The Impact of Endurance Exercise on Routine Laboratory Parameters in Young Trained Individuals
Abstract
1. Introduction
2. Materials and Methods
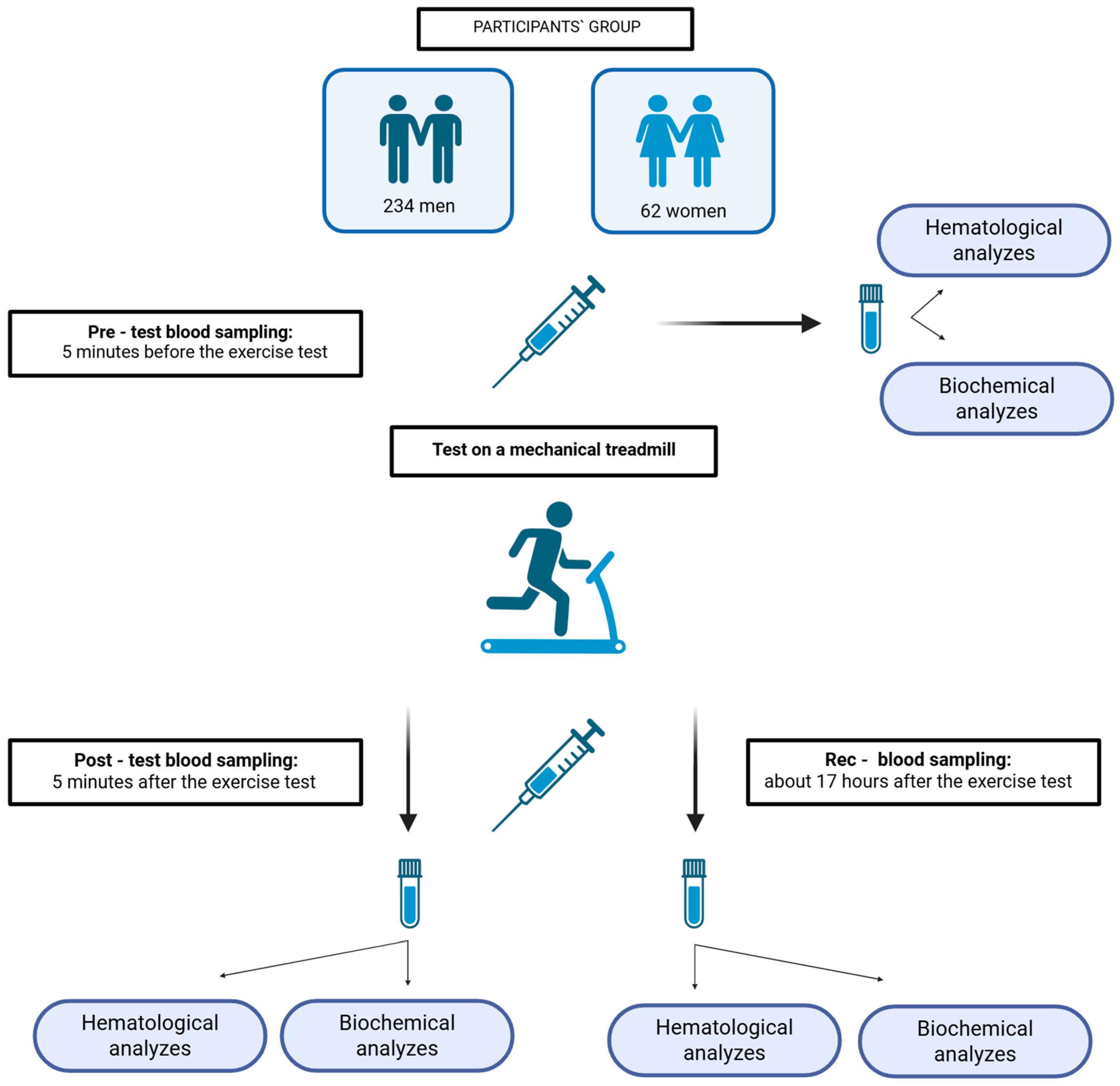
2.1. Participants
2.2. Exercise Tests and Blood Sampling
2.3. Medical Laboratory Methods
2.3.1. Hematological Analyses
2.3.2. Biochemical Analyses
2.4. Calculations and Statistical Analyses
3. Results
4. Discussion
4.1. The Impact of Endurance Effort on Blood Morphology
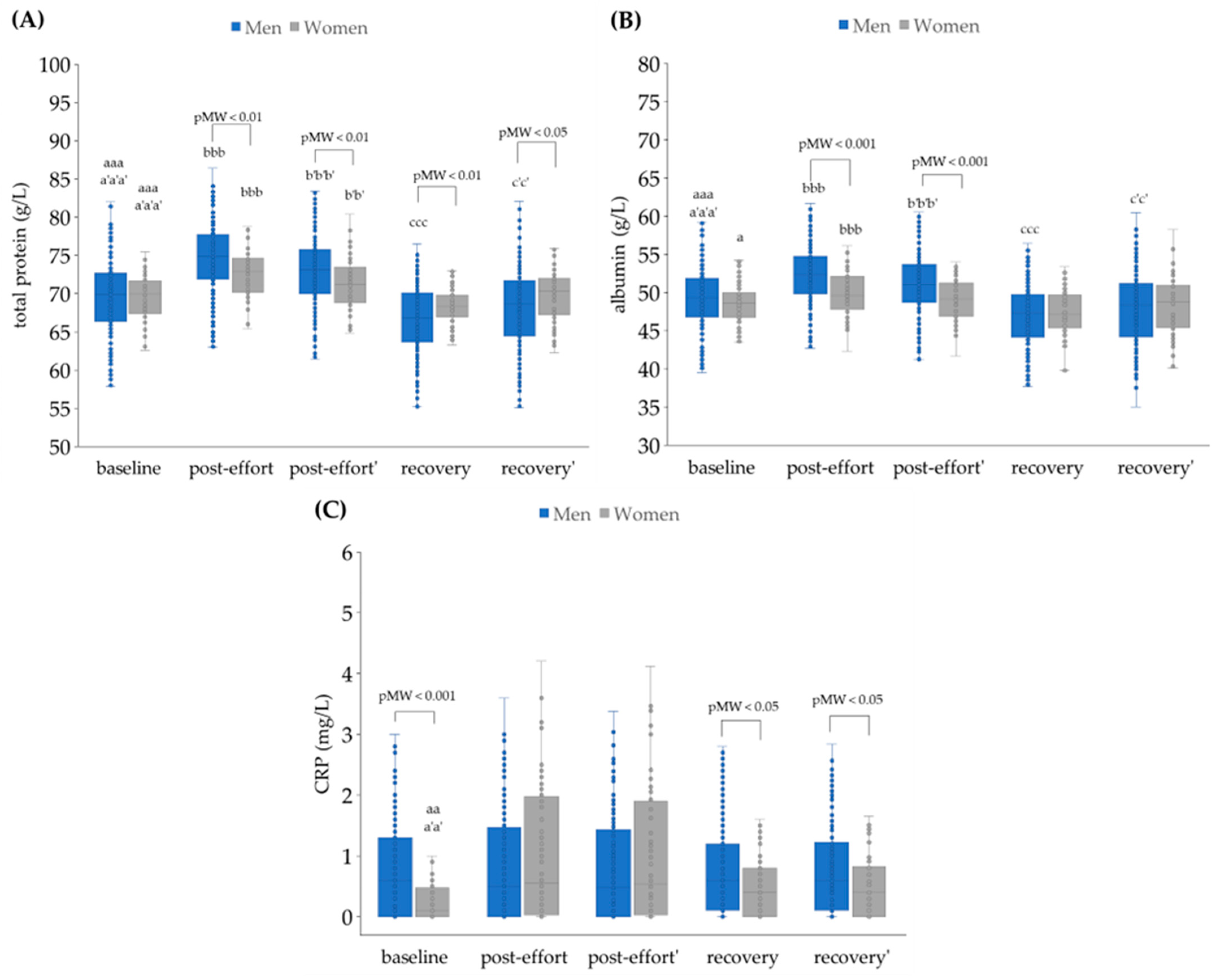
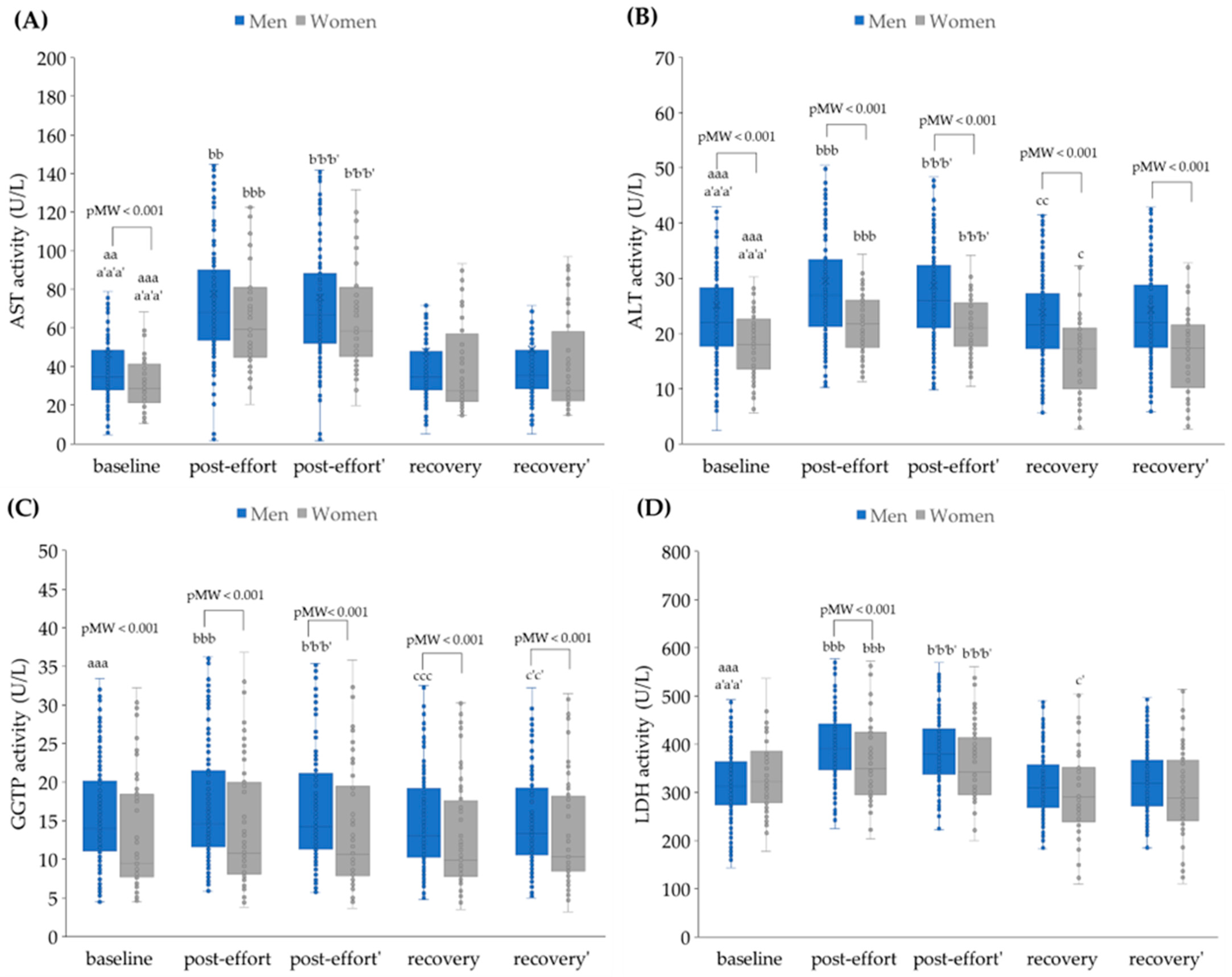
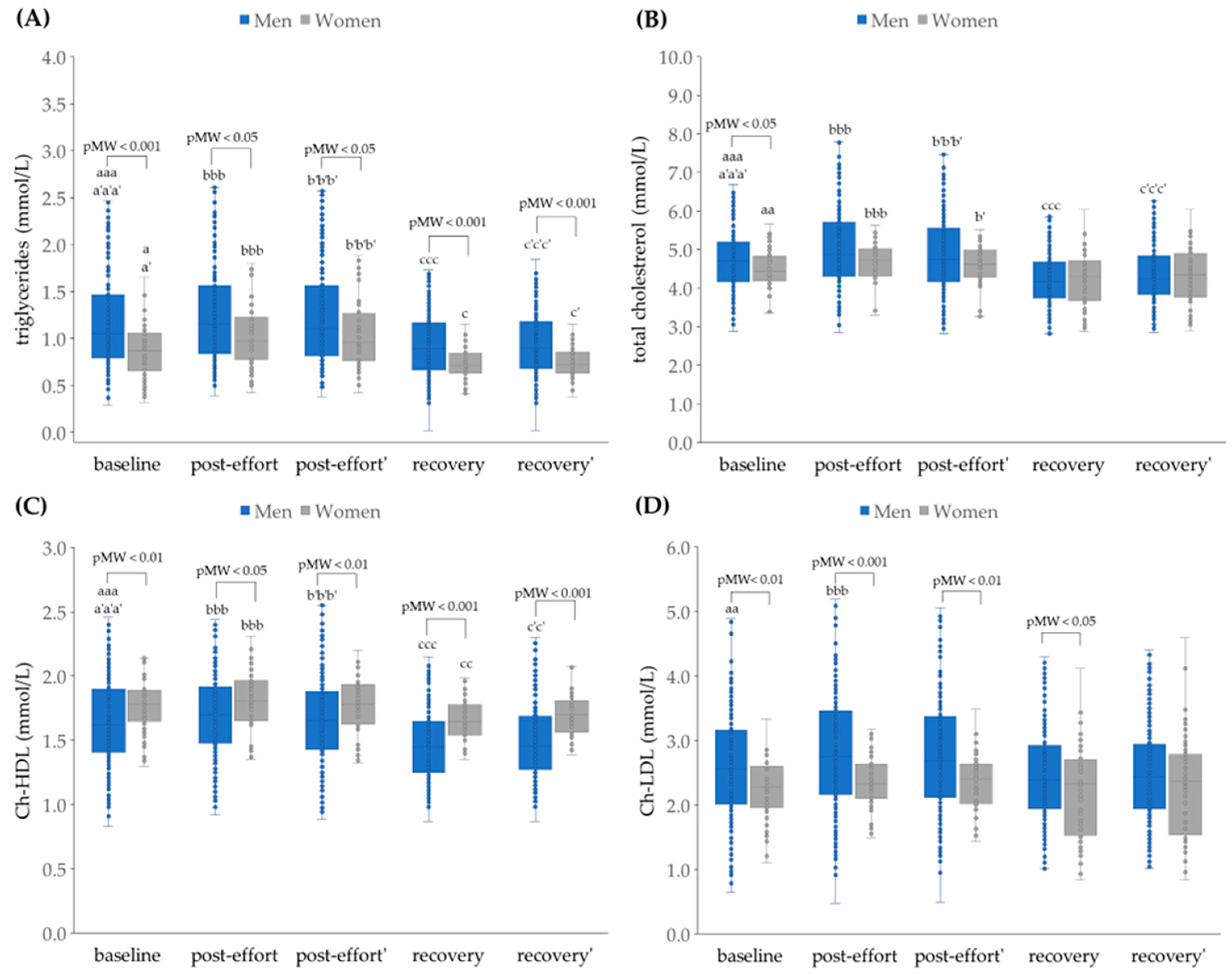
4.2. The Impact of Physical Effort on Selected Biochemical Variables
4.2.1. Plasma Volume Changes as a Post-Effort Factor
4.2.2. The Changes in Activities of Selected Enzymes as a Post-Effort Effect
4.2.3. The Changes in Concentration of Selected Metabolites as a Post-Effort Effect
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ATP | adenosine triphosphate |
| BMI | body mass index |
| Ch-HDL | high-density lipoprotein cholesterol |
| Ch-LDL | low-density lipoprotein cholesterol |
| CK | creatine kinase |
| CRP | C-reactive protein |
| EDTA | ethylenediaminetetraacetic acid |
| GGTP | gamma-glutamyltransferase |
| GRA | granulocytes |
| HGB | hemoglobin |
| HTC | hematocrit |
| HDL | high-density lipoprotein |
| iCa | ionized calcium |
| LDH | lactate dehydrogenase |
| LDL | low-density lipoprotein |
| LYM | lymphocytes |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| MCV | mean corpuscular volume |
| MON | monocytes |
| MPV | mean platelet volume |
| PCT | plateletcrit |
| PDW | platelet distribution width |
| PLT | platelet count |
| RBC | red blood cells |
| RDW | red cell distribution width |
| TC | total cholesterol |
| tCa | total calcium |
| TG | triglycerides |
| TP | total protein |
| UA | uric acid |
| WBC | white blood cells |
| ΔPV | change in plasma volume |
References
- CDC. Centers for Disease Control and Prevention. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/nchs/nhis/physical_activity/pa_glossary.htm (accessed on 10 October 2017).
- WHO. World Health Organization. Available online: https://iris.who.int/bitstream/handle/10665/341120/WHO-EURO-2021-1204-40953-58211-pol.pdf (accessed on 1 March 2025).
- Smith, D.J. A Framework for Understanding the Training Process Leading to Elite Performance. Sports Med. 2003, 33, 1103–1126. [Google Scholar] [CrossRef]
- Kojda, G.; Hambrecht, R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc. Res. 2005, 67, 187–197. [Google Scholar] [CrossRef]
- Huertas, J.R.; Casuso, R.A.; Agustín, P.H.; Cogliati, S. Stay Fit, Stay Young: Mitochondria in Movement: The Role of Exercise in the New Mitochondrial Paradigm. Oxid. Med. Cell Longev. 2019, 2019, 7058350. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef]
