Factors Related to Decline of Renal Function in Patients with Chronic Hypoparathyroidism †
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Clinical Evaluation
2.3. Biochemical Test
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Population of Study
3.2. Estimated Glomerular Filtration Rate (eGFR)
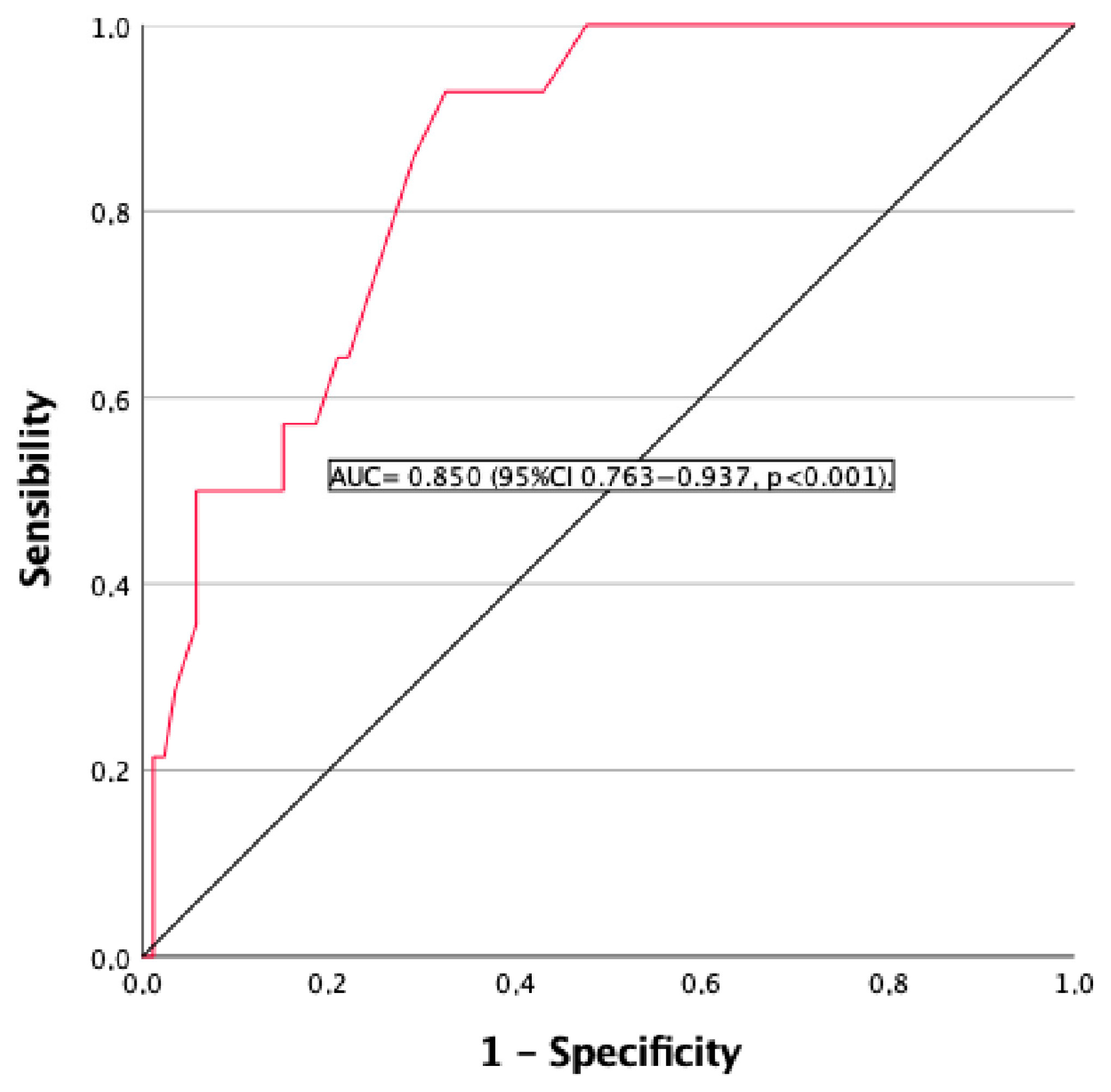
3.3. Chronic Kidney Disease (CKD)
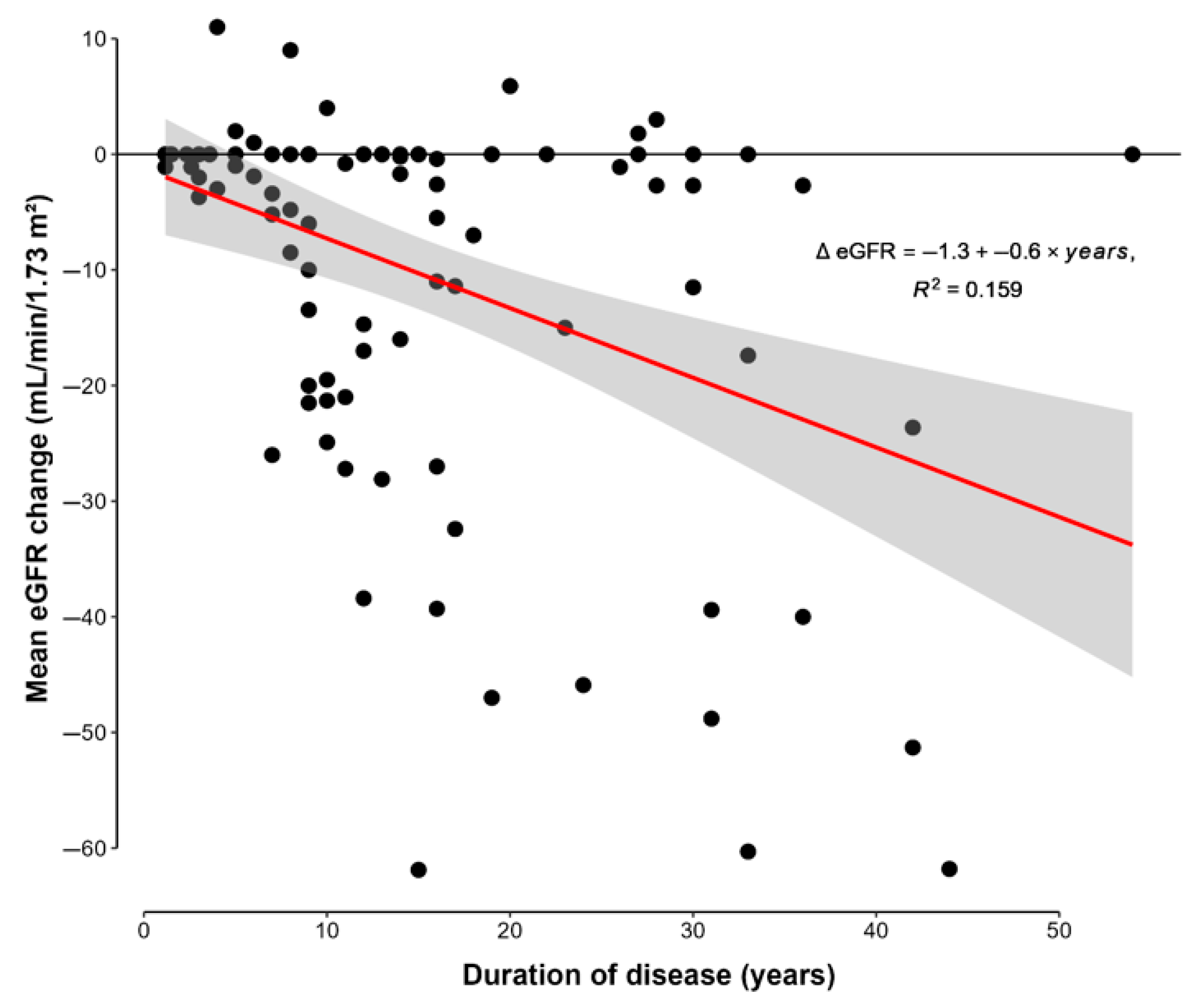
3.4. Duration of Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilezikian, J.P. Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2020, 105, 1722–1736. [Google Scholar] [CrossRef]
- Pasieka, J.L.; Wentworth, K.; Yeo, C.T.; Cremers, S.; Dempster, D.; Fukumoto, S.; Goswami, R.; Houillier, P.; Levine, M.A.; Pasternak, J.D.; et al. Etiology and pathophysiology of hypoparathyroidism: A narrative review. J. Bone Miner. Res. 2022, 37, 2586–2601. [Google Scholar] [CrossRef]
- Yao, L.; Hui, X.; Li, M.; Li, J.; Ahmed, M.M.; Lin, C.; Kandi, M.; Sreekanta, A.; Makhdami, N.; Tamilselvan, D.; et al. Complications, symptoms, presurgical predictors in patients with chronic hypoparathyroidism: A systematic review. J. Bone Miner. Res. 2022, 37, 2642–2653. [Google Scholar] [CrossRef]
- Gosmanova, E.O.; Houillier, P.; Rejnmark, L.; Marelli, C.; Bilezikian, J.P. Renal complications in patients with chronic hypoparathyroidism on conventional therapy: A systematic literature review. Rev. Endocr. Metab. Disord. 2021, 22, 297–316. [Google Scholar] [CrossRef]
- Khan, A.A.; Bilezikian, J.P.; Brandi, M.L.; Clarke, B.L.; Gittoes, N.J.; Pasieka, J.L.; Rejnmark, L.; Shoback, D.M.; Potts, J.T.; Guyatt, G.H.; et al. Evaluation and management of hypoparathyroidism summary statement and guidelines from de second international workshop. J. Bone Miner. Res. 2022, 37, 2568–2585. [Google Scholar] [CrossRef]
- Ridder, L.O.; Harsløf, T.; Sikjær, T.; Underbjerg, L.; Rejnmark, L. Determinants of hipercalciuria and renal calcifications in chronic hypoparathyroidism: A cross-sectional study. Clin. Endocrinol. 2021, 95, 286–294. [Google Scholar] [CrossRef]
- Ketteler, M.; Chen, K.; Gosmanova, E.O.; Signorovitch, J.; Mu, F.; Young, J.A.; Sherry, N.; Rejnmark, L. Risk of nephrolithiasis and nephrocalcinosis in patients with chronic hypoparathyroidism: A retrospective cohort study. Adv. Ther. 2021, 38, 1946–1957. [Google Scholar] [CrossRef]
