Bradykinin-Mediated Angioedema Induced by Drugs
Abstract
1. Introduction
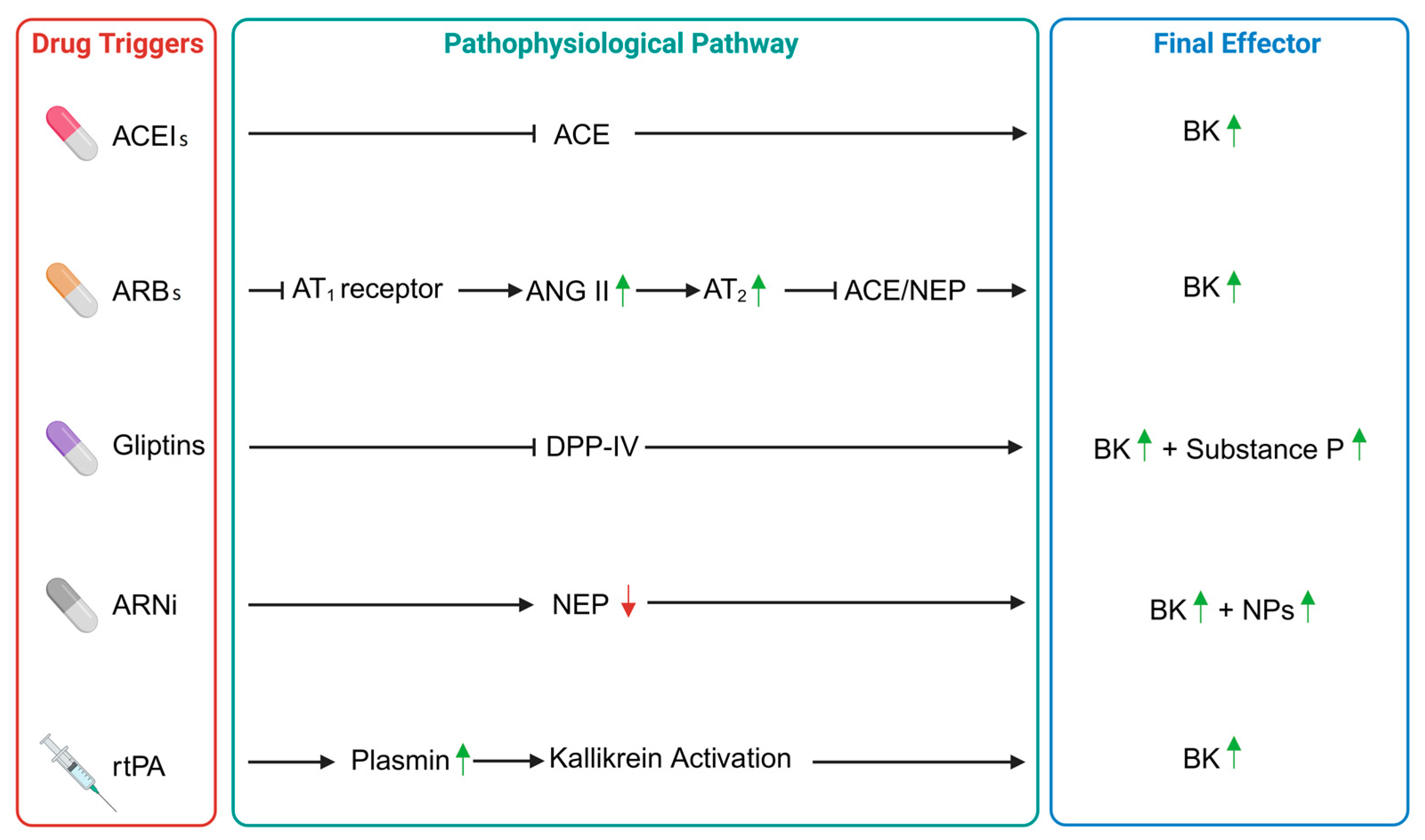
2. Angiotensin-Converting Enzyme Inhibitors-Induced Angioedema
3. Angiotensin II Receptor Blockers-Induced Angioedema
4. Angioedema in DPP-IV Inhibitors
5. Neprilysin Inhibitors-Induced Angioedema
6. Recombinant Tissue Plasminogen Activator-Induced Angioedema
7. Discussion
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reshef, A.; Buttgereit, T.; Betschel, S.D.; Caballero, T.; Farkas, H.; Grumach, A.S.; Hide, M.; Jindal, A.K.; Longhurst, H.; Peter, J.; et al. Definition, acronyms, nomenclature, and classification of angioedema (DANCE): AAAAI, ACAAI, ACARE, and APAAACI DANCE consensus. J. Allergy Clin. Immunol. 2024, 154, 398–411.e1. [Google Scholar] [CrossRef] [PubMed]
- Cicardi, M.; Aberer, W.; Banerji, A.; Bas, M.; Bernstein, J.A.; Bork, K.; Caballero, T.; Farkas, H.; Grumach, A.; Kaplan, A.P.; et al. Classification, diagnosis, and approach to treatment for angioedema: Consensus report from the Hereditary Angioedema International Working Group. Allergy 2014, 69, 602–616. [Google Scholar] [CrossRef]
- Maurer, M.; Magerl, M. Differences and Similarities in the Mechanisms and Clinical Expression of Bradykinin-Mediated vs. Mast Cell-Mediated Angioedema. Clin. Rev. Allergy Immunol. 2021, 61, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, R.; D’Andrea, G.; Maffione, A.B.; Margaglione, M.; d’Apolito, M. The Genetics of Hereditary Angioedema: A Review. J. Clin. Med. 2021, 10, 2023. [Google Scholar] [CrossRef]
- Caldwell, J.R.; Ruddy, S.; Schur, P.H.; Austen, K.F. Acquired C1 inhibitor deficiency in lymphosarcoma. Clin. Immunol. Immunopathol. 1972, 1, 39–52. [Google Scholar] [CrossRef]
- Zanichelli, A.; Azin, G.M.; Wu, M.A.; Suffritti, C.; Maggioni, L.; Caccia, S.; Perego, F.; Vacchini, R.; Cicardi, M. Diagnosis, Course, and Management of Angioedema in Patients with Acquired C1-Inhibitor Deficiency. J. Allergy Clin. Immunol. Pract. 2017, 5, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Gonzalez, J.; Monteleone, C. Angiotensin-converting enzyme inhibitor-induced angioedema: A review of the literature. J. Clin. Hypertens. 2017, 19, 1377–1382. [Google Scholar] [CrossRef]
- Maurer, M.; Bader, M.; Bas, M.; Bossi, F.; Cicardi, M.; Cugno, M.; Howarth, P.; Kaplan, A.; Kojda, G.; Leeb-Lundberg, F.; et al. New topics in bradykinin research. Allergy 2011, 66, 1397–1406. [Google Scholar] [CrossRef]
- Nussberger, J.; Cugno, M.; Amstutz, C.; Cicardi, M.; Pellacani, A.; Agostoni, A. Plasma bradykinin in angio-oedema. Lancet 1998, 351, 1693–1697. [Google Scholar] [CrossRef]
- Veronez, C.L.; Aabom, A.; Martin, R.P.; Filippelli-Silva, R.; Gonçalves, R.F.; Nicolicht, P.; Mendes, A.R.; Da Silva, J.; Guilarte, M.; Grumach, A.S.; et al. Genetic Variation of Kallikrein-Kinin System and Related Genes in Patients with Hereditary Angioedema. Front. Med. 2019, 21, 28. [Google Scholar] [CrossRef]
- Firinu, D.; Loffredo, S.; Bova, M.; Cicardi, M.; Margaglione, M.; Del Giacco, S. The role of genetics in the current diagnostic workup of idiopathic non-histaminergic angioedema. Allergy 2019, 74, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Jaiganesh, T.; Hughan, C.; Webster, A.; Bethune, C. Hereditary angioedema: A survey of UK emergency departments and recommendations for management. Eur. J. Emerg. Med. 2012, 19, 271–274. [Google Scholar] [CrossRef]
- Hébert, J.; Boursiquot, J.N.; Chapdelaine, H.; Laramée, B.; Desjardins, M.; Gagnon, R.; Payette, N.; Lepeshkina, O.; Vincent, M. Bradykinin-induced angioedema in the emergency department. Int. J. Emerg. Med. 2022, 15, 15. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Cremonesi, P.; Hoffmann, T.K.; Hollingsworth, J. Angioedema in the emergency department: A practical guide to differential diagnosis and management. Int. J. Emerg. Med. 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Khoubaeva, A.; Murray, K.; Mitchell, P.M.; Zaniboni, H.A.; Feldman, J.A.; Mycyk, M.B. Are patients aware of angiotensin-converting enzyme inhibitor-associated adverse effects? Am. J. Ther. 2012, 19, 180–184. [Google Scholar] [CrossRef]
- Fan, M.; Niu, K.; Wu, X.; Shi, H. Risk of drug-induced angioedema: A pharmacovigilance study of FDA adverse event reporting system database. Front. Pharmacol. 2024, 15, 1417596. [Google Scholar] [CrossRef]
- Carucci, L.; Bova, M.; Petraroli, A.; Ferrara, A.L.; Sutic, A.; de Crescenzo, G.; Cordisco, G.; Margaglione, M.; Gambardella, J.; Spadaro, G.; et al. Angiotensin-Converting Enzyme Inhibitor-Associated Angioedema: From Bed to Bench. J. Investig. Allergol. Clin. Immunol. 2020, 30, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Malde, B.; Regalado, J.; Greenberger, P.A. Investigation of angioedema associated with the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Ann. Allergy Asthma Immunol. 2007, 98, 57–63. [Google Scholar] [CrossRef]
- Miller, D.R.; Oliveria, S.A.; Berlowitz, D.R.; Fincke, B.G.; Stang, P.; Lillienfeld, D.E. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension 2008, 51, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Thalanayar, P.M.; Ghobrial, I.; Lubin, F.; Karnik, R.; Bhasin, R. Drug-induced visceral angioedema. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 25260. [Google Scholar] [CrossRef]