- Perry, C.G.R.; Lally, J.; Holloway, G.P.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010, 588, 4795–4810. [Google Scholar] [CrossRef]
- Davies, K.J.A.; Packer, L.; Brooks, F.G.A.; Laboratmq, P. Biochemical Adaptation of Mitochondria, Muscle, and Whole-Animal Respiration to Endurance Training. Arch. Biochem. Biophys. 1981, 209, 539–554. [Google Scholar] [CrossRef]
- Neufer, P.D. The Bioenergetics of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029678. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Holloszy John, O.; Coyle Edward, F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. 1984, 56, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Koval, J.A.; DeFronzo, R.A.; O’Doherty, R.M.; Printz, R.; Ardehali, H.; Granner, D.K.; Mandarino, L.J. Regulation of hexokinase II activity and expression in human muscle by moderate exercise. Am. J. Physiol.-Endocrinol. Metab. 1998, 274, 304–308. [Google Scholar] [CrossRef]
- Flück, M.; Hoppeler, H. Molecular basis of skeletal muscle plasticity from gene to form and function. Rev. Physiol. Biochem. Pharmacol. 2003, 146, 159–216. [Google Scholar] [CrossRef]
- Shakoor, H.; Kizhakkayil, J.; Khalid, M.; Mahgoub, A.; Platat, C. Effect of Moderate-Intense Training and Detraining on Glucose Metabolism, Lipid Profile, and Liver Enzymes in Male Wistar Rats: A Preclinical Randomized Study. Nutrients 2023, 15, 3820. [Google Scholar] [CrossRef]
- Coffey, V.G.; Zhong, Z.; Shield, A.; Canny, B.J.; Chibalin, A.V.; Zierath, J.R.; Hawley, J.A. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006, 20, 190–192. [Google Scholar] [CrossRef]
- Pi, A.; Villivalam, S.D.; Kang, S. The Molecular Mechanisms of Fuel Utilization during Exercise. Biology 2023, 12, 1450. [Google Scholar] [CrossRef]
- Ashcroft, S.P.; Stocks, B.; Egan, B.; Zierath, J.R. Exercise induces tissue-specific adaptations to enhance cardiometabolic health. Cell Metab. 2024, 36, 278–300. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef]
- Furrer, R.; Hawley, J.A.; Handschin, C. The molecular athlete: Exercise physiology from mechanisms to medals. Physiol. Rev. 2023, 103, 1693–1787. [Google Scholar] [CrossRef]
- Gillen, C.M.; Lee, R.; Mack, G.W.; Tomaselli, C.M.; Nishiyasu, T.; Nadel, E.R. Plasma volume expansion in humans after a single intense exercise protocol. J. Appl. Physiol. 1991, 71, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A. Blood volume: Its adaptation to endurance training. Med. Sci. Sports Exerc. 1991, 23, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Steinach, M.; Lichti, J.; Maggioni, M.A.; Fähling, M. A fluid shift for endurance exercise—Why hydration matters. Acta Physiol. 2019, 227, e13347. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Brock, J.; Keil, L.C.; Bernauer, E.M.; Greenleaf, J.E. Exercise training-induced hypervolemia: Role of plasma albumin, renin, and vasopressin. J. Appl. Physiol. 1980, 48, 665–669. [Google Scholar] [CrossRef]