- Rejnmark, L.; Gosmanova, E.O.; Khan, A.A.; Makita, N.; Imanishi, Y.; Takeuchi, Y.; Sprague, S.; Shoback, D.M.; Kohlmeier, L.; Rubin, M.R.; et al. Palopegteriparatide treatment improves renal function in adults with chronic hypoparathyroidism: 1-year results from the phase 3 PaTHway trial. Adv. Ther. 2024, 41, 2500–2518. [Google Scholar] [CrossRef]
- Yao, J.; Dong, Z.; Wang, Q.; Li, Z.; Zhang, W.; Lin, W.; Luo, Y.; Li, H.; Guo, X.; Zhang, L.; et al. Clinical factors associated with arterial stiffness in chronic kidney disease. J. Clin. Med. 2023, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Adamczak, M.; de Oliveira, R.B.; A Massy, Z.; Sarafidis, P.; Agarwal, R.; Mark, P.B.; Kotanko, P.; Ferro, C.J.; et al. Cardiovascular complications in chronic kidney disease: A review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc. Res. 2023, 119, 2017–2032. [Google Scholar] [CrossRef] [PubMed]
- Benson, T.; Menezes, T.; Campbell, J.; Bice, A.; Hood, B.; Prisby, R. Mechanisms of vasodilation to PTH 1-84, PTH 1-34, and PTHrP 1-34 in rat bone resistance arteries. Osteoporos. Int. 2016, 27, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.L.; Shao, J.S.; Halstead, L.R.; Distelhorst, K.; Sierra, O.; Towler, D.A. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ. Res. 2010, 107, 271–282. [Google Scholar] [CrossRef]
- Underbjerg, L.; Sikiaer, T.; Rejnmark, L. Cardiovascular findings in patients with nonsurgical hypoparathyroidism and pseudohypoparathyroidism: A cohort study. Clin. Endocrinol. 2019, 90, 592–600. [Google Scholar] [CrossRef]
- Fuss, C.T.; Gronemeyer, K.; Hermes, F.; Dörr, M.; Schmid, B.; Morbach, C.; Schmidbauer, L.; Schlegel, N.; Fassnacht, M.; Koschker, A.C.; et al. Cardiovascular status in chronic hypoparathyroidism: A systematic cross-sectional assessment in 168 patients. Eur. J. Endocrinol. 2025, 192, 373–384. [Google Scholar] [CrossRef]
- Ayodele, O.; Mu, F.; Berman, R.; Swallow, E.; Rejnmark, L.; Gosmanova, E.O.; Kaul, S. Lower risk of cardiovascular events in adult patients with chronic hypoparathyroidism treated with rpPTH(1–84): A retrospective cohort study. Adv. Ther. 2022, 39, 3845–3856. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Astor, M.C.; Løvås, K.; Debowska, A.; Eriksen, E.F.; Evang, J.A.; Fossum, C.; Fougner, K.J.; Holte, S.E.; Lima, K.; Moe, R.B.; et al. Epidemiology and health -related quality of life in hypoparathyroidism in Norway. J. Clin. Endocrinol. Metab. 2016, 101, 3045–3053. [Google Scholar] [CrossRef]
