Building the Foundation for Standardized Care Metrics in Jejunoileal Atresia: A Systematic Review of Reported Baseline Characteristics, Treatment Variables and Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
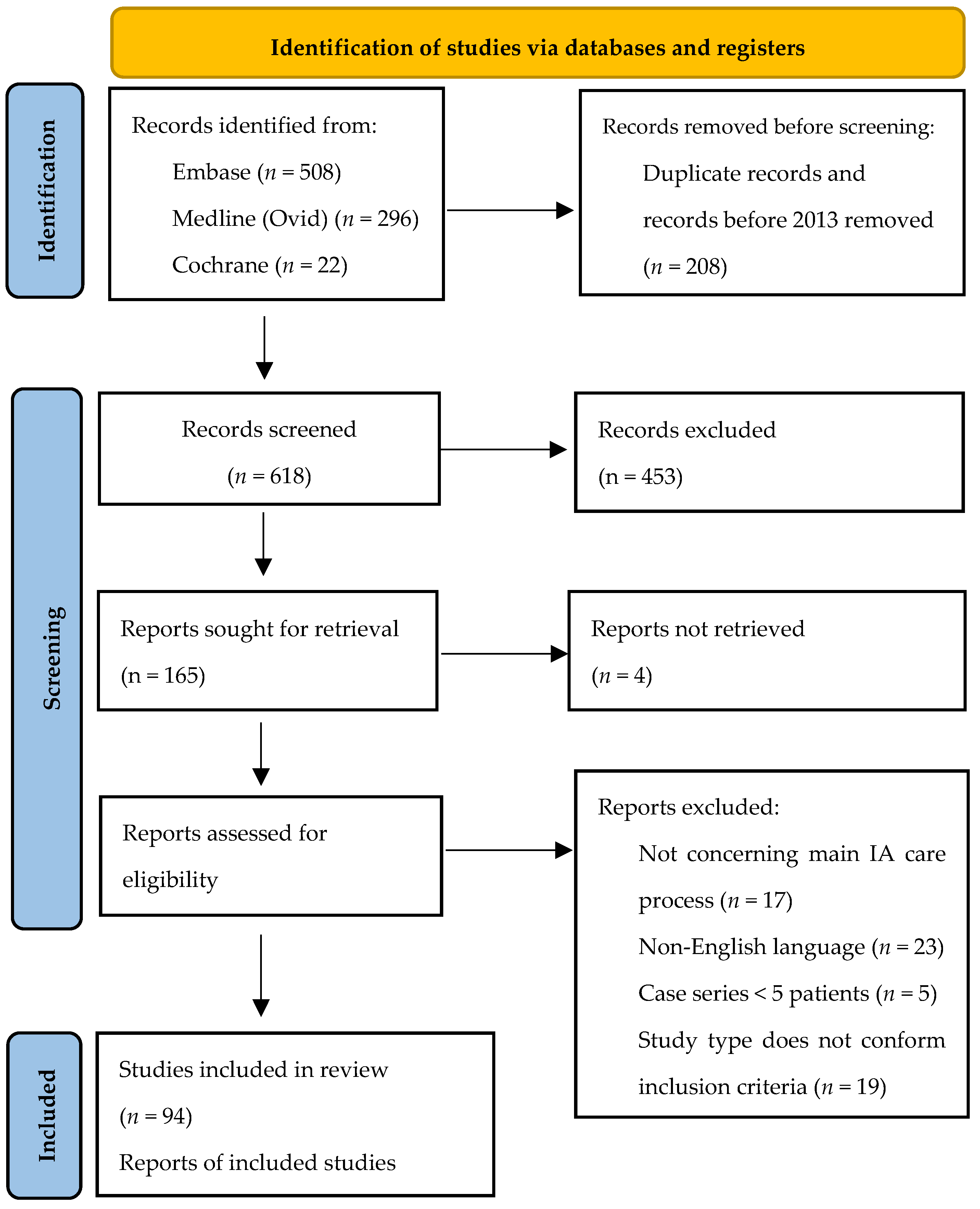
2.2. Selection Process
2.3. Data Extraction, Analysis, and Results
3. Results
3.1. Included Publications
3.2. Data Extraction
3.3. Extracted Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JIA | Jejunoileal atresia |
| EPSA | European Pediatric Surgical Audit |
References
- Vinocur, D.N.; Lee, E.Y.; Eisenberg, R.L. Neonatal intestinal obstruction. AJR Am. J. Roentgenol. 2012, 198, W1–W10. [Google Scholar] [CrossRef] [PubMed]
- Hajivassiliou, C.A. Intestinal obstruction in neonatal/pediatric surgery. Semin. Pediatr. Surg. 2003, 12, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Tam, Y.H.; Wong, Y.S.; Tsui, S.Y.; Wong, H.Y.; Pang, K.K.; Houben, C.H.; Mou, J.W.C.; Chan, K.W.; Lee, K.H. Early reoperations after primary repair of jejunoileal atresia in newborns. J. Neonat. Surg. 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Prevalence Charts and Tables. Updated Data Including Birth Year 2022—Last Updated April 2024. (Website). November 2024. Available online: https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en (accessed on 23 July 2025).