- Messerli, F.H.; Nussberger, J. Vasopeptidase inhibition and angio-oedema. Lancet 2000, 356, 608–609. [Google Scholar] [CrossRef]
- Holm, J.P.; Ovesen, T. Increasing rate of angiotensin-converting enzyme inhibitor-related upper airway angio-oedema. Dan. Med. J. 2012, 59, A4449. [Google Scholar]
- Roberts, J.R.; Lee, J.J.; Marthers, D.A. Angiotensin-converting enzyme (ACE) inhibitor angioedema: The silent epidemic. Am. J. Cardiol. 2012, 109, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Y.; Levine, R.J.; Lin, H. Adverse drug effects and angioedema hospitalizations in the United States from 2000 to 2009. Allergy Asthma Proc. 2013, 34, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.L.; Holmes, W.L.; Bell, W.A.; Finks, S.W. Life-threatening ACE inhibitor-induced angioedema after eleven years on lisinopril. J. Pharm. Pract. 2013, 26, 382–388. [Google Scholar] [CrossRef]
- Sarkar, P.; Nicholson, G.; Hall, G. Brief review: Angiotensin converting enzyme inhibitors and angioedema: Anesthetic implications. Can. J. Anaesth. 2006, 53, 994–1003. [Google Scholar] [CrossRef]
- Campo, P.; Fernandez, T.D.; Canto, G.; Mayorga, C. Angioedema induced by angiotensin-converting enzyme inhibitors. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 337–344. [Google Scholar] [CrossRef]
- Knecht, S.E.; Dunn, S.P.; Macaulay, T.E. Angioedema related to Angiotensin inhibitors. J. Pharm. Pract. 2014, 27, 461–465. [Google Scholar] [CrossRef]
- Ghouse, J.; Ahlberg, G.; Andreasen, L.; Banasik, K.; Brunak, S.; Schwinn, M.; Larsen, I.H.; Petersen, O.; Sørensen, E.; Ullum, H.; et al. Association of Variants Near the Bradykinin Receptor B2 Gene with Angioedema in Patients Taking ACE Inhibitors. J. Am. Coll. Cardiol. 2021, 78, 696–709. [Google Scholar] [CrossRef]
- Rasmussen, E.R.; Hallberg, P.; Baranova, E.V.; Eriksson, N.; Karawajczyk, M.; Johansson, C.; Cavalli, M.; Maroteau, C.; Veluchamy, A.; Islander, G.; et al. Genome-wide association study of angioedema induced by angiotensin-converting enzyme inhibitor and angiotensin receptor blocker treatment. Pharmacogenomics J. 2020, 20, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Pall, A.H.; Rasmussen, E.R.; Wadelius, M. Pharmacogenetics of angiotensin-converting enzyme inhibitor-induced angioedema. Pharmacogenomics 2021, 22, 319–321. [Google Scholar] [CrossRef]
- Mathey, C.M.; Maj, C.; Scheer, A.B.; Fazaal, J.; Wedi, B.; Wieczorek, D.; Amann, P.M.; Löffler, H.; Koch, L.; Schöffl, C.; et al. Molecular Genetic Screening in Patients with ACE Inhibitor/Angiotensin Receptor Blocker-Induced Angioedema to Explore the Role of Hereditary Angioedema Genes. Front. Genet. 2022, 13, 914376. [Google Scholar] [CrossRef] [PubMed]
- Mathey, C.M.; Maj, C.; Eriksson, N.; Krebs, K.; Westmeier, J.; David, F.S.; Koromina, M.; Scheer, A.B.; Szabo, N.; Wedi, B.; et al. Meta-analysis of ACE inhibitor-induced angioedema identifies novel risk locus. J. Allergy Clin. Immunol. 2024, 153, 1073–1082. [Google Scholar] [CrossRef]
- Lang, D.M.; Aberer, W.; Bernstein, J.A.; Chng, H.H.; Grumach, A.S.; Hide, M.; Maurer, M.; Weber, R.; Zuraw, B. International consensus on hereditary and acquired angioedema. Ann. Allergy Asthma Immunol. 2012, 109, 395–402. [Google Scholar] [CrossRef]
- Ng, K.K.; Vane, J.R. Fate of angiotensin I in the circulation. Nature 1968, 218, 144–150. [Google Scholar] [CrossRef]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Blais, C., Jr.; Rouleau, J.L.; Brown, N.J.; Lepage, Y.; Spence, D.; Munoz, C.; Friborg, J.; Geadah, D.; Gervais, N.; Adam, A. Serum metabolism of bradykinin and des-Arg9-bradykinin in patients with angiotensin-converting enzyme inhibitor-associated angioedema. Immunopharmacology 1999, 43, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, G.; Cugno, M.; Perez, M.; Lepage, Y.; Gervais, N.; Agostoni, A.; Adam, A. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J. Pharmacol. Exp. Ther. 2002, 303, 232–237. [Google Scholar] [CrossRef]
- Sinnathamby, E.S.; Issa, P.P.; Roberts, L.; Norwood, H.; Malone, K.; Vemulapalli, H.; Ahmadzadeh, S.; Cornett, E.M.; Shekoohi, S.; Kaye, A.D. Hereditary Angioedema: Diagnosis, Clinical Implications, and Pathophysiology. Adv. Ther. 2023, 40, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Bas, M.; Hoffmann, T.K.; Bier, H.; Kojda, G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br. J. Clin. Pharmacol. 2005, 59, 233–238. [Google Scholar] [CrossRef]
- Bolton, M.R.; Dooley-Hash, S.L. Angiotensin-converting enzyme inhibitor angioedema. J. Emerg. Med. 2012, 43, e261–e262. [Google Scholar] [CrossRef]
- Leung, E.; Hanna, M.Y.; Tehami, N.; Francombe, J. Isolated unilateral tongue oedema: The adverse effect of Angiotensin converting enzyme inhibitors. Curr. Drug Saf. 2012, 7, 382–383. [Google Scholar] [CrossRef]
- Palmquist, S.; Mathews, B. Isolated intestinal type angioedema due to ACE-inhibitor therapy. Clin. Case Rep. 2017, 5, 707–710. [Google Scholar] [CrossRef]
- Krause, A.J.; Patel, N.B.; Morgan, J. An unusual presentation of ACE inhibitor-induced visceral angioedema. BMJ Case Rep. 2019, 12, e230865. [Google Scholar] [CrossRef]
- Gabb, G.M.; Ryan, P.; Wing, L.M.; Hutchinson, K.A. Epidemiological study of angioedema and ACE inhibitors. Aust. N. Z. J. Med. 1996, 26, 777–782. [Google Scholar] [CrossRef]
