766–0.919) |
| WBC < 4.000 | 1.781 (1.553–2.042) |
| C-Reactive Protein | 0.691 (0.628–0.760) |
| Procalcitonin | 1.199 (1.094–1.314) |
| Hypotension | 2.140 (1.955–2.343) |
| Hypoxemia | 1.784 (1.630–1.952) |
| Oliguria | 2.609 (2.363–2.881) |
| Elevated creatinine | 1.729 (1.571–1.902) |
| Coagulopathy | 1.713 (1.431–2.051) |
| Hyperlacticaemia | 2.935 (2.61–2.665) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges-Sa, M.; Giglio, A.; Aranda, M.; Socias, A.; del Castillo, A.; Mena, J.; Franco, S.; Ortega, M.; Nieto, Y.; Estrada, V.; et al. Decoding Sepsis: A 16-Year Retrospective Analysis of Activation Patterns, Mortality Predictors, and Outcomes from a Hospital-Wide Sepsis Protocol. J. Clin. Med. 2025, 14, 5759. https://doi.org/10.3390/jcm14165759
Borges-Sa M, Giglio A, Aranda M, Socias A, del Castillo A, Mena J, Franco S, Ortega M, Nieto Y, Estrada V, et al. Decoding Sepsis: A 16-Year Retrospective Analysis of Activation Patterns, Mortality Predictors, and Outcomes from a Hospital-Wide Sepsis Protocol. Journal of Clinical Medicine. 2025; 14(16):5759. https://doi.org/10.3390/jcm14165759
Chicago/Turabian StyleBorges-Sa, Marcio, Andres Giglio, Maria Aranda, Antonia Socias, Alberto del Castillo, Joana Mena, Sara Franco, Maria Ortega, Yasmina Nieto, Victor Estrada, and et al. 2025. "Decoding Sepsis: A 16-Year Retrospective Analysis of Activation Patterns, Mortality Predictors, and Outcomes from a Hospital-Wide Sepsis Protocol" Journal of Clinical Medicine 14, no. 16: 5759. https://doi.org/10.3390/jcm14165759
APA StyleBorges-Sa, M., Giglio, A., Aranda, M., Socias, A., del Castillo, A., Mena, J., Franco, S., Ortega, M., Nieto, Y., Estrada, V., de la Rica, R., & Son Llatzer’s Multidisciplinary Sepsis Unit. (2025). Decoding Sepsis: A 16-Year Retrospective Analysis of Activation Patterns, Mortality Predictors, and Outcomes from a Hospital-Wide Sepsis Protocol. Journal of Clinical Medicine, 14(16), 5759. https://doi.org/10.3390/jcm14165759