- Maughan, R.; Shirreffs, S. Exercise in the heat: Challenges and opportunities. J. Sports Sci. 2004, 22, 917–927. [Google Scholar] [CrossRef]
- Smith, E.E.; Guyton, A.C.; Manning, R.D.; White, R.J. Integrated Mechanisms of Cardiovascular Response and Control During Exercise in the Normal Human. Prog. Cardiovasc. Dis. 1976, 18, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.D.; Eijsvogels, M.H.T.; Daanen, A.M.H. Exercise under heat stress: Thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef]
- Alis, R.; Sanchis-Gomar, F.; Lippi, G.; Roamgnoli, M. Microcentrifuge or Automated Hematological Analyzer to Assess Hematocrit in Exercise? Effect on Plasma Volume Loss Calculations. J. Lab. Autom. 2016, 21, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Mandić, M.; Forsgren, M.F.; Romu, T.; Widholm, P.; Sundblad, P.; Gustafsson, T.; Rullman, E. Interval-induced metabolic perturbation determines tissue fluid shifts into skeletal muscle. Physiol. Rep. 2021, 9, e14841. [Google Scholar] [CrossRef] [PubMed]
- Green, H.J.; Thomson, J.A.; Ball, M.E.; Hughson, R.L.; Houston, M.E.; Sharratt, M.T. Alterations in blood volume following short-term supramaximal exercise. J. Appl. Physiol. 1984, 56, 145–149. [Google Scholar] [CrossRef]
- Alis, R.; Ibañez-Sania, S.; Basterra, J.; Sanchis-Gomar, F.; Romagnoli, M. Effects of an acute high-intensity interval training protocol on plasma viscosity. J. Sports Med. Phys. Fit. 2015, 55, 647–653. [Google Scholar]
- Alis, R.; Sanchis-Gomar, F.; Primo-Carrau, C.; Lozano-Calve, S.; Dipalo, M.; Aloe, R.; Blesa, J.R.; Romagnoli, M.; Lippi, G. Hemoconcentration induced by exercise: Revisiting the Dill and Costill equation. Scand. J. Med. Sci. Sports 2015, 25, e630–e637. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Lippi, G. Physical activity—An important preanalytical variable. Biochem. Med. 2014, 24, 68–79. [Google Scholar] [CrossRef]
- Lippi, G.; Guidi, G.C.; Mattiuzzi, C.; Plebani, M. Preanalytical variability: The dark side of the moon in laboratory testing. Clin. Chem. Lab. Med. 2006, 44, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.R.; Linden, M.; Hawley, J.A. Acute changes to biomarkers as a consequence of prolonged strenuous running. Ann. Clin. Biochem. 2013, 51, 137–150. [Google Scholar] [CrossRef]
- Palacios, G.; Pedrero-Chamizo, R.; Palacios, N.; Maroto-Sánchez, B.; Aznar, S.; González-Gross, M.; EXERNET Study Group. Biomarkers of physical activity and exercise. Nutr. Hosp. 2015, 31 (Suppl. S3), 237–244. [Google Scholar] [CrossRef]