- Agostoni, A.; Cicardi, M.; Cugno, M.; Zingale, L.C.; Gioffre, D.; Nussberger, J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999, 44, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kampitak, T. Recurrent severe angioedema associated with imidapril and diclofenac. Allergol. Int. 2008, 57, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Oren, E.; Hesterberg, P.; Hsu, Y.; Camargo, C.A., Jr.; Wong, J.T. Ten-year study of causes of moderate to severe angioedema seen by an inpatient allergy/immunology consult service. Allergy Asthma Proc. 2008, 29, 88–92. [Google Scholar] [CrossRef]
- Sica, D.A.; Black, H.R. Current concepts of pharmacotherapy in hypertension: ACE inhibitor-related angioedema: Can angiotensin-receptor blockers be safely used? J. Clin. Hypertens. 2002, 4, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Lerch, M. Drug-induced angioedema. Chem. Immunol. Allergy 2012, 97, 98–105. [Google Scholar] [CrossRef]
- Zingale, L.C.; Beltrami, L.; Zanichelli, A.; Maggioni, L.; Pappalardo, E.; Cicardi, B.; Cicardi, M. Angioedema without urticaria: A large clinical survey. CMAJ 2006, 175, 1065–1070. [Google Scholar] [CrossRef]
- Kesh, S.; Bernstein, J.A. Isolated angioedema: A review of classification and update on management. Ann. Allergy Asthma Immunol. 2022, 129, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Geiger, H.; McMahon, A. Tranexamic acid for ACE inhibitor induced angioedema. Am. J. Emerg. Med. 2021, 43, 292.e5–292.e7. [Google Scholar] [CrossRef]
- Gallitelli, M.; Alzetta, M. Icatibant: A novel approach to the treatment of angioedema related to the use of angiotensin-converting enzyme inhibitors. Am. J. Emerg. Med. 2012, 30, 1664.e1–1664.e2. [Google Scholar] [CrossRef]
- Bas, M.; Greve, J.; Stelter, K.; Bier, H.; Stark, T.; Hoffmann, T.K.; Kojda, G. Therapeutic efficacy of icatibant in angioedema induced by angiotensin-converting enzyme inhibitors: A case series. Ann. Emerg. Med. 2010, 56, 278–282. [Google Scholar] [CrossRef]
- Bova, M.; Guilarte, M.; Sala-Cunill, A.; Borrelli, P.; Rizzelli, G.M.; Zanichelli, A. Treatment of ACEI-related angioedema with icatibant: A case series. Intern. Emerg. Med. 2015, 10, 345–350. [Google Scholar] [CrossRef]
- Nosbaum, A.; Bouillet, L.; Floccard, B.; Javaud, N.; Launay, D.; Boccon-Gibod, I.; Fain, O.; Groupe d’experts du CREAK; French National Center for Angioedema. Prise en charge des angiœdèmes induits par les inhibiteurs de l’enzyme de conversion de l’angiotensine: Recommandations du Centre de référence national des angiœdèmes [Management of angiotensin-converting enzyme inhibitor-related angioedema: Recommendations from the French National Center for Angioedema]. Rev. Med. Interne 2013, 34, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Lee, Y.J.; Lee, S.-Y. Effect of icatibant on angiotensin-converting enzyme inhibitor-induced angioedema: A meta-analysis of randomized controlled trials. J. Clin. Pharm. Ther. 2019, 44, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.W.; Gramstad, S. Angioedema from angiotensin-converting enzyme (ACE) inhibitor treated with complement 1 (C1) inhibitor concentrate. Acta Anaesthesiol. Scand. 2006, 50, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Pekdemir, M.; Ersel, M.; Aksay, E.; Yanturali, S.; Akturk, A.; Kiyan, S. Effective treatment of hereditary angioedema with fresh frozen plasma in an emergency department. J. Emerg. Med. 2007, 33, 137–139. [Google Scholar] [CrossRef]
- Warrier, M.R.; Copilevitz, C.A.; Dykewicz, M.S.; Slavin, R.G. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann. Allergy Asthma Immunol. 2004, 92, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Hassen, G.W.; Kalantari, H.; Parraga, M.; Chirurgi, R.; Meletiche, C.; Chan, C.; Ciarlo, J.; Gazi, F.; Lobaito, C.; Tadayon, S.; et al. Fresh frozen plasma for progressive and refractory angiotensin-converting enzyme inhibitor-induced angioedema. J. Emerg. Med. 2013, 44, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.; Cicardi, M.; Farkas, H.; Bork, K.; Longhurst, H.J.; Zuraw, B.; Aygoeren-Pürsün, E.; Craig, T.; Binkley, K.; Hebert, J.; et al. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin. Immunol. 2010, 6, 24. [Google Scholar] [CrossRef]
- Craig, T. Triggers and short-term prophylaxis in patients with hereditary angioedema. Allergy Asthma Proc. 2020, 41 (Suppl. S1), S30–S34. [Google Scholar] [CrossRef] [PubMed]
- Hamrahian, S.M.; Falkner, B. Approach to Hypertension in Adolescents and Young Adults. Curr. Cardiol. Rep. 2022, 24, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; Peruzzi, L.; Amore, A.; Piccoli, A.; Cochat, P.; Stone, R.; Kirschstein, M.; Linné, T. IgACE: A placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J. Am. Soc. Nephrol. 2007, 18, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Shima, Y.; Nakanishi, K.; Sako, M.; Saito-Oba, M.; Hamasaki, Y.; Hataya, H.; Honda, M.; Kamei, K.; Ishikura, K.; Ito, S.; et al. Lisinopril versus lisinopril and losartan for mild childhood IgA nephropathy: A randomized controlled trial (JSKDC01 study). Pediatr. Nephrol. 2019, 34, 837–846. [Google Scholar] [CrossRef]
- Nakanishi, K.; Iijima, K.; Ishikura, K.; Hataya, H.; Awazu, M.; Sako, M.; Honda, M.; Yoshikawa, N. Japanese Pediatric IgA Nephropathy Treatment Study Group. Efficacy and safety of lisinopril for mild childhood IgA nephropathy: A pilot study. Pediatr. Nephrol. 2009, 24, 845–849. [Google Scholar] [CrossRef]
