Successful Treatment of an Advanced Larynx Carcinoma Using Neo-Adjuvant Chemo-Immunotherapy and Cisplatin/Radiotherapy for a Patient with Rendu–Osler Disease
Abstract
1. Introduction
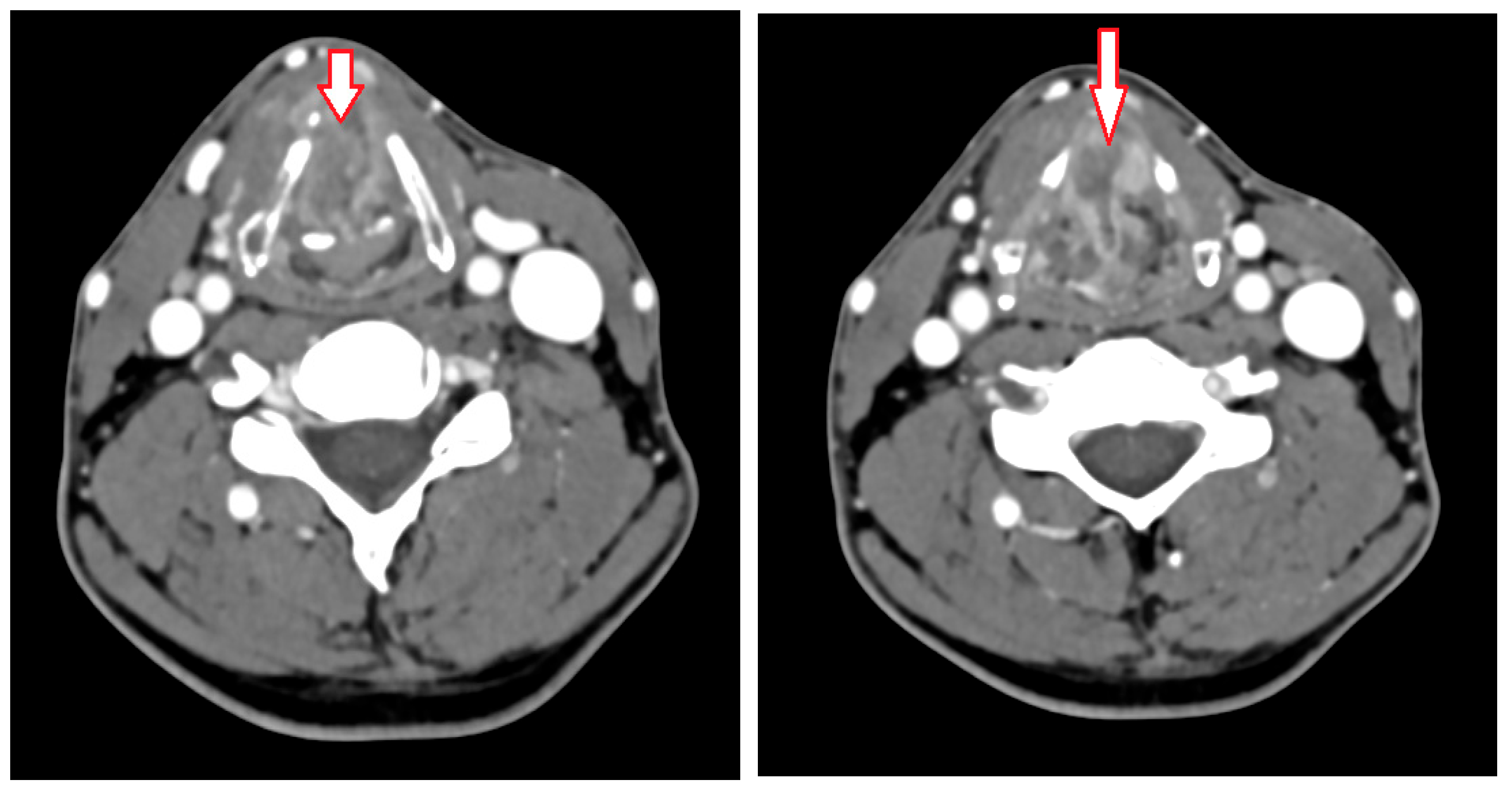
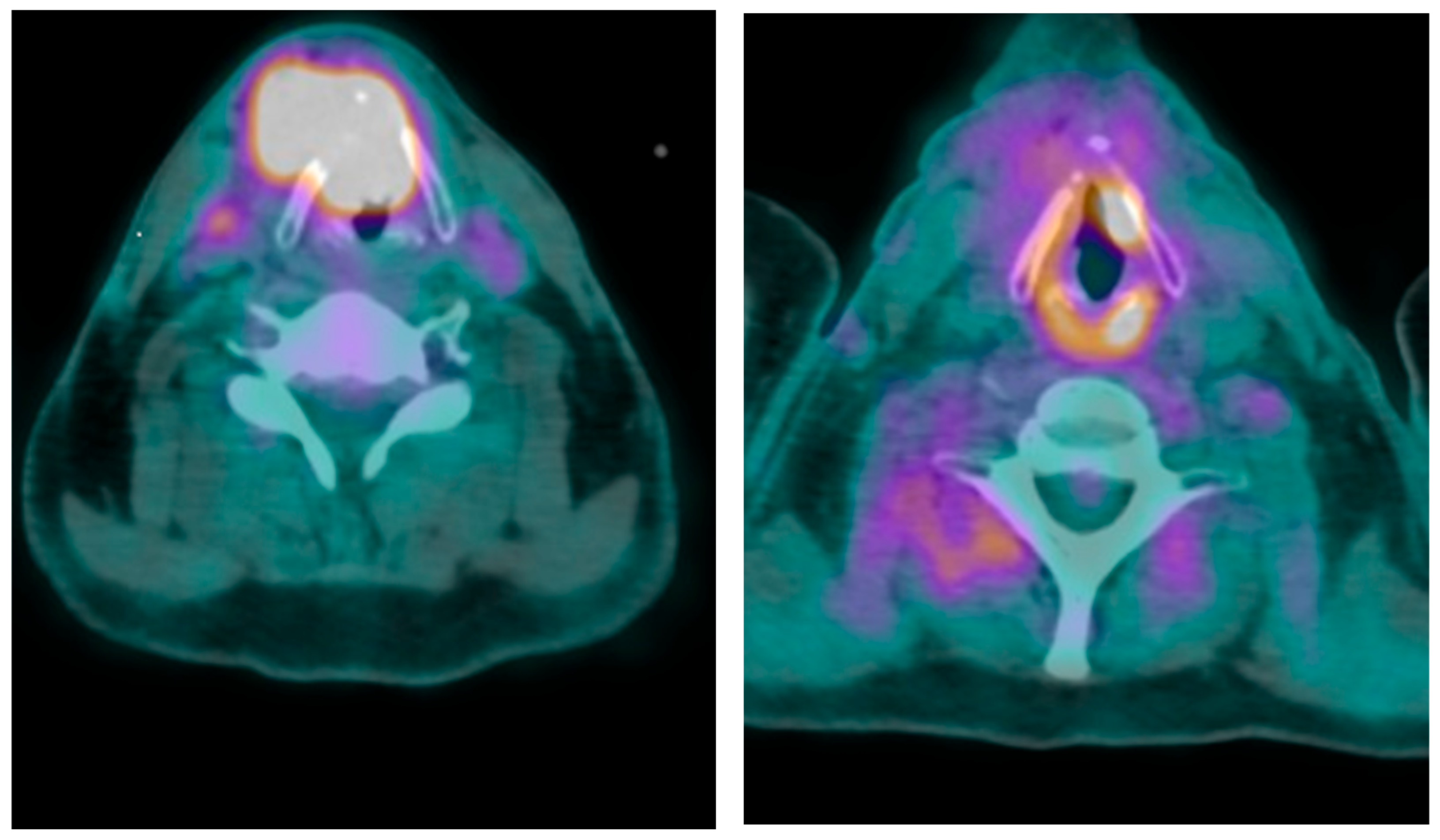
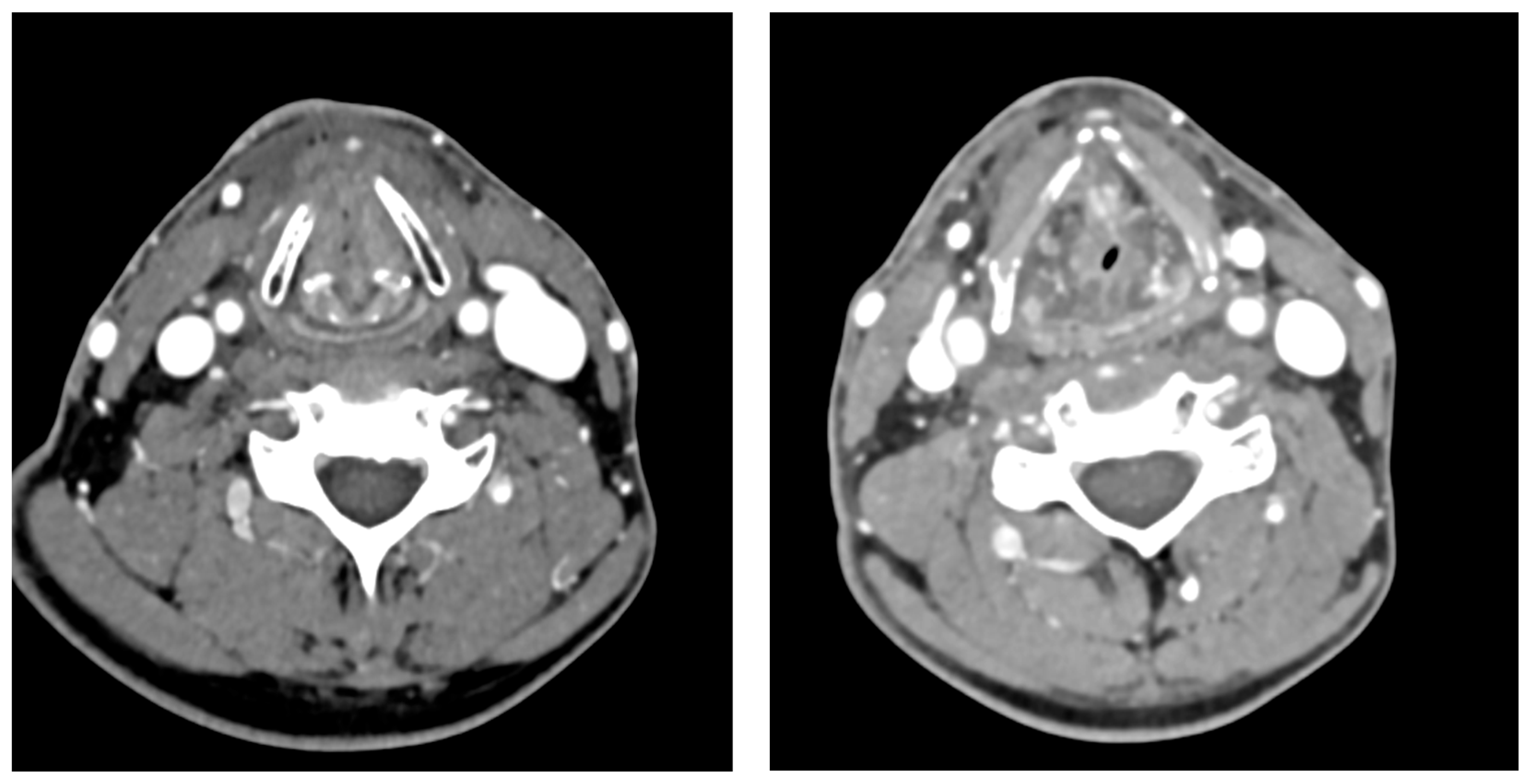
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Guarantor
References
- Geisthoff, U.W.; Mahnken, A.H.; Denzer, U.W.; Kemmling, A.; Nimsky, C.; Stuck, B.A. Hereditary hemorrhagic telangiectasia (Osler’s disease): Systemic, interdisciplinary, relatively common—And often missed. Dtsch. Ärzteblatt Int. 2024, 121, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Philp, A.; Nagji, A.S.; Kasi, A. Pathologically Complete Response after Triple Therapy in Locally Advanced Esophageal Cancer in a Hereditary Hemorrhagic Telangiectasia Patient. Case Rep Oncol. 2020, 13, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zaizaa, M.; Jamal, O.; Bahadi, N.; Sahel, N.; El Bougrini, Z.; Rkiouak, A.; Sekkach, Y. BEVACIZUMAB: Le traitement miracle des épistaxis au cours de la maladie de Rendu Osler Weber. Rev. Méd. Interne 2022, 43, A224. [Google Scholar] [CrossRef]
- Mahmoud, M.; Borthwick, G.M.; Hislop, A.A.; Arthur, H.M. Endoglin and activin receptor-like-kinase 1 are co-expressed in the distal vessels of the lung: Implications for two familial vascular dysplasias, HHT and PAH. Lab. Investig. 2009, 89, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kmeid, E.; Romanos, B.; Chammas, S. Mucoepidermoid carcinoma of the larynx: Case report and review of the literature. Otolaryngol. Case Rep. 2022, 24, 100453. [Google Scholar] [CrossRef]