- Lippi, G.; Banfi, G.; Botrè, F.; de la Torre, X.; De Vita, F.; Gomez-Cabrera, M.C.; Maffulli, N.; Marchioro, L.; Pacifici, R.; Sanchis-Gomar, F.; et al. Laboratory medicine and sports: Between Scylla and Charybdis. Clin. Chem. Lab. Med. 2012, 50, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Nowak, D.; Nowak, R. Analysis of selected T cell subsets in peripheral blood after exhaustive effort among elite soccer players. Biochem. Med. 2018, 28, 030707. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Nowak, D.; Nowak, R. Differential Th Cell-Related Immune Responses in Young Physically Active Men after an Endurance Effort. J. Clin. Med. 2020, 9, 1795. [Google Scholar] [CrossRef]
- Dorwart, W.V.; Chalmers, L. Comparison of Methods for Calculating Serum Osmolality from Chemical Concentrations, and the Prognostic Value of Such Calculations. Clin. Chem. 1975, 21, 190–194. [Google Scholar] [CrossRef]
- Khajuria, A.; Krahn, J. Osmolality revisited—Deriving and validating the best formula for calculated osmolality. Clin. Biochem. 2005, 38, 514–519. [Google Scholar] [CrossRef]
- Kratz, A.; Lewandrowski, K.B.; Siegel, A.J.; Chun, K.Y.; Flood, J.G.; Van Cott, E.M.; Lee-Lewandrowski, E. Effect of Marathon Running on Hematologic and Biochemical Laboratory Parameters, Including Cardiac Markers. Am. J. Clin. Pathol. 2002, 118, 856–863. [Google Scholar] [CrossRef]
- Diaz-Garzon, J.; Fernandez–Calle, P.; Aarsand, A.K.; Sandberg, S.; Coskun, A.; Equey, T.; Aikin, R.; Soto, A.B. Long-Term Within- and Between-Subject Biological Variation Data of Hematological Parameters in Recreational Endurance Athletes. Clin. Chem. 2023, 69, 500–509. [Google Scholar] [CrossRef]
- Skarpańska-Stejnborn, A.; Basta, P.; Trzeciak, J.; Szcześniak-Pilaczyńska, Ł. Effect of intense physical exercise on hepcidin levels and selected parameters of iron metabolism in rowing athletes. Eur. J. Appl. Physiol. 2015, 115, 345–351. [Google Scholar] [CrossRef]
- Cichoń, J.; Ostapiuk-Karolczuk, J.; Cieślicka, M.; Dziewiecka, H.; Marcinkiewicz, A.; Tafil-Klawe, M.; Basta, P.; Maciejewski, D.; Skarpańska-Stejnborn, A. Effect of an acute exercise on early responses of iron and iron regulatory proteins in young female basketball players. BMC Sports Sci. Med. Rehabil. 2022, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Chen, K.T.; Shee, B.W.; Chang, H.C.; Huang, Y.J.; Yang, R.S. Effects of 24 h ultra-marathon on biochemical and hematological parameters. World J. Gastroenterol. 2004, 10, 2711–2714. [Google Scholar] [CrossRef]
- Telford, R.D.; Sly, G.J.; Hahn, A.G.; Cunningham, R.B.; Bryant, C.; Smith, J.A. Footstrike is the major cause of hemolysis during running. J. Appl. Physiol. 2003, 94, 38–42. [Google Scholar] [CrossRef]
- Smith, J.E.; Garbutt, G.; Lopes, P.; Tunstall Pedoe, D. Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Br. J. Sports Med. 2004, 38, 292–294. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef]