- Assadi, F.K.; Wang, H.E.; Lawless, S.; McKay, C.P.; Hopp, L.; Fattori, D. Angiotensin converting enzyme inhibitor-induced angioedema: A report of two cases. Pediatr. Nephrol. 1999, 13, 917–919. [Google Scholar] [CrossRef]
- Quintana, E.C.; Attia, M.W. Angiotensin-converting enzyme inhibitor angioedema in a pediatric patient: A case report and discussion. Pediatr. Emerg. Care 2001, 17, 438–440. [Google Scholar] [CrossRef]
- El Koraichi, A.; Tadili, J.; Benjelloun, M.Y.; Benafitou, R.; El Kharraz, H.; Lahlou, J.; Chkoura, M.; El Haddoury, M.; Ech-Chérif El Kettani, S.S. Enapranil-induced angioedema in a 2-year-old infant: Case report. Cardiovasc. Toxicol. 2011, 11, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Hom, K.A.; Hirsch, R.; Elluru, R.G. Antihypertensive drug-induced angioedema causing upper airway obstruction in children. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 14–19. [Google Scholar] [CrossRef]
- Bukhari, E.; Safdar, O.Y.; Shalaby, M.; AlSharif, S.M.; Alsufiany, K.; Kari, J.A. Potentially lethal ACE-inhibitor-induced angioedema in a child. Clin. Case Rep. 2015, 3, 427–430. [Google Scholar] [CrossRef]
- Beltrami, L.; Zanichelli, A.; Zingale, L.; Vacchini, R.; Carugo, S.; Cicardi, M. Long-term follow-up of 111 patients with angiotensin-converting enzyme inhibitor-related angioedema. J. Hypertens. 2011, 29, 2273–2277. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J.; Krum, H.; Esler, M.D. Losartan Increases Bradykinin Levels in Hypertensive Humans. Circulation 2005, 111, 315–320. [Google Scholar] [CrossRef]
- Sosa-Canache, B.; Cierco, M.; Gutierrez, C.I.; Israel, A. Role of Bradykinins and Nitric Oxide in the AT2 Receptor-Mediated Hypotension. J. Hum. Hypertens. 2000, 14, S40–S46. [Google Scholar] [CrossRef][Green Version]
- Carr, A.A.; Prisant, L.M. Losartan: First of a New Class of Angiotensin Antagonists for the Management of Hypertension. J. Clin. Pharmacol. 1996, 36, 3–12. [Google Scholar] [CrossRef]
- Johnsen, S.P.; Jacobsen, J.; Monster, T.B.; Friis, S.; McLaughlin, J.K.; Sorensen, H.T. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am. J. Med. 2005, 118, 1428–1429. [Google Scholar] [CrossRef]
- Sica, D.A.; Black, H.R. Angioedema in heart failure: Occurrence with ACE inhibitors and safety of angiotensin receptor blocker therapy. Congest. Heart Fail. 2002, 8, 334–341+345. [Google Scholar] [CrossRef] [PubMed]
- Matchar, D.B.; McCrory, D.C.; Orlando, L.A.; Patel, M.R.; Patel, U.D.; Patwardhan, M.B.; Powers, B.; Samsa, G.P.; Gray, R.N. Systematic review: Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann. Intern. Med. 2008, 148, 16–29. [Google Scholar] [CrossRef]
- ONTARGET Investigators; Yusuf, S.; Teo, K.K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P.; Anderson, C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Rubattu, S. Angiotensin Receptor Blockers in the Management of Hypertension: A Real-World Perspective and Current Recommendations. Vasc. Health Risk Manag. 2022, 18, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Makani, H.; Messerli, F.H.; Romero, J.; Wever-Pinzon, O.; Korniyenko, A.; Berrios, R.S.; Bangalore, S. Meta-Analysis of Randomized Trials of Angioedema as an Adverse Event of Renin–Angiotensin System Inhibitors. Am. J. Cardiol. 2012, 110, 383–391. [Google Scholar] [CrossRef]
- Haymore, B.R.; Yoon, J.; Mikita, C.P.; Klote, M.M.; DeZee, K.J. Risk of Angioedema with Angiotensin Receptor Blockers in Patients with Prior Angioedema Associated with Angiotensin-Converting Enzyme Inhibitors: A Meta-Analysis. Ann. Allergy Asthma Immunol. 2008, 101, 495–499. [Google Scholar] [CrossRef]
- Caldeira, D.; David, C.; Sampaio, C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: A systematic review and meta-analysis. Am. J. Cardiovasc. Drugs 2012, 12, 263–277. [Google Scholar] [CrossRef]
- Granger, C.B.; McMurray, J.J.; Yusuf, S.; Held, P.; Michelson, E.L.; Olofsson, B.; Ostergren, J.; Pfeffer, M.A.; Swedberg, K.; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-Alternative trial. Lancet 2003, 362, 772–776. [Google Scholar] [CrossRef]
- Beavers, C.J.; Dunn, S.P.; Macaulay, T.E. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann. Pharmacother. 2011, 45, 520–524. [Google Scholar] [CrossRef]
- Hiyoshi, H.; Yayama, K.; Takano, M.; Okamoto, H. Stimulation of cyclic GMP production via AT2 and B2 receptors in the pressure-overloaded aorta after banding. Hypertension 2004, 43, 1258–1263. [Google Scholar] [CrossRef]
- Bas, M.; Adams, V.; Suvorava, T.; Niehues, T.; Hoffmann, T.K.; Kojda, G. Nonallergic angioedema: Role of bradykinin. Allergy 2007, 62, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Dubrall, D.; Branding, N.L.; Mathey, C.M.; Weber, A.M.; Steffens, M.; Below, M.; Schmid, M.; Wedi, B.; Wieczorek, D.; Amann, P.M.; et al. Non-genetic factors associated with ACE-inhibitor and angiotensin receptor blocker-induced angioedema. Clin. Transl. Allergy 2025, 15, e70058. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Östergren, J.; Swedberg, K.; Granger, C.B.; Held, P.; Michelson, E.L.; Olofsson, B.; Yusuf, S.; Pfeffer, M.A. Committees, for the Effects of Candesartan in Patients with Chronic Heart Failure and Reduced Left-Ventricular Systolic Function Taking Angiotensin-Converting-Enzyme Inhibitors: The CHARM-Added Trial. Lancet 2003, 362, 767–771. [Google Scholar] [CrossRef] [PubMed]