- Fuereder, T.; Kocher, F.; Vermorken, J.B. Systemic therapy for laryngeal carcinoma. Front. Oncol. 2025, 15, 1541385. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Goulart, A.P.; Moro, E.T.; Guasti, V.M.; Colares, R.F. Anesthetic Management of a Patient with Hereditary Hemorrhagic Telangiectasia (Rendu-Osler-Weber Syndrome). Case Report. Rev. Bras. Anestesiol. 2009, 59, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, K.M.Z. Anesthetic considerations for the patient with hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome). AANA J. 2009, 77, 115–118. [Google Scholar] [PubMed]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Hanna, E.Y. Optimal regimen of cisplatin in squamous cell carcinoma of head and neck yet to be determined. Ann. Transl. Med. 2018, 6, 229. [Google Scholar] [CrossRef] [PubMed]
- Janoray, G.; Pointreau, Y.; Garaud, P.; Chapet, S.; Alfonsi, M.; Sire, C.; Jadaud, E.; Calais, G. Long-Term Results of a Multicenter Randomized Phase III Trial of Induction Chemotherapy with Cisplatin, 5-Fluorouracil, ± Docetaxel for Larynx Preservation. JNCI J. Natl. Cancer Inst. 2016, 108, djv368. [Google Scholar] [CrossRef] [PubMed]