- Schild, M.; Eichner, G.; Beiter, T.; Zügel, M.; Krumholz-Wagner, I.; Hudemann, J.; Pilat, C.; Krüger, K.; Niess, A.M.; Steinacker, J.M.; et al. Effects of Acute Endurance Exercise on Plasma Protein Profiles of Endurance-Trained and Untrained Individuals over Time. Mediat. Inflamm. 2016, 2016, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Mack, G.W.; Haskell, A.; Nishiyasu, T.; Nadel, E.R. Mechanism for the posture-specific plasma volume increase after a single intense exercise protocol. J. Appl. Physiol. 1999, 86, 867–873. [Google Scholar] [CrossRef]
- Kostrzewa-Nowak, D.; Kubaszewska, J.; Nowakowska, A.; Nowak, R. Effect of Aerobic and Anaerobic Exercise on the Complement System of Proteins in Healthy Young Males. J. Clin. Med. 2020, 9, 2357. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Keil, L.C.; Bernauer, E.M.; Greenleaf, J.E. Plasma volume, osmolality, vasopressin, and renin activity during graded exercise in man. J. Appl. Physiol. 1981, 50, 123–128. [Google Scholar] [CrossRef]
- Haskell, A.; Nadel, E.R.; Stachenfeld, N.S.; Nagashima, K.; Mack, G.W. Transcapillary escape rate of albumin in humans during exercise-induced hypervolemia. J. Appl. Physiol. 1997, 83, 407–413. [Google Scholar] [CrossRef]
- Arakawa, K.; Hosono, A.; Shibata, K.; Ghadimi, R.; Fuku, M.; Goto, C.; Imaeda, N.; Tokudome, Y.; Hoshino, H.; Marumoto, M.; et al. Changes in blood biochemical markers before, during, and after a 2-day ultramarathon. Open Access J. Sports Med. 2016, 7, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.A.; Park, K.D.; Ahn, J.; Park, Y.; Kim, Y.J. Comparison of Changes in Biochemical Markers for Skeletal Muscles, Hepatic Metabolism, and Renal Function after Three Types of Long-distance Running. Medicine 2016, 95, e3657. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Wu, J.; Kavouras, S.A.; Mack, G.W. Increased renal tubular sodium reabsorption during exercise-induced hypervolemia in humans. J. Appl. Physiol. 2001, 91, 1229–1236. [Google Scholar] [CrossRef]
- Lombardo, B.; Izzo, V.; Terracciano, D.; Ranieri, A.; Mazzaccara, C.; Fimiani, F.; Cesaro, A.; Gentile, L.; Leggiero, E.; Pero, R.; et al. Laboratory medicine: Health evaluation in elite athletes. Clin. Chem. Lab. Med. 2019, 57, 1450–1473. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar] [CrossRef]
- Kargotich, S.; Goodman, C.; Keast, D.; Morton, A.R. The Influence of Exercise-Induced Plasma Volume Changes on the Interpretation of Biochemical Parameters Used for Monitoring Exercise, Training and Sport. Sports Med. 1998, 26, 101–117. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Montagnana, M.; Salvagno, G.L.; Guidi, G.C. Influence of acute physical exercise on emerging muscular biomarkers. Clin. Chem. Lab. Med. 2008, 46, 1313–1318. [Google Scholar] [CrossRef]
- Thomas, L. (Ed.) Effect of physical exercise on laboratory test results. In Clinical Laboratory Diagnostics; De Gruyter: Frankfurt am Main, Germany, 2025; Chapter 51; Available online: https://clinical-laboratory-diagnostics.com (accessed on 16 March 2025).