- Underbierg, L.; Sikiaer, T.; Rejnmark, L. Long-term complications in patients with hypoparathyroidism evaluated by biochemical findings: A case-control study. J. Bone Miner. Res. 2018, 33, 822–831. [Google Scholar] [CrossRef]
- Meola, A.; Vignali, E.; Matrone, A.; Cetani, F.; Marcocci, C. Efficacy and safety of long-term management of patients with chronic post-surgical hypoparathyroidism. J. Endocrinol. Investig. 2018, 41, 1221–1226. [Google Scholar] [CrossRef]
- Leidig-Bruckner, G.; Bruckner, T.; Raue, F.; Frank-Raue, K. Long-term follow-up and treatment of postoperative permanent hypoparathyroidism in patients with medullary thyroid carcinoma: Differences in complete and partial disease. Horm. Metab. Res. 2016, 48, 806–813. [Google Scholar] [CrossRef]
- Mitchell, D.M.; Regan, S.; Cooley, M.R.; Lauter, K.B.; Vrla, M.C.; Becker, C.B.; Burnett-Bowie, S.-A.M.; Mannstadt, M. Long-term follow-up of patients with hypoparathyroidism. J. Clin. Endocrinol. Metab. 2012, 97, 4507–4514. [Google Scholar] [CrossRef]
- Underbierg, L.; Sikiaer, T.; Mosekilde, L.; Reinmark, L. The epidemiology of nonsurgical hypoparathyroidism in Denmark: A nationwide case finding study. J. Bone Miner. Res. 2015, 30, 1738–1744. [Google Scholar] [CrossRef]
- Swartling, O.; Evans, M.; Spelman, T.; Kamal, W.; Kämpe, O.; Mannstadt, M.; Lagerros, Y.T.; Björnsdottir, S. Kidney complications and hospitalization in patients with chronic hypoparathyroidism: A cohort study in Sweden. J. Clin. Endocrinol. Metab. 2022, 107, e4098–e4105. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.J.; Anda, E.; Pérez-Corral, B.; Paja, M.; Alcázar, V.; Sánchez-Ragnarsson, C.; Orois, A.; Romero-Lluch, A.R.; Sambo, M.; Oleaga, A.; et al. Impaired renal function in patients with permanent hypoparathyroidism after thyroidectomy: Analysis of a nationwide cohort in Spain. Endocrine 2025, 88, 826–835. [Google Scholar] [CrossRef]