- TRANSCEND Investigators; Yusuf, S.; Teo, K.; Anderson, C.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P. Effects of the Angiotensin-Receptor Blocker Telmisartan on Cardiovascular Events in High-Risk Patients Intolerant to Angiotensin-Converting Enzyme Inhibitors: A Randomised Controlled Trial. Lancet 2008, 372, 1174–1183. [Google Scholar] [CrossRef]
- Julius, S.; Nesbitt, S.D.; Egan, B.M.; Weber, M.A.; Michelson, E.L.; Kaciroti, N.; Black, H.R.; Grimm, R.H., Jr.; Messerli, F.H.; Oparil, S.; et al. Trial of Preventing Hypertension (TROPHY) Study Investigators. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N. Engl. J. Med. 2006, 354, 1685–1697. [Google Scholar] [CrossRef]
- Sridharan, K.; Sivaramakrishnan, G. A pharmacovigilance study assessing risk of angioedema with angiotensin receptor blockers using the US FDA Adverse Event Reporting System. Expert. Opin. Drug Saf. 2024, 1–8. [Google Scholar] [CrossRef]
- Warner, K.K.; Visconti, J.A.; Tschampel, M.M. Angiotensin II Receptor Blockers in Patients with ACE Inhibitor–Induced Angioedema. Ann. Pharmacother. 2000, 34, 526–528. [Google Scholar] [CrossRef]
- van Rijnsoever, E.W.; Kwee-Zuiderwijk, W.J.; Feenstra, J. Angioneurotic edema attributed to the use of losartan. Arch. Intern. Med. 1998, 158, 2063–2065. [Google Scholar] [CrossRef]
- Douillard, M.; Deheb, Z.; Bozon, A.; Raison-Peyron, N.; Dereure, O.; Moulis, L.; Soria, A.; Du-Thanh, A. Over Diagnosis of Bradykinin Angioedema in Patients Treated with Angiotensin-Converting Enzyme Inhibitors or Angiotensin II Receptor Blockers. World Allergy Organ. J. 2023, 16, 100809. [Google Scholar] [CrossRef]
- Cicardi, M.; Zingale, L.C.; Bergamaschini, L.; Agostoni, A. Angioedema Associated with Angiotensin-Converting Enzyme Inhibitor Use: Outcome After Switching to a Different Treatment. Arch. Intern. Med. 2004, 164, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, E.R.; Pottegård, A.; Bygum, A.; Buchwald, C.; Homøe, P.; Hallas, J. Angiotensin II Receptor Blockers Are Safe in Patients with Prior Angioedema Related to Angiotensin-converting Enzyme Inhibitors—A Nationwide Registry-based Cohort Study. J. Int. Med. 2019, 285, 553–561. [Google Scholar] [CrossRef]
- Boonacker, E.; Van Noorden, C.J. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell Biol. 2003, 82, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tong, X.; Zhang, S.; Wang, D.; Wang, L.; Wang, Q.; Fan, H. The Roles of Dipeptidyl Peptidase 4 (DPP4) and DPP4 Inhibitors in Different Lung Diseases: New Evidence. Front. Pharmacol. 2021, 12, 731453. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Lambertz, A.M.; McCray, P.B., Jr. Dipeptidyl Peptidase 4 Distribution in the Human Respiratory Tract: Implications for the Middle East Respiratory Syndrome. Am. J. Pathol. 2016, 186, 78–86. [Google Scholar] [CrossRef]
- Trajenta, INN-Linagliptin, EMA. Available online: https://www.ema.europa.eu/en/documents/product-information/trajenta-epar-product-information_en.pdf (accessed on 27 March 2025).
- Vipidia, INN-Alogliptin—EMA. Available online: https://www.ema.europa.eu/en/documents/product-information/vipidia-epar-product-information_en.pdf (accessed on 27 March 2025).
- Galvus, INN-Vildagliptin—EMA. Available online: https://www.ema.europa.eu/en/documents/product-information/galvus-epar-product-information_en.pdf (accessed on 27 March 2025).
- Onglyza, EMA. Available online: https://www.ema.europa.eu/en/documents/product-information/onglyza-epar-product-information_en.pdf (accessed on 27 March 2025).
- Januvia, INN-Sitagliptin—EMA. Available online: https://www.ema.europa.eu/en/documents/product-information/januvia-epar-product-information_en.pdf (accessed on 27 March 2025).
- Sridharan, K.; Sivaramakrishnan, G. Interaction between dipeptidyl-peptidase-4 inhibitors and drugs acting on renin angiotensin aldosterone system for the risk of angioedema: A pharmacovigilance assessment using disproportionality and interaction analyses. Diabetol. Metab. Syndr. 2025, 17, 7. [Google Scholar] [CrossRef]
- Ohyama, K.; Shindo, J.; Takahashi, T.; Takeuchi, H.; Hori, Y. Pharmacovigilance study of the association between dipeptidyl peptidase-4 inhibitors and angioedema using the FDA Adverse Event Reporting System (FAERS). Sci. Rep. 2022, 12, 13122. [Google Scholar] [CrossRef]
- Brown, N.J.; Byiers, S.; Carr, D.; Maldonado, M.; Warner, B.A. Dipeptidyl peptidase-IV inhibitor use associated with increased risk of ACE inhibitor-associated angioedema. Hypertension 2009, 54, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.B.; Touzin, K.; Sile, S.; Gainer, J.V.; Yu, C.; Nadeau, J.; Adam, A.; Brown, N.J. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension 2008, 51, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hermanrud, T.; Bygum, A.; Rasmussen, E.R. Recurrent angioedema associated with pharmacological inhibition of dipeptidyl peptidase IV. BMJ Case Rep. 2017, 2017, bcr2016217802. [Google Scholar] [CrossRef]
