Comparative Study of Automated Real-Time Left Atrial Appendage Sizing Using Patient-Specific 3D Heart Models Versus Transesophageal Echocardiography and Multidetector Computed Tomography in Patients with Nonvalvular Atrial Fibrillation: Implications for Device Selection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Multislice Computed Tomography
2.3. Transesophageal Echocardiography
2.4. Angiography
2.5. Fusion Imaging and LAA Sizing Based on a 3D-Heart Model
2.6. Study Endpoints
2.6.1. Feasibility and Accuracy
2.6.2. Efficacy and Safety
2.6.3. Antithrombotic Management and Follow-Up
2.6.4. Statistical Analysis
3. Results
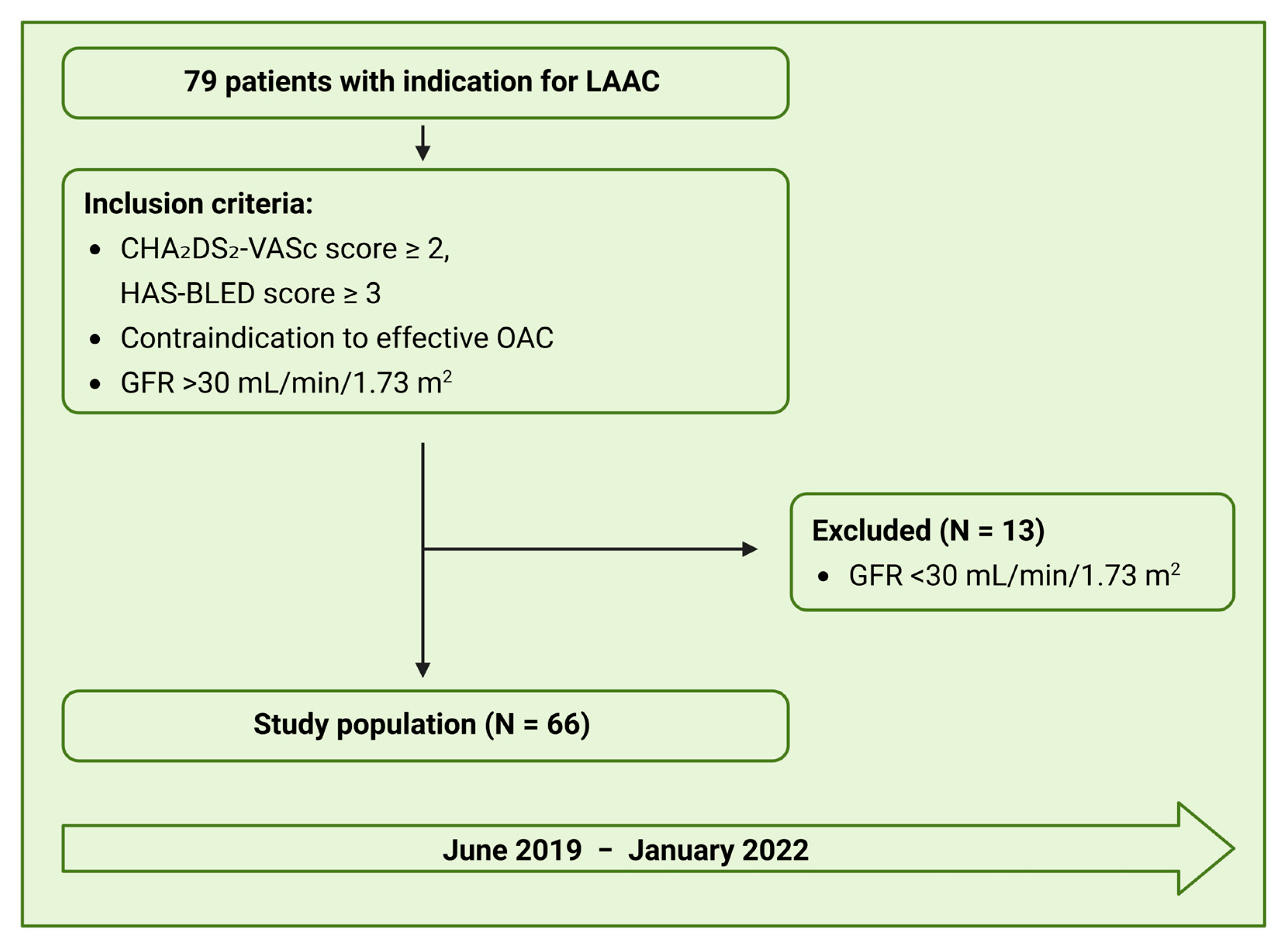
3.1. Patient Population
3.2. Feasibility and Accuracy
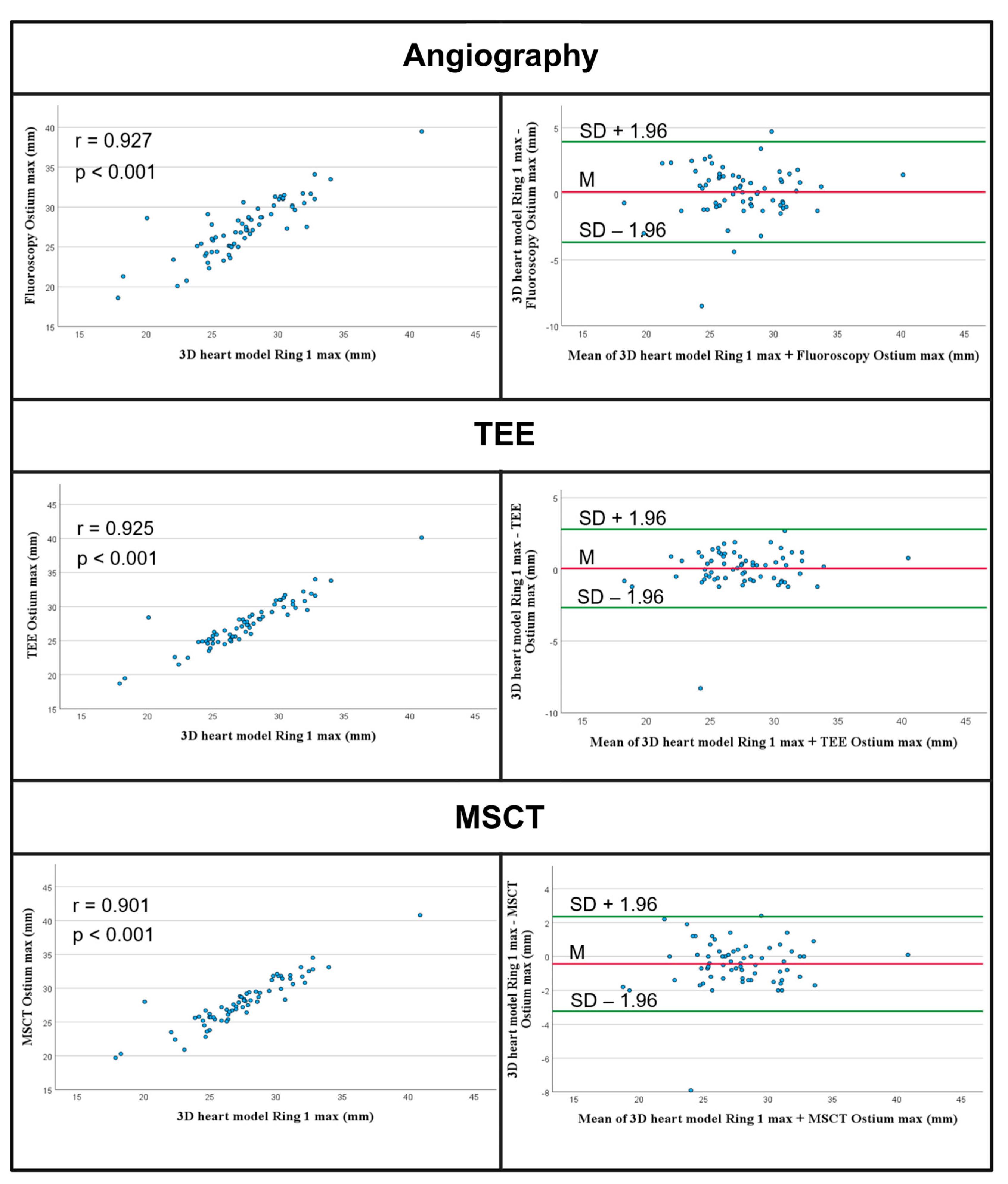
3.2.1. Comparison of Ostium Measurements
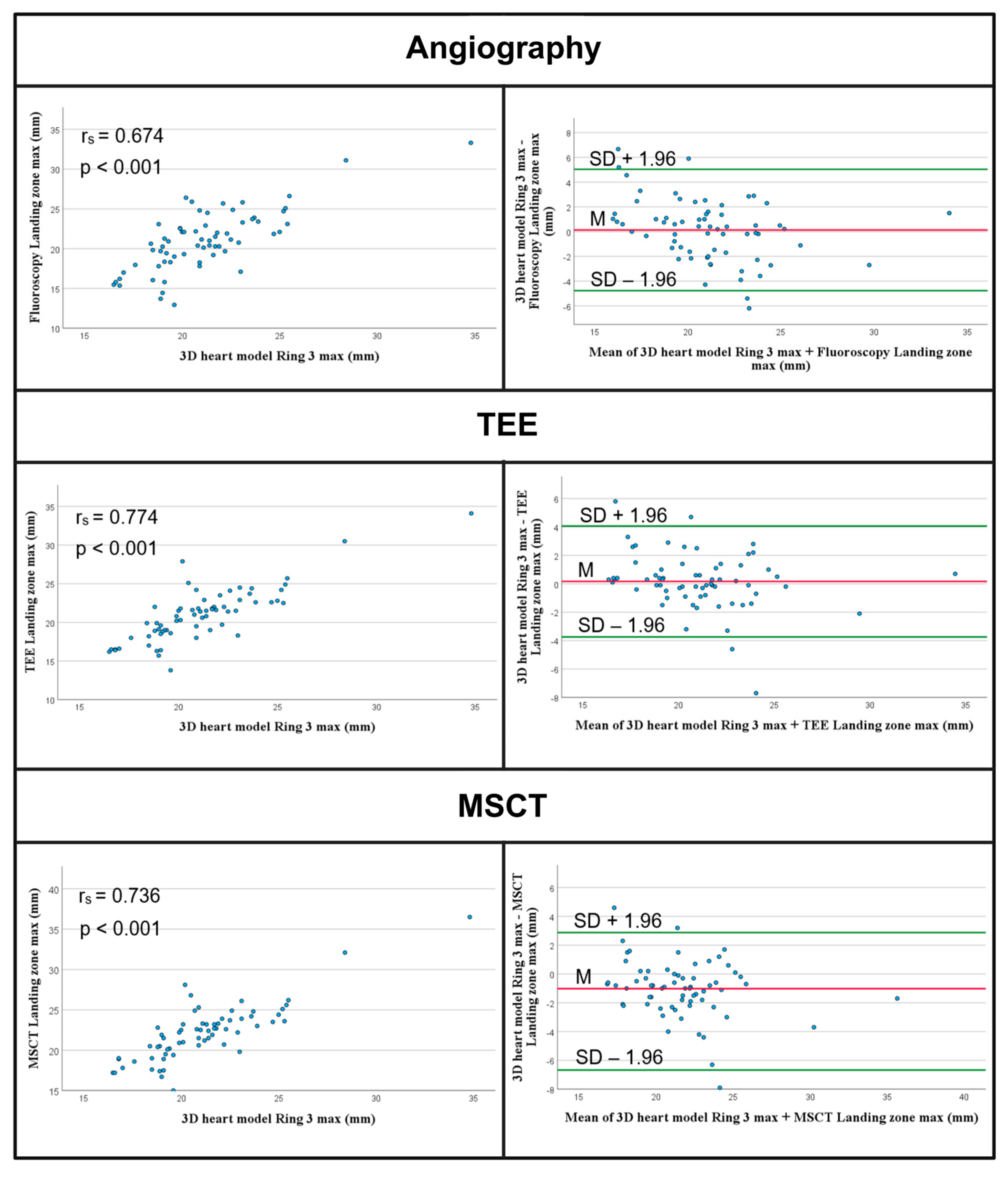
3.2.2. Comparison of Landing Zone Measurements
3.2.3. Analysis of Selected Device Size
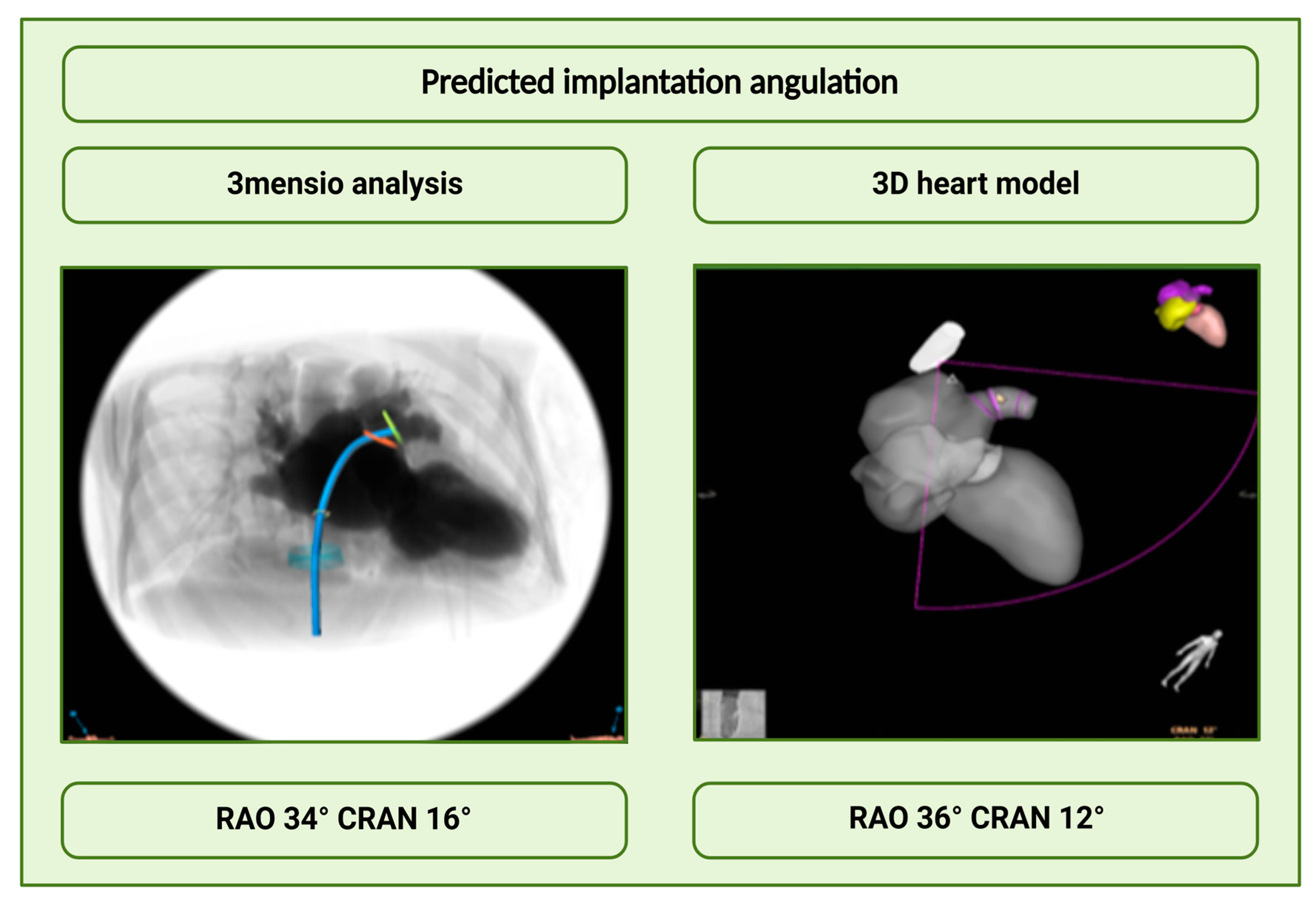
3.2.4. Analysis of the Angulations
3.2.5. Inter-Rater Reliability Analysis of the MSCT Measurements
3.2.6. Efficacy and Safety
Procedure Parameters
3.2.7. Procedural Safety
4. Discussion
- (1)
- Automated echocardiographic LAA sizing based on a patient-specific 3D heart model prototype in real-time is safe and feasible.