- Koenig, S.M.; Russell, R.T.; Quevedo, O.G.; Chen, M.K. Intestinal Atresias: A Ten-Year Evaluation of Outcomes. J. Surg. Res. 2024, 296, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, P.; Lui, V.C.H.; Xie, X.; Li, Y.; Song, Y.; Li, L.; Jin, Z.W. Gut lumen formation defect can cause intestinal atresia: Evidence from histological studies of human embryos and intestinal atresia septum. J. Dev. Orig. Health Dis. 2022, 13, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.D.; Stanton, M.P. Malrotation and intestinal atresias. Early Hum. Dev. 2014, 90, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Stollman, T.H.; de Blaauw, I.; Wijnen, M.H.; van der Staak, F.H.; Rieu, P.N.; Draaisma, J.M.; Wijnen, R.M. Decreased mortality but increased morbidity in neonates with jejunoileal atresia; a study of 114 cases over a 34-year period. J. Pediatr. Surg. 2009, 44, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Beck, N.; van Bommel, A.C.; Eddes, E.H.; van Leersum, N.J.; Tollenaar, R.A.; Wouters, M.W. Dutch Clinical Auditing Group*. The Dutch Institute for Clinical Auditing: Achieving Codman’s Dream on a Nationwide Basis. Ann. Surg. 2020, 271, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Voeten, S.C.; Krijnen, P.; Voeten, D.M.; Hegeman, J.H.; Wouters, M.W.J.M.; Schipper, I.B. Quality indicators for hip fracture care, a systematic review. Osteoporos. Int. 2018, 29, 1963–1985. Available online: http://link.springer.com/10.1007/s00198-018-4558-x (accessed on 4 June 2025). [CrossRef] [PubMed]
- Courrech Staal, E.F.W.; Wouters, M.W.J.M.; Boot, H.; Tollenaar, R.A.E.M.; van Sandick, J.W. Quality-of-care indicators for oesophageal cancer surgery: A review. Eur. J. Surg. Oncol. 2010, 36, 1035–1043. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0748798310004737 (accessed on 28 May 2025). [CrossRef] [PubMed]
- Teunissen, N.M.; Brendel, J.; Heurn, L.W.E.V.; Ure, B.; Wijnen, R.; Eaton, S. EPSA|ERNICA Registry Group; EA Quality of Care Initiative. Selection of Quality Indicators to Evaluate Quality of Care for Patients with Esophageal Atresia Using a Delphi Method. Eur. J. Pediatr. Surg. 2024, 34, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Löf Granström, A.; Pakarinen, M.; Bjørnland, K.; De Blaauw, I.; Ellebæk, M.; Fascetti Leon, F.; Gloudemans, D.; Pini Prato, A.; Rolle, U.; et al. Defining excellence: The first core set of Quality Indicators for the European Pediatric Surgical Audit on Hirschsprung’s disease care. Acta Paediatr. 2025, 114, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Zhang, H.; Chen, C. Definition and grading of anastomotic stricture/stenosis following low anastomosis after total mesorectal excision: A single-center study. Asian J. Surg. 2023, 46, 3722–3726. [Google Scholar] [CrossRef] [PubMed]
- Kodra, Y.; Weinbach, J.; Posada-de-la-Paz, M.; Coi, A.; Lemonnier, S.L.; Van Enckevort, D.; Roos, M.; Jacobsen, A.; Cornet, R.; Ahmed, S.F.; et al. Recommendations for Improving the Quality of Rare Disease Registries. Int. J. Environ. Res. Public Health 2018, 15, 1644. [Google Scholar] [CrossRef] [PubMed]