- Trajkovic, N.; Sporis, G.; Vlahovic, T.; Madic, D.; Gusic, M. Post-match changes in muscle damage markers among U-21 soccer players. Monten. J. Sports Sci. Med. 2018, 7, 49–53. [Google Scholar] [CrossRef]
- Diaz-Garzon, J.; Fernandez-Calle, P.; Aarsand, A.K.; Sandberg, S.; Coskun, A.; Carobene, A.; Jonker, N.; Itkonen, O.; Bartlett, W.A.; Buno, A.; et al. Long-term within- and between-subject biological variation of 29 routine laboratory measurands in athletes. Clin. Chem. Lab. Med. 2021, 60, 618–628. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Salvagno, G.L.; Montagnana, M.; Gelati, M.; Tarperi, C.; Banfi, G.; Guidi, G.C. Acute variation of biochemical markers of muscle damage following a 21 km, half-marathon run. Scand. J. Clin. Lab. Invest. 2008, 68, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Chamera, T.; Spieszny, M.; Klocek, T.; Kostrzewa-Nowak, D.; Nowak, R.; Lachowicz, M.; Buryta, R.; Ficek, K.; Eider, J.; Moska, W.; et al. Post-effort changes in activity of traditional diagnostic enzymatic markers in football players’ blood. J. Med. Biochem. 2015, 34, 179–190. [Google Scholar] [CrossRef]
- Kostrzewa-Nowak, D.; Nowakowska, A.; Zwierko, T.; Rybak, M.; Nowak, R. The Influence of a Health-Related Fitness Training Program on Motor Performance as Well as Hematological and Biochemical Parameters. Int. J. Env. Res. Public. Health 2020, 17, 578. [Google Scholar] [CrossRef]
- Chamera, T.; Spieszny, M.; Klocek, T.; Kostrzewa-Nowak, D.; Nowak, R.; Lachowicz, M.; Buryta, R.; Cięszczyk, P. Could Biochemical Liver Profile Help to Assess Metabolic Response to Aerobic Effort in Athletes? J. Strength. Cond. Res. 2014, 28, 2180–2186. [Google Scholar] [CrossRef]
- Nie, J.; Tong, T.K.; George, K.; Fu, F.H.; Lin, H.; Shi, Q. Resting and post-exercise serum biomarkers of cardiac and skeletal muscle damage in adolescent runners. Scand. J. Med. Sci. Sports 2011, 21, 625–629. [Google Scholar] [CrossRef]
- Tirabassi, J.N.; Olewinski, L.; Khodaee, M. Variation of Traditional Biomarkers of Liver Injury After an Ultramarathon at Altitude. Sports Health 2018, 10, 361–365. [Google Scholar] [CrossRef]
- Neumayr, G.; Pfister, R.; Hoertnagl, H.; Mitterbauer, G.; Getzner, W.; Ulmer, H.; Gaenzer, H.; Joannidis, M. The effect of marathon cycling on renal function. Int. J. Sports Med. 2003, 24, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Nowak, D.; Nowak, R.; Jastrzębski, Z.; Zarębska, A.; Bichowska, M.; Drobnik-Kozakiewicz, I.; Radzimiński, Ł.; Leońska-Duniec, A.; Ficek, K.; Cięszczyk, P. Effect of 12-week-long aerobic training programme on body composition, aerobic capacity, complete blood count and blood lipid profile among young women. Biochem. Med. 2015, 25, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Neumayr, C.; Pfister, R.; Hoertnagl, H.; Mitterbauer, G.; Prokop, W.; Joannidis, M. Renal Function and Plasma Volume Following Ultramarathon Cycling. Int. J. Sports Med. 2005, 26, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Schena, F.; Montagnana, M.; Salvagno, G.L.; Banfi, G.; Guidi, G.C. Significant variation of traditional markers of liver injury after a half-marathon run. Eur. J. Intern. Med. 2011, 22, 36–38. [Google Scholar] [CrossRef]
- Leers, M.P.G.; Schepers, R.; Baumgarten, R. Effects of a long-distance run on cardiac markers in healthy athletes. Clin. Chem. Lab. Med. 2006, 44, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Jassal, D.S.; Moffat, D.; Krahn, J.; Ahmadie, R.; Fang, T.; Eschun, G.; Sharma, S. Cardiac injury markers in non-elite marathon runners. Int. J. Sports Med. 2009, 30, 75–79. [Google Scholar] [CrossRef]
- Berković, M.C.; Matanović, L.; Buljubašić, R.; Marijančević, D.; Žarak, M.; Perović, A.; Šimac, B.; Marević, S.; Biljak, V.R.; Đerek, L. Laboratory medicine and sports: Where are we now? Biochem. Med. 2024, 34, 030501. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, O.; Borén, J.; et al. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 2016, 37, 1944–1958. [Google Scholar] [CrossRef] [PubMed]