- (2)
- The automated landing zone measurements demonstrated good agreement with the TEE and angiographic measurements, but not with the MSCT measurements.
- (3)
- Intraprocedural implantation angulations performed with the 3D heart model matched very well with the predicted angulations using the 3mensio software.
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two dimensional |
| 3D | Three dimensional |
| AI | Artificial intelligence |
| CRA | Cranial |
| CAU | Caudal |
| FI | Fusion imaging |
| GFR | Glomerular filtration rate |
| ICC | Intraclass correlation coefficient |
| LAA | Left atrial appendage |
| LAAC | Left atrial appendage closure |
| MRI | Magnetic resonance imaging |
| MSCT | Multislice computed tomography |
| RAO | Right anterior oblique |
| TEE | Transesophageal echocardiography |
References
- Tabata, N.; Sinning, J.M.; Kaikita, K.; Tsujita, K.; Nickenig, G.; Werner, N. Current status and future perspective of structural heart disease intervention. J. Cardiol. 2019, 74, 1–12. [Google Scholar] [CrossRef]
- Saw, J.; Holmes, D.R.; Cavalcante, J.L.; Freeman, J.V.; Goldsweig, A.M.; Kavinsky, C.J.; Moussa, I.D.; Munger, T.M.; Price, M.J.; Reisman, M.; et al. SCAI/HRS Expert Consensus Statement on Transcatheter Left Atrial Appendage Closure. JACC Cardiovasc. Interv. 2023, 16, 1384–1400. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.; Roehrich, A.; Boenner, F.; Aissa, J.; Kröpil, P.; Antoch, G.; Verde, P.; Ohmann, C.; Balzer, J.; Shin, D.I.; et al. Value of 3D TEE for LAA Morphology. JACC Cardiovasc. Imaging 2015, 8, 1107–1110. [Google Scholar] [CrossRef][Green Version]
- Fiore, G.; Gaspardone, C.; Ingallina, G.; Rizza, V.; Melillo, F.; Stella, S.; Ancona, F.; Biondi, F.; Margonato, D.; Palmisano, A.; et al. Accuracy and Reliability of Left Atrial Appendage Morphology Assessment by Three-Dimensional Transesophageal Echocardiographic Glass Rendering Modality: A Comparative Study With Computed Tomography. J. Am. Soc. Echocardiogr. 2023, 36, 1083–1091. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Korsholm, K.; Rodés-Cabau, J.; Saw, J.; Berti, S.; Alkhouli, M.A. Left atrial appendage occlusion. EuroIntervention 2023, 18, e1038–e1065. [Google Scholar] [CrossRef]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. EuroIntervention 2020, 15, 1133–1180. [Google Scholar] [CrossRef]
- DeCampos, D.; Teixeira, R.; Saleiro, C.; Oliveira-Santos, M.; Paiva, L.; Costa, M.; Botelho, A.; Gonçalves, L. 3D printing for left atrial appendage closure: A meta-analysis and systematic review. Int. J. Cardiol. 2022, 356, 38–43. [Google Scholar] [CrossRef]
- Heidari, H.; Kanschik, D.; Erkens, R.; Maier, O.; Wolff, G.; Bruno, R.R.; Werner, N.; Daniel Reinartz, S.; Antoch, G.; Kelm, M.; et al. Left atrial appendage sizing for percutaneous closure in virtual reality-a feasibility study. Front. Cardiovasc. Med. 2023, 10, 1188571. [Google Scholar] [CrossRef] [PubMed]