- Guigay, J.; Aupérin, A.; Fayette, J.; Saada-Bouzid, E.; Lafond, C.; Taberna, M.; Geoffrois, L.; Martin, L.; Capitain, O.; Cupissol, D.; et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021, 22, 463–475. [Google Scholar] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; De Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: An open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Chauffert, B.; Zhou, Y.; Medjkoune, L.; Ouikene, A.; Galez, A.; Belkhir, F.; Saint-Germain, P.; Youssef, A.; Chehimi, M. High Response Rate to Carboplatin-Paclitaxel-Cetuximab and Pembrolizumab in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Case Rep. Oncol. 2023, 16, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chauffert, B.; Gognard, M.; Ouikene, A.; Chehimi, M.; Galez, A.; Garnier, T.; Belkhir, F.; Oueslati, H.; Abdaoui, A.; Youssef, A.; et al. Weekly Carboplatin, Paclitaxel and Cetuximab with Pembrolizumab Every 3 Weeks Is Active and Tolerated in Patients with Locally Advanced and/or Metastatic/Recurrent Head and Neck Squamous Cell Carcinoma. J. Cancer Res. Cell. Ther. 2024, 8, 1–4. [Google Scholar] [CrossRef]
- De Carvalho E Silva, R.M.; Mendes, F.M.; Degasperi, G.R.; Pinheiro, S.L. Photobiomodulation for the management of xerostomia and oral mucositis in patients with cancer: A randomized clinical trial. Lasers Med. Sci. 2023, 38, 101. