Trends and Projections of the Prevalence of Diabetes Mellitus in Pregnancy and Fetal–Neonatal Metabolic Disorders, 2010–2035: A Nationwide Population-Based Study from Hungary
Abstract
1. Introduction
DMP Care in Hungary
2. Materials and Methods
2.1. Methods
2.2. Data
2.3. Statistical Analysis
2.4. Ethical Considerations
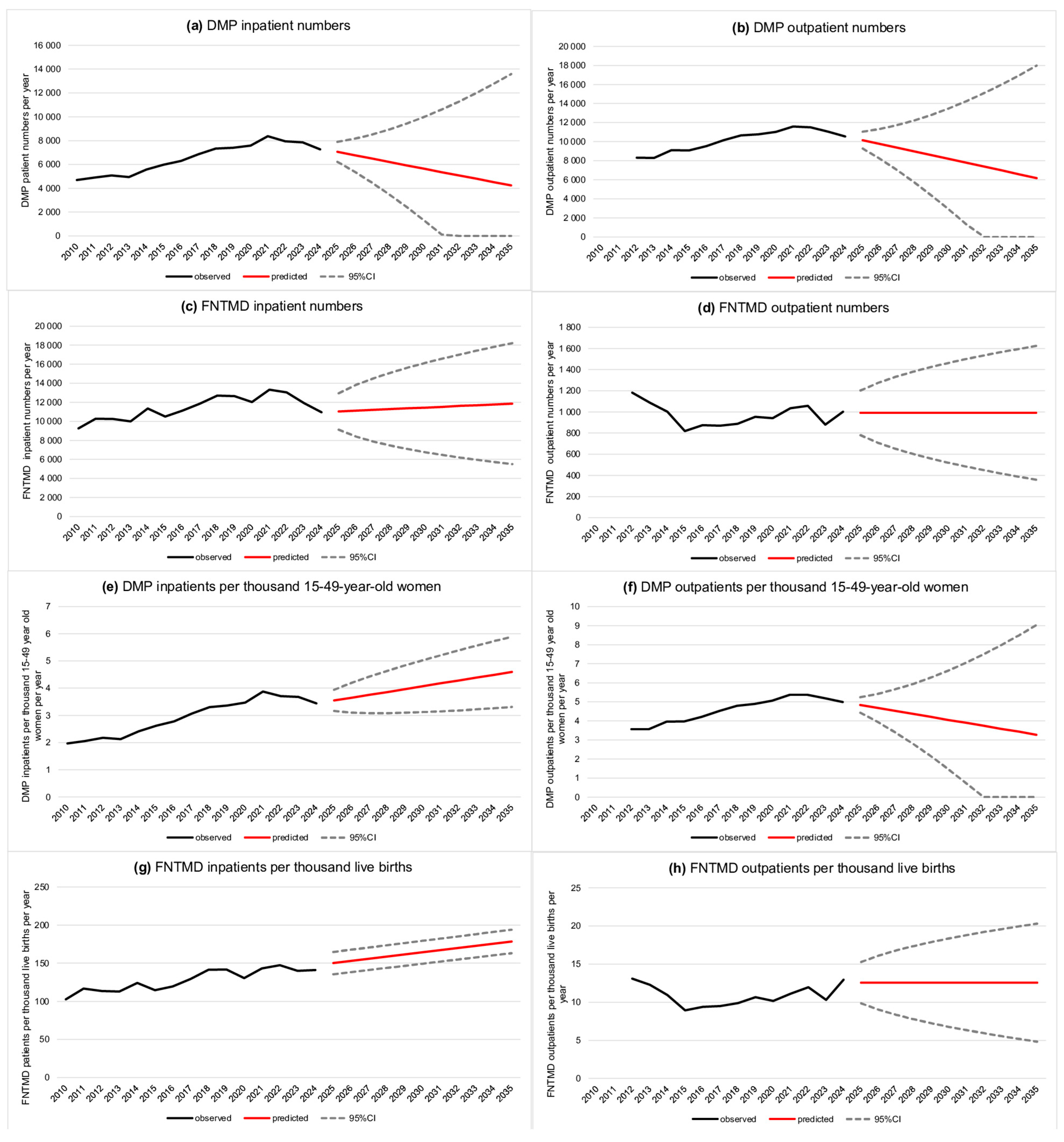
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAPC | Annual average percentage change |
| APC | Annual percentage change |
| DMP | Diabetes mellitus in pregnancy |
| FNTMDs | Fetal and neonatal transient metabolic disorders |
| GDM | Gestational diabetes mellitus |
| NDGH | National Directorate General for Hospitals |
| NHIFA | National Health Insurance Fund Administration |
References
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://diabetesatlas.org (accessed on 19 June 2025).