- Mo, B.F.; Wan, Y.; Alimu, A.; Sun, J.; Zhang, P.P.; Yu, Y.; Chen, M.; Li, W.; Wang, Z.Q.; Wang, Q.S.; et al. Image fusion of integrating fluoroscopy into 3D computed tomography in guidance of left atrial appendage closure. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 92–101. [Google Scholar] [CrossRef]
- Afzal, S.; Piayda, K.; Hellhammer, K.; Veulemans, V.; Wolff, G.; Heidari, H.; Stüwe, D.; Kanschik, D.; Polzin, A.; Kelm, M.; et al. Real-time echocardiography-fluoroscopy fusion imaging for left atrial appendage closure: Prime time for fusion imaging? Acta Cardiol. 2021, 76, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Soetemann, D.; Nijhof, N.; Kelm, M.; Zeus, T. First experience with real-time 3D anatomical fusion imaging during left atrial appendage occluder implantation. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 719–720. [Google Scholar] [CrossRef]
- Biaggi, P.; Sager, D.F.; Külling, J.; Küest, S.; Wyss, C.; Hürlimann, D.; Reho, I.; Bühler, I.; Noll, G.; Huber, M.; et al. Potential Value of Fusion Imaging and Automated Three-Dimensional Heart Segmentation During Transcatheter Aortic Valve Replacement. J. Am. Soc. Echocardiogr. 2020, 33, 516–517.e511. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Tosetto, A.; Abshire, T.; Arnold, D.M.; Coller, B.; James, P.; Neunert, C.; Lillicrap, D. ISTH/SSC bleeding assessment tool: A standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J. Thromb. Haemost. 2010, 8, 2063–2065. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Laterra, G.; Dattilo, G.; Correale, M.; Brunetti, N.D.; Artale, C.; Sacchetta, G.; Pistelli, L.; Borgi, M.; Campanella, F.; Cocuzza, F.; et al. Imaging Modality to Guide Left Atrial Appendage Closure: Current Status and Future Perspectives. J. Clin. Med. 2023, 12, 3756. [Google Scholar] [CrossRef] [PubMed]
- Freixa, X.; Aminian, A.; Tzikas, A.; Saw, J.; Nielsen-Kudsk, J.E.; Ghanem, A.; Schmidt, B.; Hildick-Smith, D. Left atrial appendage occlusion with the Amplatzer Amulet: Update on device sizing. J. Interv. Card. Electrophysiol. 2020, 59, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Fahmy, P.; DeJong, P.; Lempereur, M.; Spencer, R.; Tsang, M.; Gin, K.; Jue, J.; Mayo, J.; McLaughlin, P.; et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Morais, P.; Fan, Y.; Queirós, S.; D’Hooge, J.; Lee, A.P.; Vilaça, J.L. Feasibility and Accuracy of Automated Three-Dimensional Echocardiographic Analysis of Left Atrial Appendage for Transcatheter Closure. J. Am. Soc. Echocardiogr. 2022, 35, 124–133. [Google Scholar] [CrossRef]
- Sun, A.; Ren, S.; Xiao, Y.; Chen, Y.; Wang, N.; Li, C.; Tan, X.; Pan, Y.; Sun, F.; Ren, W. Real-time 3D echocardiographic transilluminated imaging combined with artificially intelligent left atrial appendage measurement for atrial fibrillation interventional procedures. Front. Physiol. 2022, 13, 1043551. [Google Scholar] [CrossRef]
- Michiels, K.; Heffinck, E.; Astudillo, P.; Wong, I.; Mortier, P.; Bavo, A.M. Automated MSCT Analysis for Planning Left Atrial Appendage Occlusion Using Artificial Intelligence. J. Interv. Cardiol. 2022, 2022, 5797431. [Google Scholar] [CrossRef]