- Luk, Y.; Fung, M.M.H.; Lui, D.T.W.; Liu, X.; Li, L.; Wong, C.K.H.; Lang, B.H.H. Long-term kidney outcomes in patients with permanent hypoparathyroidism after total thyroidectomy for benign disease: A population-based study. Surgery 2024, 176, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Gosmanova, E.O.; Chen, K.; Ketteler, M.; Rejnmark, L.; Mu, F.; Swallow, E.; Briggs, A.; Sherry, N.; Kaul, S. Risk of cardiovascular conditions in patients with chronic hypoparathyroidism: A retrospective cohort study. Adv. Ther. 2021, 38, 4246–4257. [Google Scholar] [CrossRef]
- Shoback, D.M.; Bilezikian, J.P.; Costa, A.G.; Dempster, D.; Dralle, H.; Khan, A.A.; Peacock, M.; Raffaelli, M.; Silva, B.C.; Thakker, R.V.; et al. Presentation of hypoparathyroidism: Etiologies and clinical features. J. Clin. Endocrinol. Metab. 2016, 101, 2300–2312. [Google Scholar] [CrossRef]
- Tabacco, G.; Naciu, A.M.; Maggi, D.; Santonati, A.; Pedone, C.; Cesareo, R.; Bosco, D.; Gaspa, G.; Napoli, N.; Pozzilli, P.; et al. Cardiovascular autonomic neuropathy as a new complication of postsurgical chronic hypoparathyroidism. J. Bone Miner. Res. 2019, 34, 475–481. [Google Scholar] [CrossRef]
- Vadiveloo, T.; Donnan, P.T.; Leese, G.P. A population-based study of the epidemiology of chronic hypoparathyroidism. J. Bone Miner. Res. 2018, 33, 478–485. [Google Scholar] [CrossRef]
- Anderson, J.J.; Kruszka, B.; Delaney, J.A.; He, K.; Burke, G.L.; Alonso, A.; Bild, D.E.; Budoff, M.; Michos, E.D. Calcium intake from diet and supplments and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, K.; Melhus, H.; Lemming, E.W.; Wolk, A.; Byberg, L. Long term calcium intake and rates of all cause and cardiovascular mortality: Community based prospective longitudinal cohort study. BMJ 2013, 346, f228. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Reid, I.R. Calcium supplements and cariovascular risk: 5 years on. Ther. Adv. Drug Saf. 2013, 4, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Xie, H.; Yang, W.; Xie, D.; Anderson, A.H.; Scialla, J.; Wahl, P.; Gutiérrez, O.M.; Steigerwalt, S.; He, J.; et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011, 305, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Xie, H.; Rahman, M.; Anderson, A.H.; Isakova, T.; Ojo, A.; Zhang, X.; Nessel, L.; Hamano, T.; Grunwald, J.E.; et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J. Am. Soc. Nephrol. 2014, 25, 349–360. [Google Scholar] [CrossRef]