- Tönnies, T.; Rathmann, W.; Hoyer, A.; Brinks, R.; Kuss, O. Quantifying the underestimation of projected global diabetes prevalence by the International Diabetes Federation (IDF) Diabetes Atlas. BMJ Open Diabetes Res. Care 2021, 9, e002122. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and management of gestational diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef]
- Damm, P.; Houshmand-Oeregaard, A.; Kelstrup, L.; Lauenborg, J.; Mathiesen, E.R.; Clausen, T.D. Gestational diabetes mellitus and long-term consequences for mother and offspring: A view from Denmark. Diabetologia 2016, 59, 1396–1399. [Google Scholar] [CrossRef]
- Bartáková, V.; Chalásová, K.; Pácal, L.; Ťápalová, V.; Máchal, J.; Janků, P.; Kaňková, K. Metabolic Syndrome Prevalence in Women with Gestational Diabetes Mellitus in the Second Trimester of Gravidity. J. Clin. Med. 2024, 13, 1260. [Google Scholar] [CrossRef]
- Tsironikos, G.I.; Zakynthinos, G.E.; Tatsioni, A.; Tsolaki, V.; Kagias, I.G.; Potamianos, P.; Bargiota, A. Gestational Metabolic Risk: A Narrative Review of Pregnancy-Related Complications and of the Effectiveness of Dietary, Exercise and Lifestyle Interventions during Pregnancy on Reducing Gestational Weight Gain and Preventing Gestational Diabetes Mellitus. J. Clin. Med. 2024, 13, 3462. [Google Scholar] [CrossRef]
- Kempler, P.; Putz, Z.; Kiss, Z.; Wittmann, I.; Abonyi-Tóth, Z.; Rokszin, G.; Jermendy, G. A 2-es típusú diabetes előfordulása és költségterheinek alakulása Magyarországon 2001–2014 között—Az Országos Egészségbiztosítási Pénztár adatbázis-elemzésének eredményei. Diabetol. Hung. 2016, 24, 177–188. [Google Scholar]
- Bedros, J.R.; Jermendy, G.; Gaál, Z.; Gerő, L.; Hidvégi, T.; Kempler, P.; Lengyel, C.; Várkonyi, T.; Winkler, G.; Wittmann, I. Clinical Practice Guideline—Diagnosis of diabetes, and antihyperglycaemic treatment and care of patients with diabetes in adulthood. Diabetol. Hung. 2023, 31, 335–443. [Google Scholar] [CrossRef]
- Zhang, C.; Ning, Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: Review of epidemiologic evidence. Am. J. Clin. Nutr. 2011, 94, 1975S–1979S. [Google Scholar] [CrossRef]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet. Med. 2004, 21, 103–113. [Google Scholar] [CrossRef]
- Bao, W.; Michels, K.B.; Tobias, D.K.; Li, S.; Chavarro, J.E.; Gaskins, A.J.; Vaag, A.A.; Hu, F.B.; Zhang, C. Parental smoking during pregnancy and the risk of gestational diabetes in the daughter. Int. J. Epidemiol. 2016, 45, 160–169. [Google Scholar] [CrossRef]
- Zhang, C.; Rawal, S.; Chong, Y.S. Risk factors for gestational diabetes: Is prevention possible? Diabetologia 2016, 59, 1385–1390. [Google Scholar] [CrossRef]
- Malaza, N.; Masete, M.; Adam, S.; Dias, S.; Nyawo, T.; Pheiffer, C. A Systematic Review to Compare Adverse Pregnancy Outcomes in Women with Pregestational Diabetes and Gestational Diabetes. Int. J. Environ. Res. Public Health 2022, 19, 10846. [Google Scholar] [CrossRef]
- Linder, T.; Eder, A.; Monod, C.; Rosicky, I.; Eppel, D.; Redling, K.; Geissler, F.; Huhn, E.A.; Hösli, I.; Göbl, C.S. Impact of prepregnancy overweight and obesity on treatment modality and pregnancy outcome in women with gestational diabetes mellitus. Front. Endocrinol. 2022, 13, 799625. [Google Scholar] [CrossRef]