- Cruz González, I.; Antúnez Muiños, P.J.; López Tejero, S.; Núñez García, J.C.; Rodríguez Collado, J.; Martín Moreiras, J.; Diego Nieto, A.; Herrero Garibi, J.; Díaz Peláez, E.; Sánchez Fernández, P.L. Left Atrial Appendage Occlusion Using the Novel Amplatzer Steerable Delivery Sheath Combine With FEops HEARTguide. JACC Cardiovasc. Interv. 2021, 14, e301–e304. [Google Scholar] [CrossRef] [PubMed]
- Bavo, A.M.; Wilkins, B.T.; Garot, P.; De Bock, S.; Saw, J.; Søndergaard, L.; De Backer, O.; Iannaccone, F. Validation of a computational model aiming to optimize preprocedural planning in percutaneous left atrial appendage closure. J. Cardiovasc. Comput. Tomogr. 2020, 14, 149–154. [Google Scholar] [CrossRef] [PubMed]
- De Backer, O.; Iriart, X.; Kefer, J.; Nielsen-Kudsk, J.E.; Aminian, A.; Rosseel, L.; Kofoed, K.F.; Odenstedt, J.; Berti, S.; Saw, J.; et al. Impact of Computational Modeling on Transcatheter Left Atrial Appendage Closure Efficiency and Outcomes. JACC Cardiovasc. Interv. 2023, 16, 655–666. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | N = 66 |
|---|---|
| Male gender, n (%) | 38 (57.6%) |
| Age, M (years) ± SD | 75 ± 7 |
| Height, M (m) ± SD | 1.73 ± 0.1 |
| Weight, M (kg) ± SD | 77 ± 23 |
| BMI, M (kg/m2) ± SD | 26 ± 6 |
| CHA2DS2-VASc-Score, M ± SD | 4 ± 2 |
| HAS-BLED-Score, M ± SD | 3 ± 2 |
| Atrial fibrillation, n (%) | 66 (100) |
| Paroxysmal, n (%) | 39 (59.1) |
| Persistent, n (%) | 6 (9.1) |
| Permanent, n (%) | 21 (31.8) |
| Arterial hypertension, n (%) | 55 (83.3) |
| Heart failure, n (%) | 50 (75.8) |
| Coronary artery disease, n (%) | 33 (50.0) |
| s/p. MI, n (%) | 6 (9.1) |
| s/p. CABG, n (%) | 9 (13.6) |
| s/p PCI, n (%) | 24 (36.4) |
| Chronic kidney disease, n (%) | 22 (33.3) |
| Diabetes mellitus, n (%) | 30 (45.5) |
| Morphology | |
| Chicken Wing, n (%) | 42 (62.6) |
| Cactus, n (%) | 10 (15.2) |
| Windsock, n (%) | 12 (18.2) |
| Cauliflower, n (%) | 2 (3) |
| Indication for LAAC | |
| Contraindications to OAC | |
| Increased risk of bleeding, n (%) | 26 (39.4 %) |
| Gastrointestinal bleeding, n (%) | 19 (28.8 %) |
| Other bleeding, n (%) | 17 (25.8 %) |
| INR instability, n(%) | 4 (6.1 %) |
| Variable | M ± SD | p-Value |
|---|---|---|
| 3D heart model Ring 1 min, M (mm) ± SD | 18.18 ± 2.57 | |
| Angiography, M (mm) ± SD | 19.97 ± 3.02 | p < 0.001 |
| TEE, M (mm) ± SD | 18.94 ± 2.70 | p < 0.001 |
| MSCT, M (mm) ± SD | 20.33 ± 3.35 | p < 0.001 |
| 3D heart model Ring 1 max, M (mm) ± SD | 27.54 ± 3.71 | |
| Angiography, M (mm) ± SD | 27.41 ± 3.59 | 0.239 |
| TEE, M (mm) ± SD | 27.47 ± 3.51 | 0.243 |
| MSCT, M (mm) ± SD | 27.98 ± 3.60 | 0.244 |
| Variable | M ± IQR | p-Wert |
|---|---|---|
| Ring 2 | ||
| 3D heart model Ring 2 max, M (mm) ± IQR | 23.10 ± 3.65 | |
| Angiography, M (mm) ± IQR | 20.96 ± 4.81 | p < 0.001 |
| TEE, M (mm) ± IQR | 21.20 ± 3.90 | p < 0.001 |