- Chu, D.Q.; Cox, H.M.; Costa, S.K.; Herzog, H.; Brain, S.D. The ability of neuropeptide Y to mediate responses in the murine cutaneous microvasculature: An analysis of the contribution of Y1 and Y2 receptors. Br. J. Pharmacol. 2003, 140, 422–430. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Stanojević, S.; Vujić, V.; Kovacević-Jovanović, V.; Beck-Sickinger, A.; Demuth, H.; von Hörsten, S. Effect of neuropeptide Y on inflammatory paw edema in the rat: Involvement of peripheral NPY Y1 and Y5 receptors and interaction with dipeptidyl-peptidase IV (CD26). J. Neuroimmunol. 2002, 129, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.F.; Gall, M.G.; Bachovchin, W.W.; McCaughan, G.W.; Keane, F.M.; Gorrell, M.D. Neuropeptide Y is a physiological substrate of fibroblast activation protein: Enzyme kinetics in blood plasma and expression of Y2R and Y5R in human liver cirrhosis and hepatocellular carcinoma. Peptides 2016, 75, 80–95. [Google Scholar] [CrossRef]
- Pedrosa, M.; Prieto-García, A.; Sala-Cunill, A.; Spanish Group for the Study of Bradykinin-Mediated Angioedema (SGBA) and the Spanish Committee of Cutaneous Allergy (CCA). Management of angioedema without urticaria in the emergency department. Ann. Med. 2014, 46, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Hudey, S.N.; Westermann-Clark, E.; Lockey, R.F. Cardiovascular and Diabetic Medications That Cause Bradykinin-Mediated Angioedema. J. Allergy Clin. Immunol. Pract. 2017, 5, 610–615. [Google Scholar] [CrossRef]
- Cassano, N.; Nettis, E.; Di Leo, E.; Ambrogio, F.; Vena, G.A.; Foti, C. Angioedema associated with dipeptidyl peptidase-IV inhibitors. Clin. Mol. Allergy 2021, 19, 24. [Google Scholar] [CrossRef]
- Sharma, N.R.; Sharma, B.; Lamichhane, S.; Pokhrel, M.; Shrestha, P. A Rare Case Report of Sitagliptin-Induced Angioedema. Cureus 2022, 14, e30077. [Google Scholar] [CrossRef]
- Scott, S.I.; Andersen, M.F.; Aagaard, L.; Buchwald, C.V.; Rasmussen, E.R. Dipeptidyl Peptidase-4 Inhibitor Induced Angioedema—An Overlooked Adverse Drug Reaction? Curr. Diabetes Rev. 2018, 14, 327–333. [Google Scholar] [CrossRef]
- Poddar, S.; Chandra, S.; Podder, I. Vildagliptin-Induced Tongue Angioedema: An Uncommon Occurrence. Indian Dermatol. Online J. 2024, 15, 685–686. [Google Scholar] [CrossRef]
- Ejikeme, C.; Nwachukwu, C.; Viechweg, J.L.; Ejikeme, I.; Brescia, M. DPP-IV Inhibitor-Associated Angioedema in Patient with Known History of ACE Inhibitor Angioedema. J. Investig. Med. High. Impact Case Rep. 2021, 9, 23247096211033049. [Google Scholar] [CrossRef] [PubMed]
- Bas, M.; Greve, J.; Strassen, U.; Khosravani, F.; Hoffmann, T.K.; Kojda, G. Angioedema induced by cardiovascular drugs: New players join old friends. Allergy 2015, 70, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Arcani, R.; Martinez, S.; Gayet, S. Sitagliptin and Angioedema. Ann. Intern. Med. 2017, 167, 142–143. [Google Scholar] [CrossRef]
- Williams-Herman, D.; Engel, S.S.; Round, E.; Johnson, J.; Golm, G.T.; Guo, H.; Musser, B.J.; Davies, M.J.; Kaufman, K.D.; Goldstein, B.J. Safety and tolerability of sitagliptin in clinical studies: A pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr. Disord. 2010, 10, 7. [Google Scholar] [CrossRef]
- Membrane Metalloendopeptidase. Available online: https://www.ncbi.nlm.nih.gov/gene/4311 (accessed on 6 April 2025).
- Nalivaeva, N.N.; Zhuravin, I.A.; Turner, A. Neprilysin expression and functions in development, ageing and disease. Mech. Ageing Dev. 2020, 192, 111363. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiao, Y.; An, X.; Luo, L.; Gong, K.; Yu, D. A comprehensive review of the literature on CD10: Its function, clinical application, and prospects. Front. Pharmacol. 2024, 15, 1336310. [Google Scholar] [CrossRef]
- Kalled, S.; Siva, N.; Stein, H.; Reinherz, E.L. The distribution of Cd10 (nep 24.11, calla) in humans and mice is similar in non-lymphoid organs but differs within the hematopoietic system: Absence on murine T and B lymphoid progenitors. Eur. J. Immunol. 1995, 25, 677–687. [Google Scholar] [CrossRef]
- de Leval, L.; Ferry, J.A.; Falini, B.; Shipp, M.; Harris, N.L. Expression of bcl-6 and Cd10 in primary mediastinal large B-cell lymphoma: Evidence for derivation from germinal center B cells? Am. J. Surg. Pathol. 2001, 25, 1277–1282. [Google Scholar] [CrossRef]
- Chu, P.; Arber, D.A. Paraffin-section detection of Cd10 in 505 nonhematopoietic neoplasms. Frequent expression in renal cell carcinoma and endometrial stromal sarcoma. Am. J. Clin. Pathol. 2000, 113, 374–382. [Google Scholar] [CrossRef]
- Thong, A.; Müller, D.; Feuerstacke, C.; Mietens, A.; Stammler, A.; Middendorff, R. Neutral endopeptidase (Cd10) is abundantly expressed in the epididymis and localized to a distinct population of epithelial cells--its relevance for cnp degradation. Mol. Cell. Endocrinol. 2014, 382, 234–243. [Google Scholar] [CrossRef]
- Feygina, E.E.; Katrukha, A.G.; Semenov, A.G. Neutral endopeptidase (neprilysin) in therapy and diagnostics: Yin and yang. Biochemistry 2019, 84, 1346–1358. [Google Scholar] [CrossRef]
- Bayés-Genís, A.; Barallat, J.; Galán, A.; de Antonio, M.; Domingo, M.; Zamora, E.; Urrutia, A.; Lupón, J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J. Am. Coll. Cardiol. 2015, 65, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Entresto, INN-Sacubitril/Valsartan. Available online: https://www.ema.europa.eu/en/documents/product-information/entresto-epar-product-information_en.pdf (accessed on 6 April 2025).