- Callaway, L.K.; Britten, F. Managing pre-existing diabetes prior to and during pregnancy. Aust. Prescr. 2024, 47, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, S.Y.; Deryabina, E.G.; Ladoshina, K.S. Gestational diabetes mellitus as a predictor of health disorders and lipid metabolism in newborns and infants (literature review). Bull. Matern. Child Care 2024, 1, 37–47. (In Russian) [Google Scholar] [CrossRef]
- Vohr, B.R.; Boney, C.M. Gestational diabetes: The forerunner for the development of maternal and childhood obesity and metabolic syndrome? J. Matern. Fetal Neonatal Med. 2008, 21, 149–157. [Google Scholar] [CrossRef]
- Nijs, H.; Benhalima, K. Gestational Diabetes Mellitus and the Long-Term Risk for Glucose Intolerance and Overweight in the Offspring: A Narrative Review. J. Clin. Med. 2020, 9, 599. [Google Scholar] [CrossRef]
- Damm, P. Future risk of diabetes in mother and child after gestational diabetes mellitus. Int. J. Gynaecol. Obs. 2009, 104, S25–S26. [Google Scholar] [CrossRef] [PubMed]
- Bardenheier, B.H.; Imperatore, G.; Gilboa, S.M.; Geiss, L.S.; Saydah, S.H.; Devlin, H.M.; Kim, S.Y.; Gregg, E.W. Trends in Gestational Diabetes Among Hospital Deliveries in 19 U.S. States, 2000–2010. Am. J. Prev. Med. 2015, 49, 12–19. [Google Scholar] [CrossRef]
- Joinpoint Regression Program, Version 4.9.0.0. Statistical Research and Applications Branch, National Cancer Institute. 2021. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 20 April 2025).
- Chivese, T.; Hoegfeldt, C.A.; Werfalli, M.; Yuen, L.; Sun, H.; Karuranga, S.; Li, N.; Gupta, A.; Immanuel, J.; Divakar, H.; et al. IDF Diabetes Atlas: The prevalence of pre-existing diabetes in pregnancy–A systematic reviewand meta-analysis of studies published during 2010–2020. Diabetes Res. Clin. Pract. 2022, 183, 109049. [Google Scholar] [CrossRef]
- International Diabetes Atlas. Diabetes Evidence Initiative Central Europe: An Expert Declaration. Available online: https://idf.org/europe/media/uploads/sites/2/2023/06/DEICE-Expert-Declaration_A4-Brochure_FINAL.pdf (accessed on 31 July 2025).
- Bromiker, R.; Perry, A.; Kasirer, Y.; Einav, S.; Klinger, G.; Levy-Khademi, F. Early neonatal hypoglycemia: Incidence of and risk factors. A cohort study using universal point of care screening. J. Matern. Fetal Neonatal Med. 2019, 32, 786–792. [Google Scholar] [CrossRef]
- Chen, Y.S.; Ho, C.H.; Lin, S.J.; Tsai, W.H. Identifying additional risk factors for early asymptomatic neonatal hypoglycemia in term and late preterm babies. Pediatr. Neonatol. 2022, 63, 625–632. [Google Scholar] [CrossRef]
- O’Brien, M.; Gilchrist, C.; Sadler, L.; Hegarty, J.E.; Alsweiler, J.M. Infants Eligible for Neonatal Hypoglycemia Screening: A Systematic Review. JAMA Pediatr. 2023, 177, 1187–1196. [Google Scholar] [CrossRef]
- Mirsky, E.L.; Mastronardi, A.; Paudel, A.M.; Young, M.; Zite, N.; Maples, J. Comparison of the Prevalence of Gestational Diabetes Pre-COVID-19 Pandemic Versus During COVID-19 [A196]. Obstet. Gynecol. 2022, 139, 57S. [Google Scholar] [CrossRef]
- La Verde, M.; Torella, M.; Riemma, G.; Narciso, G.; Iavarone, I.; Gliubizzi, L.; Palma, M.; Morlando, M.; Colacurci, N.; De Franciscis, P. Incidence of gestational diabetes mellitus before and after the Covid-19 lockdown: A retrospective cohort study. Obstet. Gynaecol. Res. 2022, 48, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Wilmot, E.; Idris, I. Early onset type 2 diabetes: Risk factors, clinical impact and management. Ther. Adv. Chronic Dis. 2014, 5, 234–244. [Google Scholar] [CrossRef]