- Langsted, A.; Nordestgaard, B.G. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology 2019, 51, 131–141. [Google Scholar] [CrossRef] [PubMed]


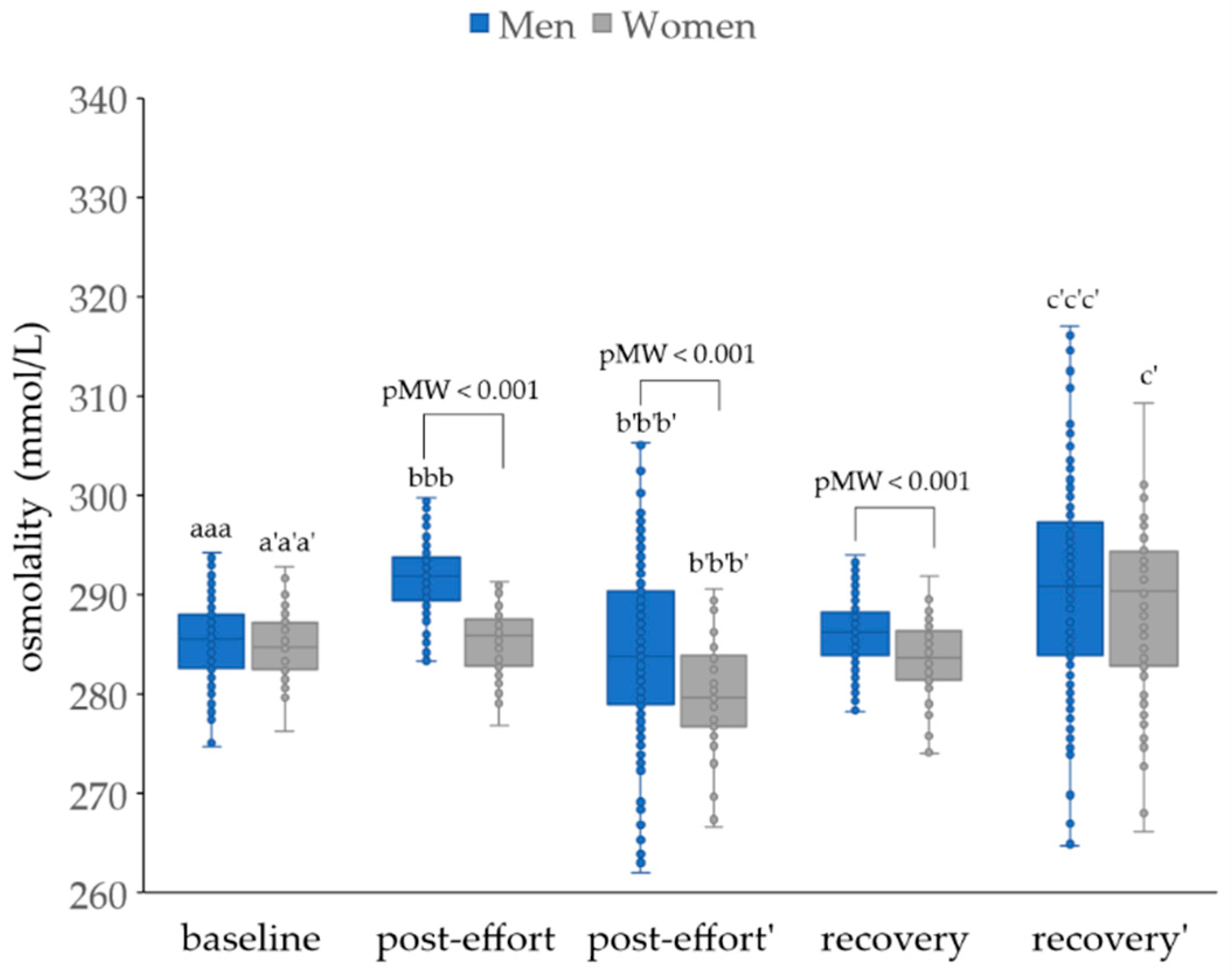
| Parameter | Men | Women |
|---|---|---|
| n = 234 | n = 62 | |
| Age (years) | 19 (16–36) | 21 (16–29) |
| Height (cm) | 182 (179–185) | 172 (165–176) |
| Weight (kg) | 74.9 (69.7–80.0) | 64.6 (61.0–68.7) |
| BMI (kg/m2) | 22.6 (21.5–23.6) | 22.3 (20.8–23.2) |
| VO2max | 60.9 (58.4–64.4) | 46.5 (42.5–50.4) |
| Length of training experience (years) | 12 (10–14) | 9 (6–10.5) |
| Weekly training volumes (h) | 12 (10–14) | 9 (6–12) |
| Variable | Time Point | Men n = 234 | Women n = 62 | pMW |
|---|---|---|---|---|
| RBC (109/L) | baseline | 5.1 aaa (4.8–5.3) | 4.5 aaa (4.3–4.7) | <0.001 |
| post-effort | 5.1 bbb (5.0–5.4) | 4.6 bbb (4.4–4.9) | <0.001 | |
| recovery | 4.9 ccc (4.7–5.2) | 4.4 ccc (4.2–4.6) | <0.001 | |
| HGB (mmol/L) | baseline | 9.3 aaa (8.8–9.6) | 8.0 aaa (7.6–8.3) | <0.001 |
| post-effort | 9.5 bbb (9.0–9.8) | 8.1 bbb (7.7–8.4) | <0.001 | |
| recovery | 9.1 ccc (8.5–9.5) | 7.9 cc (7.3–8.2) | <0.001 | |
| HTC (L/L) | baseline | 0.45 aaa (0.44–0.47) | 0.40 aaa (0.38–0.41) | <0.001 |
| post-effort | 0.47 bbb (0.45–0.49) | 0.41 bbb (0.39–0.42) | <0.001 | |
| recovery | 0.45 ccc (0.43–0.46) | 0.39 ccc (0.38–0.40) | <0.001 | |
| MCV (fL) | baseline | 91 aaa (88–93) | 88 (86–91) | <0.001 |
| post-effort | 91 bbb (89–93) | 88 bbb (86–91) | <0.001 | |
| recovery | 90 (88–93) | 88 ccc (85–92) | 0.001 | |
| MCH (fmol) | baseline | 1.8 (1.8–1.9) | 1.8 (1.7–1.8) | <0.001 |
| post-effort | 1.8 (1.8–1.9) | 1.8 (1.7–1.8) | <0.001 | |
| recovery | 1.8 (1.8–1.9) | 1.8 c (1.8–1.9) | 0.002 | |
| MCHC (mol/L) | baseline | 20.3 a (19.6–20.8) | 20.2 (19.1–20.5) | 0.067 |
| post-effort | 20.2 bbb (19.4–20.6) | 20.2 (19.2–20.5) | 0.234 | |
| recovery | 20.3 (19.5–20.8) | 20.4 c (19.9–20.5) | 0.316 | |
| RDW (%) | baseline | 13.0 a (12.5–13.4) | 13.5 (13.0–14.1) | <0.001 |
| post-effort | 13.2 bbb (12.6–13.5) | 13.6 (13.1–14.3) | <0.001 | |
| recovery | 13.0 (12.4–13.4) | 13.5 (13.1–14.1) | <0.001 | |
| WBC (109/L) | baseline | 5.8 aaa (5.1–6.6) | 6.6 aaa (5.9–7.5) | <0.001 |
| post-effort | 9.0 bbb (7.9–10.3) | 10.1 bbb (8.5–11.2) | 0.002 | |
| recovery | 5.7 (5.0–6.5) | 5.5 cc (4.9–6.5) | 0.448 | |
| LYM (%) | baseline | 36.6 aaa (30.6–41.5) | 34.4 aaa (28.0–39.7) | 0.077 |
| post-effort | 42.2 bbb (36.6–47.9) | 40.3 (35.1–46.0) | 0.121 | |
| recovery | 37.2 (31.0–42.1) | 38.9 ccc (33.4–45.0) | 0.048 | |
| LYM (109/L) | baseline | 2.0 aaa (1.7–2.4) | 2.2 aaa (1.9–2.6) | 0.017 |
| post-effort | 3.8 bbb (3.0–4.4) | 4.0 bbb (3.4–4.4) | 0.214 | |
| recovery | 2.0 c (1.6–2.3) | 1.9 (1.7–2.4) | 0.202 | |
| MON (%) | baseline | 4.6 aaa (4.0–6.0) | 3.8 aaa (3.1–4.5) | <0.001 |
| post-effort | 5.2 bbb (4.5–6.5) | 4.3 (3.7–5.1) | <0.001 | |
| recovery | 4.7 (3.9–6.1) | 4.3 cc (3.7–4.9) | 0.014 | |
| MON (109/L) | baseline | 0.2 aaa (0.2–0.3) | 0.2 aaa (0.2–0.3) | 0.005 |
| post-effort | 0.4 bbb (0.3–0.6) | 0.4 bbb (0.3–0.5) | <0.001 | |
| recovery | 0.2 (0.1–0.3) | 0.2 (0.1–0.2) | 0.063 | |
| GRA (%) | baseline | 57.4 aaa (52.2–63.5) | 61.3 aaa (56.0–68.9) | 0.001 |
| post-effort | 51.2 bbb (45.2–57.2) | 55.5 (49.7–61.2) | 0.001 | |
| recovery | 56.5 cc (51.4–62.2) | 56.2 cc (49.2–62.2) | 0.495 | |
| GRA (109/L) | baseline | 3.4 aaa (2.8–4.2) | 4.3 aaa (3.5–5.0) | <0.001 |
| post-effort | 4.6 bbb (3.8–5.5) | 5.7 bbb (4.4–7.1) | <0.001 | |
| recovery | 3.3 (2.8–3.7) | 3.2 ccc (2.6–3.9) | 0.415 | |
| PLT (109/L) | baseline | 277 aaa (198–263) | 261 aaa (227–298) | <0.001 |
| post-effort | 292 bbb (247–319) | 321 bbb (277–355) | 0.001 | |
| recovery | 218 ccc (191–249) | 241 cc (211–276) | <0.001 | |
| MPV (fL) | baseline | 7.5 aa (6.9–7.9) | 7.6 (7.3–8.2) | 0.014 |
| post-effort | 7.5 bbb (7.1–8.0) | 7.8 bbb (7.2–8.4) | 0.017 | |
| recovery | 7.4 c (6.9–7.8) | 7.5 ccc (6.9–7.9) | 0.353 | |
| PCT (10−2 L/L) | baseline | 0.17 aaa (0.15–0.19) | 0.20 aaa (0.18–0.22) | <0.001 |
| post-effort | 0.21 bbb (0.19–0.24) | 0.24 bbb (0.21–0.27) | <0.001 | |