- Raheja, H.; Kumar, V.; Kamholz, S.; Hollander, G.; Shani, J. Life Threatening Angioedema Due to Valsartan/Sacubitril with Previously Well-Tolerated ACE Inhibitor. Am. J. Ther. 2018, 25, e508–e509. [Google Scholar] [CrossRef]
- Hahn, J.; Greve, J.; Bas, M.; Kojda, G. Bradykinin-Mediated Angioedema Induced by Commonly Used Cardiovascular Drugs. Drugs Drug Candidates 2023, 2, 708–727. [Google Scholar] [CrossRef]
- Hahn, J.; Bas, M.; Hoffmann, T.K.; Greve, J. Bradykinin-induced angioedema: Definition, pathogenesis, clinical presentation, diagnosis and therapy. HNO 2015, 63, 885–893; quiz 894–895. [Google Scholar] [CrossRef]
- Lochbaum, R.; Hoffmann, T.K.; Greve, J.; Hahn, J. Concomitant medication in patients with bradykinin-mediated angioedema-there’s more than ACE inhibitors. J. Dtsch. Dermatol. Ges. = J. Ger. Soc. Dermatol. JDDG 2023, 21, 1283–1289. [Google Scholar] [CrossRef]
- Eworuke, E.; Welch, E.C.; Haug, N.; Horgan, C.; Lee, H.S.; Zhao, Y.; Huang, T.Y. Comparative Risk of Angioedema with Sacubitril-Valsartan vs. Renin-Angiotensin-Aldosterone Inhibitors. JACC 2023, 81, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Gurman, P.; Miranda, O.R.; Nathan, A.; Washington, C.; Rosen, Y.; Elman, N.M. Recombinant tissue plasminogen activators (rtPA): A review. Clin. Pharmacol. Ther. 2015, 97, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, K.M. Angioedema after thrombolysis with tissue plasminogen activator: An airway emergency. Oxf. Med. Case Rep. 2019, 2019, omy112. [Google Scholar] [CrossRef] [PubMed]
- Minami, C.; Araki, R.; Hamamoto, T.; Yamada, H. Orolingual Angioedema after Recombinant Tissue Plasminogen Activator Treatment in Acute Cardiogenic Cerebral Embolism Patient Using Olmesartan: A Case Report. Yakugaku Zasshi 2022, 142, 85–89. (In Japanese) [Google Scholar] [CrossRef]
- Mazzoli, C.A.; D’Angelo, M.I.; Simonetti, L.; Cirillo, L.; Zini, A.; Gentile, M.; Gordini, G.; Coniglio, C. Angioedema after rt-PA infusion led to airway emergency: A case report of rescue treatment with fresh frozen plasma. Braz. J. Anesthesiol. 2023, 73, 223–226. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, Y.Q.; Chen, Y.; Zhang, C.H.; Dong, Z.; Wang, Z.; Zhao, S.N.; Li, C.H.; Zhang, P.L. Analysis of related factors of orolingual angioedema after rt-PA intravenous thrombolytic therapy. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Lin, H. Alteplase associated Orolingual angioedema: A case report and literature review. Medicine 2022, 101, e32474. [Google Scholar] [CrossRef]
- Pisani, E.; Gaudiano, C.; Petrone, A.; Stancati, F.; Siniscalchi, A. Isolated Tongue Angioedema after Alteplase Infusion in Acute Ischemic Stroke. Curr. Drug Saf. 2022, 17, 75–77. [Google Scholar] [CrossRef]
- Gauberti, M.; Potzeha, F.; Vivien, D.; Martinez de Lizarrondo, S. Impact of Bradykinin Generation During Thrombolysis in Ischemic Stroke. Front. Med. 2018, 5, 195. [Google Scholar] [CrossRef]
- Hurford, R.; Rezvani, S.; Kreimei, M.; Herbert, A.; Vail, A.; Parry-Jones, A.R.; Douglass, C.; Molloy, J.; Alachkar, H.; Tyrrell, P.J.; et al. Incidence, predictors and clinical characteristics of orolingual angio-oedema complicating thrombolysis with tissue plasminogen activator for ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2015, 86, 520–523. [Google Scholar] [CrossRef]
- Mas-Serrano, M.; García-Pastor, A.; Iglesias-Mohedano, A.M.; Díaz-Otero, F.; Vázquez-Alén, P.; Fernández-Bullido, Y.; Vales-Montero, M.; Amaya-Pascasio, L.; Portela-Sánchez, S.; Cátedra-Caramé, C.; et al. Related factors with orolingual angioedema after intravenous alteplase in acute ischemic stroke: Results from a single-center cohort and meta-analysis. Neurol. Sci. 2022, 43, 441–452. [Google Scholar] [CrossRef]
- Mormile, I.; Palestra, F.; Petraroli, A.; Loffredo, S.; Rossi, F.W.; Spadaro, G.; de Paulis, A.; Bova, M. Neurologic and Psychiatric Manifestations of Bradykinin-Mediated Angioedema: Old and New Challenges. Int. J. Mol. Sci. 2023, 24, 12184. [Google Scholar] [CrossRef]
- O’Carroll, C.B.; Aguilar, M.I. Management of Postthrombolysis Hemorrhagic and Orolingual Angioedema Complications. Neurohospitalist 2015, 5, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ewald, G.A.; Eisenberg, P.R. Plasmin-mediated activation of contact system in response to pharmacological thrombolysis. Circulation 1995, 91, 28–36. [Google Scholar] [CrossRef]
- Cheong, E.; Dodd, L.; Smith, W.; Kleinig, T. Icatibant as a Potential Treatment of Life-Threatening Alteplase-Induced Angioedema. J. Stroke Cerebrovasc. Dis. 2018, 27, e36–e37. [Google Scholar] [CrossRef]
- Mas-Serrano, M.; Garcia-Pastor, A.; Tornero-Molina, P.; Vazquez-Alen, P.; Palacios-Mendoza, M.A.; Gil-Nunez, A.C. Tratamiento del angioedema orolingual inducido por alteplasa mediante icatibant [Treatment of alteplase-induced orolingual angioedema by means of icatibant]. Rev. Neurol. 2019, 69, 261–262. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Bertazzoni, G.; Bresciani, E.; Cipollone, L.; Fante, E.; Galandrini, R. Treatment with icatibant in the management of drug induced angioedema. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 149–153. [Google Scholar] [PubMed]
- Brown, E.; Campana, C.; Zimmerman, J.; Brooks, S. Icatibant for the treatment of orolingual angioedema following the administration of tissue plasminogen activator. Am. J. Emerg. Med. 2018, 36, e1–e1125. [Google Scholar] [CrossRef]
- Wollmach, A.D.; Zehnder, D.; Schwendinger, M.; Tarnutzer, A.A. Unilateral orolingual angioedema in a patient with sarcoidosis after intravenous thrombolysis due to acute stroke without improvement after treatment with icatibant. BMJ Case Rep. 2020, 13, e236643. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, A.; Dimitriadou, E.M.; Tzanetakos, D.; Bakola, E.; Chondrogianni, M.; Palaiodimou, L.; Keramida, A.; Vassilopoulou, S.; Makris, M.; Paraskevas, G.P.; et al. Icatibant averting mechanical ventilation in acute ischemic stroke patient with alteplase-induced orolingual angioedema. Eur. J. Neurol. 2024, 31, e16173. [Google Scholar] [CrossRef] [PubMed]
- Pahs, L.; Droege, C.; Kneale, H.; Pancioli, A. A Novel Approach to the Treatment of Orolingual Angioedema After Tissue Plasminogen Activator Administration. Ann. Emerg. Med. 2016, 68, 345–348. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418, Erratum in Stroke 2019, 50, e440–e441. https://doi.org/10.1161/STR.0000000000000215. [Google Scholar] [CrossRef]