- Richards, S.E.; Wijeweera, C.; Wijeweera, A. Lifestyle and socioeconomic determinants of diabetes: Evidence from country-level data. PLoS ONE 2022, 17, e0270476. [Google Scholar] [CrossRef]
- Tseng, E.; Hsu, Y.J.; Nigrin, C.; Clark, J.M.; Marsteller, J.A.; Maruthur, N.M. Improving Diabetes Screening in the Primary Care Clinic. Jt. Comm. J. Qual. Patient Saf. 2023, 49, 698–705. [Google Scholar] [CrossRef]
- Mirabelli, M.; Tocci, V.; Chiefari, E.; Iuliano, S.; Brunetti, F.S.; Misiti, R.; Giuliano, S.; Greco, M.; Foti, D.P.; Brunetti, A. Clinical Risk Factors and First Gestational 75 g OGTT May Predict Recurrent and New-Onset Gestational Diabetes in Multiparous Women. J. Clin. Med. 2024, 13, 5200. [Google Scholar] [CrossRef] [PubMed]
| Variables | 2010 a | 2024 | Change (%) |
|---|---|---|---|
| Number of live births (n) | 90,335 | 77,500 | −14.21% |
| Mean age of women at first childbirth (years) | 28.23 | 29.43 | 4.25% |
| Number of 15–49-year-old women (n) | 2,385,782 | 2,112,676 | −11.45% |
| Number of live births per thousand 15–49-year-old women (n) | 37.86 | 36.68 | −3.10% |
| Number of DMP patients (inpatient care) (n) Gestational Pre-existing Unspecified | 4693 4168 (88.81%) 334 (7.12%) 191 (4.07%) | 7271 6853 (94.25%) 370 (5.09%) 48 (0.66%) | 54.93% 64.42% 10.78% −74.87% |
| Number of DMP patients per thousand 15–49-year-old women (inpatient care) (n) Gestational Pre-existing Unspecified | 1.97 1.75 0.14 0.08 | 3.44 3.24 0.18 0.02 | 74.96% 85.67% 25.10% −71.62% |
| Age distribution in inpatient care (n, [%]) | |||
| 5–18 years Gestational Pre-existing Unspecified | 37 32 (86.49%) 5 (13.51%) - | 42 40 (95.24%) 2 (4.76%) - | 13.51% 25.00% −60.00% - |
| 19–30 years Gestational Pre-existing Unspecified | 1858 1662 (89.45%) 131 (7.05%) 65 (3.50%) | 2729 2578 (94.47%) 132 (4.84%) 19 (0.70%) | 46.88% 55.11% 0.76% −70.77% |
| 31–40 years Gestational Pre-existing Unspecified | 2649 2340 (88.34%) 187 (7.06%) 122 (4.61%) | 4085 3842 (94.05%) 216 (5.29%) 27 (0.66%) | 54.21% 64.19% 15.51% −77.87% |
| 41–50 years Gestational Pre-existing Unspecified | 149 134 (89.93%) 11 (7.38%) 4 (2.68%) | 415 393 (94.70%) 20 (4.82%) 2 (0.48%) | 178.52% 193.28% 81.82% −50.00% |
| DMP mean length of hospital stays (days) Gestational Pre-existing Unspecified | 8.43 7.99 10.44 12.34 | 5.96 5.86 10.02 5.23 | −29.32% −26.66% −4.02% −57.62% |
| Number of DMP patients (outpatient care) (n) Gestational Pre-existing Unspecified | 8319 7098 (85.32%) 681 (8.19%) 540 (6.49%) | 10,530 9513 (90.34%) 558 (5.30%) 459 (4.36%) | 26.58% 34.02% −18.06% −15.00% |
| Number of DMP patients per thousand 15–49-year-old women (outpatient care) (n) Gestational Pre-existing Unspecified | 3.56 3.04 0.29 0.23 | 4.98 4.50 0.26 0.22 | 39.84% 48.03% −10.34% −4.35% |
| Number of FNTMD patients (inpatient care) (n) Syndrome due to GDM mother Syndrome due to diabetic mother Neonatal diabetes Neonatal hypoglycemia Other, unspecified | 9276 2250 (24.26%) 289 (3.12%) 4 (0.04%) 3214 (34.65%) 3519 (37.94%) | 10,951 4909 (44.83%) 300 (2.74%) 4 (0.04%) 1537 (14.04%) 4201 (38.36%) | 18.06% 118.18% 3.81% 0.00% −52.18% 19.38% |