| recovery | 0.16 ccc (0.14–0.18) | 0.18 ccc (0.16–0.20) | <0.001 | |
| PDW (%) | baseline | 16.0 aaa (15.1–16.8) | 15.4 aa (14.8–15.9) | <0.001 |
| post-effort | 16.0 b (15.2–17.0) | 15.8 (15.1–16.7) | 0.218 | |
| recovery | 15.8 (15.0–16.6) | 15.3 c (14.8–:15.9) | 0.012 | |
| ΔPV (%) | post-effort | −2.54 bbb (−3.99–0.709) | −1.92 bbb (−2.84–0.662) | 0.027 |
| recovery | 1.62 (−0.359–3.33) | 1.86 (0.338–3.64) | 0.411 |
| Variable | Time Point | Men n = 234 | Women n = 62 | pMW |
|---|---|---|---|---|
| Na+ (mmol/L) | baseline | 137 aaa (136–138) | 138 (137–139) | 0.059 |
| post-effort | 139 bbb (138–140) | 139 bb (137–140) | 0.166 | |
| recovery | 137 (136–138) | 137 (136–139) | 0.604 | |
| Corrected Na+ (mmol/L) | baseline | 137 aaa (136–138) | 138 aaa (137–139) | 0.589 |
| post-effort | 135 bbb (133–138) | 136 bbb (134–138) | 0.416 | |
| recovery | 140 ccc (136–143) | 141 (137–142) | 0.708 | |
| K+ (mmol/L) | baseline | 4.02 aaa (3.89–4.21) | 3.94 (3.83–4.12) | 0.011 |
| post-effort | 3.89 bbb (3.74–4.05) | 3.89 (3.69–4.06) | 0.839 | |
| recovery | 4.25 ccc (4.09–4.49) | 3.95 (3.76–4.06) | <0.001 | |
| Corrected K+ (mmol/L) | baseline | 4.02 aaa (3.89–4.21) | 3.94 aa (3.83–4.12) | 0.011 |
| post-effort | 3.79 bbb (3.62–3.99) | 3.83 bb (3.64–4.02) | 0.496 | |
| recovery | 4.32 ccc (4.15–4.58) | 3.98 (3.78–4.17) | <0.001 | |
| Cl− (mmol/L) | baseline | 98.9 aaa (97.9–100) | 101 a (99.8–102) | <0.001 |
| post-effort | 99.8 (98.6–101) | 101 (99.9–102) | <0.001 | |
| recovery | 100 ccc (98.6–101) | 101 (99.8–102) | <0.001 | |
| Corrected Cl− (mmol/L) | baseline | 98.9 aaa (97.9–100) | 100.8 (99.8–102) | <0.001 |
| post-effort | 97.2 bbb (95–99.5) | 99.7 bbb (98.3–101) | <0.001 | |
| recovery | 102 ccc (98.6–104) | 103 ccc (101–105) | 0.010 | |
| tCa (mmol/L) | baseline | 2.41 aaa (2.28–2.57) | 2.31 (2.27–2.39) | <0.001 |
| post-effort | 2.34 bbb (2.24–2.55) | 2.29 bb (2.21–2.40) | 0.003 | |
| recovery | 2.46 ccc (2.37–2.60) | 2.33 (2.30–2.48) | <0.001 | |
| Corrected tCa (mmol/L) | baseline | 2.41 aaa (2.28–2.57) | 2.31 aa (2.27–2.39) | <0.001 |
| post-effort | 2.29 bbb (2.19–2.51) | 2.29 bbb (2.21–2.40) | 0.003 | |
| recovery | 2.5 ccc (2.39–2.67) | 2.38 c (2.30–2.48) | <0.001 | |
| iCa (mmol/L) | baseline | 1.24 aaa (1.17–1.31) | 1.19 aa (1.16–1.23) | <0.001 |
| post-effort | 1.20 bbb (1.15–1.30) | 1.17 bb (1.13–1.19) | <0.001 | |
| recovery | 1.25 ccc (1.21–1.31) | 1.19 (1.17–1.23) | <0.001 | |
| Corrected iCa (mmol/L) | baseline | 1.24 aaa (1.17–1.31) | 1.19 aaa (1.16–1.23) | <0.001 |
| post-effort | 1.17 bbb (1.11–1.26) | 1.15 bbb (1.11–1.18) | 0.029 | |
| recovery | 1.27 ccc (1.22–1.36) | 1.22 (1.19–1.25) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, R.; Turkiewicz, K.; Sławiński, M.; Clark, J.S.C.; Szylińska, A.; Proia, P.; Jodko, Ł.; Wojciuk, B.; Sulżyc-Bielicka, V.; Kostrzewa-Nowak, D. The Impact of Endurance Exercise on Routine Laboratory Parameters in Young Trained Individuals. J. Clin. Med. 2025, 14, 5703. https://doi.org/10.3390/jcm14165703
Nowak R, Turkiewicz K, Sławiński M, Clark JSC, Szylińska A, Proia P, Jodko Ł, Wojciuk B, Sulżyc-Bielicka V, Kostrzewa-Nowak D. The Impact of Endurance Exercise on Routine Laboratory Parameters in Young Trained Individuals. Journal of Clinical Medicine. 2025; 14(16):5703. https://doi.org/10.3390/jcm14165703
Chicago/Turabian StyleNowak, Robert, Karolina Turkiewicz, Michał Sławiński, Jeremy S. C. Clark, Aleksandra Szylińska, Patrizia Proia, Łukasz Jodko, Bartosz Wojciuk, Violetta Sulżyc-Bielicka, and Dorota Kostrzewa-Nowak. 2025. "The Impact of Endurance Exercise on Routine Laboratory Parameters in Young Trained Individuals" Journal of Clinical Medicine 14, no. 16: 5703. https://doi.org/10.3390/jcm14165703
APA StyleNowak, R., Turkiewicz, K., Sławiński, M., Clark, J. S. C., Szylińska, A., Proia, P., Jodko, Ł., Wojciuk, B., Sulżyc-Bielicka, V., & Kostrzewa-Nowak, D. (2025). The Impact of Endurance Exercise on Routine Laboratory Parameters in Young Trained Individuals. Journal of Clinical Medicine, 14(16), 5703. https://doi.org/10.3390/jcm14165703