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauffert, B.; Alsuliman, T.; Oueslati, H.; Ouikene, A.; Belkhir, F.; Nemmaoui, S.; Cau, A.; Galez, A.; Garnier, T.; Salle, V.; et al. Successful Treatment of an Advanced Larynx Carcinoma Using Neo-Adjuvant Chemo-Immunotherapy and Cisplatin/Radiotherapy for a Patient with Rendu–Osler Disease. J. Clin. Med. 2025, 14, 5694. https://doi.org/10.3390/jcm14165694
Chauffert B, Alsuliman T, Oueslati H, Ouikene A, Belkhir F, Nemmaoui S, Cau A, Galez A, Garnier T, Salle V, et al. Successful Treatment of an Advanced Larynx Carcinoma Using Neo-Adjuvant Chemo-Immunotherapy and Cisplatin/Radiotherapy for a Patient with Rendu–Osler Disease. Journal of Clinical Medicine. 2025; 14(16):5694. https://doi.org/10.3390/jcm14165694
Chicago/Turabian StyleChauffert, Bruno, Tamim Alsuliman, Hanene Oueslati, Abdenour Ouikene, Farid Belkhir, Sana Nemmaoui, Alexandre Cau, Agnes Galez, Thomas Garnier, Valéry Salle, and et al. 2025. "Successful Treatment of an Advanced Larynx Carcinoma Using Neo-Adjuvant Chemo-Immunotherapy and Cisplatin/Radiotherapy for a Patient with Rendu–Osler Disease" Journal of Clinical Medicine 14, no. 16: 5694. https://doi.org/10.3390/jcm14165694
APA StyleChauffert, B., Alsuliman, T., Oueslati, H., Ouikene, A., Belkhir, F., Nemmaoui, S., Cau, A., Galez, A., Garnier, T., Salle, V., & Garidi, R. (2025). Successful Treatment of an Advanced Larynx Carcinoma Using Neo-Adjuvant Chemo-Immunotherapy and Cisplatin/Radiotherapy for a Patient with Rendu–Osler Disease. Journal of Clinical Medicine, 14(16), 5694. https://doi.org/10.3390/jcm14165694