- European Society of Endocrinology. In Proceedings of the Joint Congress of European Society of Paediatric Endocrinology (ESPE) and European Society of Endocrinology (ESE) 2025, Copenhagen, Denmark, 10–13 May 2025.
| Population Study | Range | |
|---|---|---|
| Age (years) | 57 (14) | 29–86 |
| Duration of disease (years) | 15 (11) | 1–54 |
| Serum calcium (mg/dL) | 8.6 (0.7) | 6.3–10.0 |
| Albumin-adjusted serum calcium levels (mg/dL) | 8.3 (0.7) | 6.2–9.9 |
| Serum phosphorus (mg/dL) | 4.3 (0.3) | 2.5–6.5 |
| Serum magnesium (mg/dL) | 1.8 (0.2) | 1.1–2.3 |
| Calcium phosphate product (mg2/dL2) | 35.7 (5.9) | 19.8–61.7 |
| PTH levels (mg/dL) | 11.5 (11.6) | 1.0–49.0 |
| 25 OH vitamin D (mg/dL) | 32.3 (12) | 13.0–63.0 |
| Creatinine (mg/dL) | 0.86 (0.27) | 0.56–1.81 |
| eGFR (mL/min/1.73 m2) | 78.2 (17.2) | 28.1–91.8 |
| ΔeGFR (mL/min/1.73 m2) | −10.36 (16.63) | −61.88–11.00 |
| ΔeGFR/yr (ΔeGFR/year) | −0.64 (1.04) | −4.13–2.75 |
| Calciuria (mg/24 h) | 243.9 (137.9) | 5.4–635.0 |
| Hypercalciuria | 44% | NA |
| Urolithiasis | 10% | NA |
| Nephrocalcinosis | 6% | NA |
| eGFR < 60 mL/min/1.73 m2 | 14% | NA |
| Hypertension | 38% | NA |
| Type 2 diabetes | 18% | NA |
| Dyslipidemia | 44% | NA |
| Coronary heart disease | 3% | NA |
| Cerebrovascular disease | 3% | NA |
| Peripheral arterial disease | 2% | NA |
| Arrhythmia | 3% | NA |
| eGFR ≥ 60 mL/min/1.72 m2 n: 86 | eGFR < 60 mL/min/1.72 m2 n: 14 | p Value | |
|---|---|---|---|
| Etiology post-surgery (%) | 89.5 | 87.5 | 0.672 |
| Age (years) | 55 (13) | 75 (7) | <0.001 |
| Gender (% females) | 86 | 85 | 0.468 |
| Duration of disease (years) | 13 (10) | 27 (11) | <0.001 |
| Serum calcium (mg/dL) | 8.5 (0.7) | 9.1 (0.7) | <0.001 |
| Albumin-adjusted serum calcium levels (mg/dL) | 8.2 (0.7) | 8.9 (0.7) | <0.001 |
| Serum phosphorus (mg/dL) | 4.3 (0.7) | 3.8 (0.5) | 0.002 |
| Serum magnesium (mg/dL) | 1.8 (0.2) | 1.8 (0.3) | 0.441 |
| Calcium phosphate product (mg2/dL2) | 36.0 (6.0) | 33.9 (4.9) | 0.662 |
| PTH levels (mg/dL) | 11.9 (11.5) | 9.2 (11.3) | 0.383 |
| 25 OH vitamin D (mg/dL) | 32.8 (12.0) | 28.9 (11.0) | 0.975 |
| Creatinine (mg/dL) | 0.78 (0.14) | 1.38 (0.27) | <0.001 |
| eGFR (mL/min/1.73 m2) | 84.2 (8.1) | 40.9 (9.2) | <0.001 |