| MSCT, M (mm) ± IQR | 22.15 ± 3.49 | p < 0.001 |
| Ring 3 | ||
| 3D heart model Ring 3 max, M (mm) ± IQR | 20.90 ± 3.42 | |
| Angiography, M (mm) ± IQR | 20.96 ± 4.81 | 0.563 |
| TEE, M (mm) ± IQR | 21.20 ± 3.90 | 0.291 |
| MSCT, M (mm) ± IQR | 22.15 ± 3.49 | p < 0.001 |
| Ring 4 | ||
| 3D heart model Ring 4 max, M (mm) ± IQR | 20.10 ± 3.35 | |
| Angiography, M (mm) ± IQR | 20.96 ± 4.81 | 0.010 |
| TEE, M (mm) ± IQR | 21.20 ± 3.90 | p < 0.001 |
| MSCT, M (mm) ± IQR | 22.15 ± 3.49 | p < 0.001 |
| Ring 5 | ||
| 3D heart model Ring 5 max, M (mm) ± IQR | 18.90 ± 3.60 | |
| Angiography, M (mm) ± IQR | 20.96 ± 4.81 | p < 0.001 |
| TEE, M (mm) ± IQR | 21.20 ± 3.90 | p < 0.001 |
| MSCT, M (mm) ± IQR | 22.15 ± 3.49 | p < 0.001 |
| Ring 6 | ||
| 3D heart model Ring 6 max, M (mm) ± IQR | 17.60 ± 3.20 | |
| Angiography, M (mm) ± IQR | 20.96 ± 4.81 | p < 0.001 |
| TEE, M (mm) ± IQR | 21.20 ± 3.90 | p < 0.001 |
| MSCT, M (mm) ± IQR | 22.15 ± 3.49 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanschik, D.; Polzin, A.; Heidari, H.; Dannenberg, L.; Phinicarides, R.; Klein, K.; Werner, N.; Kelm, M.; Jung, C.; Zeus, T.; et al. Comparative Study of Automated Real-Time Left Atrial Appendage Sizing Using Patient-Specific 3D Heart Models Versus Transesophageal Echocardiography and Multidetector Computed Tomography in Patients with Nonvalvular Atrial Fibrillation: Implications for Device Selection. J. Clin. Med. 2025, 14, 5696. https://doi.org/10.3390/jcm14165696
Kanschik D, Polzin A, Heidari H, Dannenberg L, Phinicarides R, Klein K, Werner N, Kelm M, Jung C, Zeus T, et al. Comparative Study of Automated Real-Time Left Atrial Appendage Sizing Using Patient-Specific 3D Heart Models Versus Transesophageal Echocardiography and Multidetector Computed Tomography in Patients with Nonvalvular Atrial Fibrillation: Implications for Device Selection. Journal of Clinical Medicine. 2025; 14(16):5696. https://doi.org/10.3390/jcm14165696
Chicago/Turabian StyleKanschik, Dominika, Amin Polzin, Houtan Heidari, Lisa Dannenberg, Raphael Phinicarides, Kathrin Klein, Nikos Werner, Malte Kelm, Christian Jung, Tobias Zeus, and et al. 2025. "Comparative Study of Automated Real-Time Left Atrial Appendage Sizing Using Patient-Specific 3D Heart Models Versus Transesophageal Echocardiography and Multidetector Computed Tomography in Patients with Nonvalvular Atrial Fibrillation: Implications for Device Selection" Journal of Clinical Medicine 14, no. 16: 5696. https://doi.org/10.3390/jcm14165696
APA StyleKanschik, D., Polzin, A., Heidari, H., Dannenberg, L., Phinicarides, R., Klein, K., Werner, N., Kelm, M., Jung, C., Zeus, T., & Afzal, S. (2025). Comparative Study of Automated Real-Time Left Atrial Appendage Sizing Using Patient-Specific 3D Heart Models Versus Transesophageal Echocardiography and Multidetector Computed Tomography in Patients with Nonvalvular Atrial Fibrillation: Implications for Device Selection. Journal of Clinical Medicine, 14(16), 5696. https://doi.org/10.3390/jcm14165696