- Li, S.; Gu, H.Q.; Li, H.; Wang, X.; Jin, A.; Guo, S.; Lu, G.; Che, F.; Wang, W.; Wei, Y.; et al. Reteplase versus Alteplase for Acute Ischemic Stroke. N. Engl. J. Med. 2024, 390, 2264–2273. [Google Scholar] [CrossRef]
- Zhu, A.; Rajendram, P.; Tseng, E.; Coutts, S.B.; Yu, A.Y.X. Alteplase or tenecteplase for thrombolysis in ischemic stroke: An illustrated review. Res. Pract. Thromb. Haemost. 2022, 6, e12795. [Google Scholar] [CrossRef]
- Rose, D.; Cavalier, A.; Kam, W.; Cantrell, S.; Lusk, J.; Schrag, M.; Yaghi, S.; Stretz, C.; de Havenon, A.; Saldanha, I.J.; et al. Complications of Intravenous Tenecteplase Versus Alteplase for the Treatment of Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Stroke 2023, 54, 1192–1204. [Google Scholar] [CrossRef]
- Menon, B.K.; Buck, B.H.; Singh, N.; Deschaintre, Y.; Almekhlafi, M.A.; Coutts, S.B.; Thirunavukkarasu, S.; Khosravani, H.; Appireddy, R.; Moreau, F.; et al. AcT Trial Investigators. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): A pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 2022, 400, 161–169. [Google Scholar] [CrossRef]
- Xiang, H.; Ma, Y.; Luo, X.; Guo, J.; Yao, M.; Liu, Y.; Deng, K.; Sun, X.; Li, L. Risk of angioedema and thrombolytic therapy among stroke patients: An analysis of data from the FDA Adverse Event Reporting System database. Neurotherapeutics 2025, 22, e00474. [Google Scholar] [CrossRef]
- Sekita, A.; Siedler, G.; Sembill, J.A.; Schmidt, M.; Singer, L.; Kallmuenzer, B.; Mers, L.; Bogdanova, A.; Schwab, S.; Gerner, S.T. Switch to tenecteplase for intravenous thrombolysis in stroke patients: Experience from a German high-volume stroke center. Neurol. Res. Pract. 2025, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Firinu, D.; Bafunno, V.; Vecchione, G.; Barca, M.P.; Manconi, P.E.; Santacroce, R.; Margaglione, M.; Del Giacco, S.R. Characterization of patients with angioedema without wheals: The importance of F12 gene screening. Clin. Immunol. 2015, 157, 239–248. [Google Scholar] [CrossRef]
- Bova, M.; Suffritti, C.; Bafunno, V.; Loffredo, S.; Cordisco, G.; Del Giacco, S.; De Pasquale, T.M.A.; Firinu, D.; Margaglione, M.; Montinaro, V.; et al. Impaired control of the contact system in hereditary angioedema with normal C1-inhibitor. Allergy 2020, 75, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Baş, M.; Greve, J.; Stelter, K.; Havel, M.; Strassen, U.; Rotter, N.; Veit, J.; Schossow, B.; Hapfelmeier, A.; Kehl, V.; et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N. Engl. J. Med. 2015, 372, 418–425. [Google Scholar] [CrossRef]
- Straka, B.T.; Ramirez, C.E.; Byrd, J.B.; Stone, E.; Woodard-Grice, A.; Nian, H.; Yu, C.; Banerji, A.; Brown, N.J. Effect of bradykinin receptor antagonism on ACE inhibitor-associated angioedema. J. Allergy Clin. Immunol. 2017, 140, 242–248.e2. [Google Scholar] [CrossRef]
- Sinert, R.; Levy, P.; Bernstein, J.A.; Body, R.; Sivilotti, M.L.A.; Moellman, J.; Schranz, J.; Baptista, J.; Kimura, A.; Nothaft, W.; et al. Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema. J. Allergy Clin. Immunol. Pract. 2017, 5, 1402–1409.e3. [Google Scholar] [CrossRef]
- Pitts, J.K.; Burns, D.M.; Patellos, K.R. Tenecteplase-associated orolingual angioedema: A case report and literature review. Am. J. Health Syst. Pharm. 2024, 81, e220–e225. [Google Scholar] [CrossRef] [PubMed]
- Lapostolle, A.; Weisenburger-Lile, D.; Yger, M.; Alamowitch, S.; Fain, O. Bradykinin-Mediated Angioedema Following Tenecteplase Administration in an Acute Ischemic Stroke. Stroke 2022, 53, e446–e447. [Google Scholar] [CrossRef] [PubMed]
| ACEIs | ARBs | DPP-IV Inhibitors | Neprilysin Inhibitors | Alteplase | |
|---|---|---|---|---|---|
| Drug class | Antihypertensive | Antihypertensive | Gliptins | Antihypertensive | Thrombolytic agent |
| Mechanism of Action | Inhibition of ACE | Selective blocking of angiotensin II | Inhibition of DPP-IV | Inhibition of NEP (neutral endopeptidase) | Conversion of plasminogen in plasmin |
| Reported frequency | 0.1–6% | 0.03–0.2% | ≥1/10,000– <1/1000 | ≥1/1000– <1/100 | 0.2–7.9% |
| Underlying pathomechanisms | Defect of des-Arg-9-BK | Increased activity of RAAS | Scarce degradation of BK | Scarce degradation of BK | Kinin system activation by plasminogen |
| Risk Factors: | |||||
| BDKRB2 | ||||
| KCNMA1 | KCNMA1 | ||||
| XPNPEP2 | |||||
| F5 | F5 | ||||
| 20q11.22 | |||||
| PROCR | PROCR | ||||
| EDEM2 | EDEM2 | ||||
| African American race | Concomitant use of ACEIs | Switching from ACEIs or ARBs | Caucasian race | |
| Smoking | Smoking | Renal dysfunction | Female sex | ||
| Older age | Older age | History of drug-induced AE | Hypertension | ||
| Female sex | Allergies | Diabetes | |||
| Heart failure | Dyslipidemia | ||||
| History of drug hypersensibility | ACEIs treatment | ||||
| Immunosuppression | |||||
| Localization of AE | Face, oral mucosa, tongue, lips, pharynx, larynx | Face, lips, tongue | Tongue, lips, oropharynx | ||
| Therapy | ACEIs discontinuation | Fresh frozen plasma | |||
| Fresh frozen plasma | C1INH concentrate | ||||
| Ecallantide | Icatibant | ||||
| Icatibant | |||||
| Tranexamic acid | |||||
| C1INH concentrate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suffritti, C.; Chan, S.; Ferrara, A.L.; Lekli, E.; Palestra, F.; Tuncay, G.; Loffredo, S.; Bova, M. Bradykinin-Mediated Angioedema Induced by Drugs. J. Clin. Med. 2025, 14, 5712. https://doi.org/10.3390/jcm14165712
Suffritti C, Chan S, Ferrara AL, Lekli E, Palestra F, Tuncay G, Loffredo S, Bova M. Bradykinin-Mediated Angioedema Induced by Drugs. Journal of Clinical Medicine. 2025; 14(16):5712. https://doi.org/10.3390/jcm14165712
Chicago/Turabian StyleSuffritti, Chiara, Samantha Chan, Anne Lise Ferrara, Eralda Lekli, Francesco Palestra, Gülseren Tuncay, Stefania Loffredo, and Maria Bova. 2025. "Bradykinin-Mediated Angioedema Induced by Drugs" Journal of Clinical Medicine 14, no. 16: 5712. https://doi.org/10.3390/jcm14165712
APA StyleSuffritti, C., Chan, S., Ferrara, A. L., Lekli, E., Palestra, F., Tuncay, G., Loffredo, S., & Bova, M. (2025). Bradykinin-Mediated Angioedema Induced by Drugs. Journal of Clinical Medicine, 14(16), 5712. https://doi.org/10.3390/jcm14165712