| ΔeGFR (mL/min/1.73 m2) | −4.6 (8.4) | −44.0 (12.0) | <0.001 |
| ΔeGFR/yr (ΔeGFR/year) | −0.43 (0.89) | −1.90 (0.92) | <0.001 |
| Calciuria (mg/24 h) | 248.1(135.6) | 219.4 (154.4) | 0.864 |
| Calcium carbonate doses (mg/24 h) | 2311 (1592) | 1713 (785) | 0.213 |
| Magnesium treatment (%) Doses (mg/24 h) | 20.9 187 (120) | 28.6 87 (74) | 0.522 0.423 |
| Calcitriol treatment doses (mcg/24 h) | 0.627 (0.494) | 0.404 (0.127) | 0.101 |
| Colecalciferol treatment (%) Doses (UI/24 h) | 57.0 1944 (3474) | 42.9 893 (454) | 0.325 0.432 |
| Calcifediol treatment (%) Doses (mcg/24 h) | 10.5 0.296 (0.088) | 7.7 0.266 | 0.757 - |
| Tiazide treatment (%) Doses (mg/24 h) | 27.9 22.4 (13.8) | 35.7 27.5 (13.7) | 0.342 0.684 |
| Hypercalciuria (%) | 45.0 | 37.7 | 0.487 |
| Urolithiasis (%) | 7.0 | 28.6 | 0.003 |
| Nephrocalcinosis (%) | 4.7 | 14.3 | 0.008 |
| Hypertension (%) | 32.6 | 71.4 | <0.005 |
| Type 2 diabetes (%) | 12.8 | 50 | <0.001 |
| Dyslipidemia (%) | 36.0 | 92.2 | <0.001 |
| Coronary disease (%) | 1.2 | 14.3 | 0.008 |
| Cerebrovascular disease (%) | 2.3 | 7.1 | 0.327 |
| Peripheral arterial disease (%) | 1.2 | 7.1 | 0.138 |
| Arrhythmia (%) | 0 | 21.4 | <0.001 |
| <15.5 Years of Evolution n: 63 | ≥15.5 Years Evolution n: 37 | p Value | |
|---|---|---|---|
| Etiology post-surgery (%) | 94 | 81 | 0.052 |
| Age (years) | 54 (13) | 63 (14) | 0.002 |
| Gender (% females) | 86 | 84 | 0.794 |
| Duration of disease (years) | 8.5 (4.1) | 26.7 (9.6) | <0.001 |
| Serum calcium (mg/dL) | 8.8 (0.6) | 8.5 (0.7) | 0.031 |
| Albumin-adjusted serum calcium levels (mg/dL) | 8.5 (0.7) | 8.2 (0.7) | 0.014 |
| Serum phosphorus (mg/dL) | 4.4 (0.7) | 4.1 (0.6) | 0.024 |
| Serum magnesium (mg/dL) | 1.8 (0.2) | 1.8 (0.2) | 0.125 |
| Calcium phosphate product (mg2/dL2) | 36 (6) | 35.2 (5.8) | 0.892 |
| PTH levels (mg/dL) | 13.5 (12.5) | 8.3 (8.6) | 0.008 |
| 25 OH vitamin D (mg/dL) | 32.7 (11.1) | 31.7 (13.3) | 0.111 |
| Creatinine (mg/dL) | 0.8 (0.22) | 0.96 (0.31) | 0.006 |
| eGFR (mL/min/1.73) | 82.1 (12.8) | 71.4 (21.4) | <0.001 |
| ΔeGFR (mL/min/1.73 m2) | −6.5 (12.3) | −17.1 (20.8) | 0.009 |
| ΔeGFR/yr (ΔeGFR/year) | −0.63 (1.15) | −0.66 (0.8) | 0.365 |
| Calciuria (mg/24 h) | 244.3 (146.7) | 243 (124) | 0.217 |
| Calcium carbonate doses (mg/24 h) | 2408 (1739) | 1915 (960) | 0.037 |