| Number of FNTMD patients per thousand live births (inpatient care) (n) Syndrome due to GDM mother Syndrome due to diabetic mother Neonatal diabetes Neonatal hypoglycemia Other, unspecified | 102.68 24.91 3.20 0.04 35.58 38.96 | 141.30 63.34 3.87 0.05 19.83 54.21 | 37.61% 154.28% 20.94% 25.00% −44.27% 39.14% |
| FNTMDmean length of hospital stays (days) Syndrome due to GDM mother Syndrome due to diabetic mother Neonatal diabetes Neonatal hypoglycemia Other, unspecified | 6.94 4.62 5.18 11.67 5.75 7.29 | 5.24 3.60 4.05 - 6.31 6.92 | −24.47% −22.08% −21.81% - 9.74% −5.08% |
| Number ofFNTMDpatients (outpatient care) (n) Syndrome due to GDM mother Syndrome due to diabetic mother Neonatal diabetes Neonatal hypoglycemia Other, unspecified | 1182 518 (43.82%) 103 (8.71%) 4 (0.34%) 398 (33.67%) 159 (13.45%) | 1003 177 (17.65%) 43 (4.29%) 3 (0.30%) 176 (17.55%) 604 (60.22%) | −15.14% −12.66% −58.25% −25.00% −55.78% −279.87% |
| Number ofFNTMDpatients per thousand live births (outpatient care) (n) Syndrome due to GDM mother Syndrome due to diabetic mother Neonatal diabetes Neonatal hypoglycemia Other, unspecified | 13.09 5.74 1.14 0.04 4.41 1.76 | 12.94 2.28 0.55 0.04 2.27 7.79 | −1.16% −60.28% −51.75% 0.00% −48.53% 342.61% |
| Variable | AAPC (95%CI) | Trends | |||
|---|---|---|---|---|---|
| Trend 1 | Trend 2 | ||||
| APC (95%CI) | Period | APC (95%CI) | Period | ||
| Number of live births | −0.9 * | 0.3 | 2010–2021 | −5.1 * | 2021–2024 |
| (−1.5–−1.3) | (−0.1–0.7) | (−7.7–−2.4) | |||
| Number of live births per 1000 15–49-year-old women | 0.0 | 1.2 *** | 2010–2021 | −4.3 ** | 2021–2024 |
| (−0.6–0.6) | (0.9–1.6) | (−7.0–−1.6) | |||
| Mean age of women at first childbirth | 0.3 * | 0.2 | 2010–2014 | 0.4 *** | 2014–2024 |
| (0.2–0.3) | (−0.2–0.2) | (0.3–0.4) | |||
| Number of 15–49-year-old women | −0.9 * | −0.9 * | 2010–2021 | −0.7 * | 2021–2024 |
| (−0.9–−0.8) | (−0.9–−0.9) | (−1.0–−0.4) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csákvári, T.; Elmer, D.; Palkovics, K.; Sántics-Kajos, L.F.; Kovács, B.; Kovács, K.; Bódis, J.; Boncz, I. Trends and Projections of the Prevalence of Diabetes Mellitus in Pregnancy and Fetal–Neonatal Metabolic Disorders, 2010–2035: A Nationwide Population-Based Study from Hungary. J. Clin. Med. 2025, 14, 5740. https://doi.org/10.3390/jcm14165740
Csákvári T, Elmer D, Palkovics K, Sántics-Kajos LF, Kovács B, Kovács K, Bódis J, Boncz I. Trends and Projections of the Prevalence of Diabetes Mellitus in Pregnancy and Fetal–Neonatal Metabolic Disorders, 2010–2035: A Nationwide Population-Based Study from Hungary. Journal of Clinical Medicine. 2025; 14(16):5740. https://doi.org/10.3390/jcm14165740
Chicago/Turabian StyleCsákvári, Tímea, Diána Elmer, Krisztina Palkovics, Luca Fanni Sántics-Kajos, Bettina Kovács, Kálmán Kovács, József Bódis, and Imre Boncz. 2025. "Trends and Projections of the Prevalence of Diabetes Mellitus in Pregnancy and Fetal–Neonatal Metabolic Disorders, 2010–2035: A Nationwide Population-Based Study from Hungary" Journal of Clinical Medicine 14, no. 16: 5740. https://doi.org/10.3390/jcm14165740
APA StyleCsákvári, T., Elmer, D., Palkovics, K., Sántics-Kajos, L. F., Kovács, B., Kovács, K., Bódis, J., & Boncz, I. (2025). Trends and Projections of the Prevalence of Diabetes Mellitus in Pregnancy and Fetal–Neonatal Metabolic Disorders, 2010–2035: A Nationwide Population-Based Study from Hungary. Journal of Clinical Medicine, 14(16), 5740. https://doi.org/10.3390/jcm14165740