| Magnesium treatment (%) Doses (mg/24 h) | 22.2 208 (127) | 21.6 103 (68) | 0.922 0.022 |
| Calcitriol treatment doses (mcg/24 h) | 0.644 (0.57) | 0.514 (0.191) | 0.13 |
| Colecalciferol treatment (%) Doses (UI/24 h) | 54 1405 (836) | 56.8 2514 (5231) | 0.787 0.59 |
| Calcifediol treatment (%) Doses (mg/24 h) | 14.3 0.296 (0.088) | 2.8 0.266 | 0.068 - |
| Tiazide treatment (%) Doses (mg/24 h) | 25.4 24.2 (16.1) | 35.1 22.1 (10.4) | 0.3 0.61 |
| Hypercalciuria (%) | 42.9 | 45.9 | 0.875 |
| Urolithiasis (%) | 7.9 | 13.5 | 0.085 |
| Nephrocalcinosis (%) | 3.2 | 10.8 | 0.049 |
| Hypertension (%) | 31.7 | 48.6 | 0.093 |
| Type 2 diabetes (%) | 12.7 | 27 | 0.072 |
| Dyslipidemia (%) | 38.1 | 54.1 | 0.121 |
| Coronary disease (%) | 1.6 | 5.4 | 0.28 |
| Cerebrovascular disease (%) | 1.6 | 5.4 | 0.28 |
| Peripheral arterial disease (%) | 1.6 | 2.7 | 0.7 |
| Arrhythmia (%) | 0 | 8.1 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Mezquita Torres, E.; García-Martín, A.; Andreo-López, M.d.C.; Contreras-Bolívar, V.; García-Fontana, C.; García-Fontana, B.; Muñoz-Torres, M. Factors Related to Decline of Renal Function in Patients with Chronic Hypoparathyroidism. J. Clin. Med. 2025, 14, 5732. https://doi.org/10.3390/jcm14165732
López-Mezquita Torres E, García-Martín A, Andreo-López MdC, Contreras-Bolívar V, García-Fontana C, García-Fontana B, Muñoz-Torres M. Factors Related to Decline of Renal Function in Patients with Chronic Hypoparathyroidism. Journal of Clinical Medicine. 2025; 14(16):5732. https://doi.org/10.3390/jcm14165732
Chicago/Turabian StyleLópez-Mezquita Torres, Elena, Antonia García-Martín, María del Carmen Andreo-López, Victoria Contreras-Bolívar, Cristina García-Fontana, Beatriz García-Fontana, and Manuel Muñoz-Torres. 2025. "Factors Related to Decline of Renal Function in Patients with Chronic Hypoparathyroidism" Journal of Clinical Medicine 14, no. 16: 5732. https://doi.org/10.3390/jcm14165732
APA StyleLópez-Mezquita Torres, E., García-Martín, A., Andreo-López, M. d. C., Contreras-Bolívar, V., García-Fontana, C., García-Fontana, B., & Muñoz-Torres, M. (2025). Factors Related to Decline of Renal Function in Patients with Chronic Hypoparathyroidism. Journal of Clinical Medicine, 14(16), 5732. https://doi.org/10.3390/jcm14165732