- Elsinga, R.M.; Roze, E.; Van Braeckel, K.N.; Hulscher, J.B.; Bos, A.F. Motor and cognitive outcome at school age of children with surgically treated intestinal obstructions in the neonatal period. Early Hum. Dev. 2013, 89, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Nusinovich, Y.; Revenis, M.; Torres, C. Long-term outcomes for infants with intestinal atresia studied at Children’s National Medical Center. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Batta, V.; Rao, S.; Wagh, D.; Tan, J.K.G.; Gollow, I.; Simmer, K.; Bulsara, M.K.; Patole, S. Early neurodevelopmental outcomes of congenital gastrointestinal surgical conditions: A single-centre retrospective study. BMJ Paediatr. Open. 2020, 4, e000736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyake, Y.; Lum Min, S.A.; Yamataka, A.; Keijzer, R. The impact of intestinal atresia on educational and mental health outcomes in school-aged children: A case-control cohort study. Pediatr. Surg. Int. 2023, 39, 86. [Google Scholar] [CrossRef] [PubMed]

| N | % | ||
|---|---|---|---|
| Originated in | Africa | 16 | 17 |
| Asia | 40 | 43 | |
| Central America | 1 | 1 | |
| Europe | 17 | 18 | |
| North America | 17 | 18 | |
| Oceania | 1 | 1 | |
| South America | 2 | 2 | |
| Study timing | Retrospective | 83 | 88 |
| Prospective | 9 | 10 | |
| Cross-sectional | 2 | 2 | |
| Type of study | Observational | 62 | 66 |
| Comparative | 32 | 34 | |
| Study design | Cohort | 88 | 94 |
| Case-control | 5 | 5 | |
| Randomized controlled Trial | 1 | 1 | |
| Year of publication | 2013 | 2 | 2 |
| 2014 | 6 | 6 | |
| 2015 | 3 | 3 | |
| 2016 | 12 | 13 | |
| 2017 | 10 | 11 | |
| 2018 | 10 | 11 | |
| 2019 | 16 | 17 | |
| 2020 | 5 | 5 | |
| 2021 | 7 | 7 | |
| 2022 | 15 | 16 | |
| 2023 | 8 | 9 | |
| Number of included patients | <10 | 11 | 12 |
| <25 | 19 | 20 | |
| 25–50 | 31 | 33 | |
| 50–100 | 20 | 21 | |
| 100–300 | 8 | 9 | |
| 300–1000 | 2 | 2 | |
| >1000 | 3 | 3 |
| Patient Characteristics | n | % |
|---|---|---|
| Sex | 81 | 86 |
| Gestational age | 67 | 71 |
| Any associated anomalies | 62 | 66 |
| Birth weight (grams) | 60 | 64 |
| Age at surgery (days) | 41 | 44 |
| Type of atresia (I: mucosal web, II atretic fibrous cord, IIIa: V-shaped mesenteric defect, type IIIb apple peel atresia, type IV: multiple atresias) | 39 | 41 |
| Cardiac anomalies | 38 | 40 |
| Prematurity | 33 | 35 |
| Level of obstruction (duodenal, jejuno-ileal, colonic) | 31 | 33 |
| Gastrointestinal anomalies | 28 | 30 |
| Urogenital anomalies | 22 | 23 |
| Site of intestinal atresia (anatomical location of atresia) | 21 | 22 |
| Age at presentation (days) | 18 | 19 |
| Respiratory anomalies | 16 | 17 |
| Anomalies of the Central Nervous System | 15 | 16 |
| Vomiting (presenting symptom) | 15 | 16 |
| Weight at surgery (grams) | 14 | 15 |
| Musculoskeletal anomalies | 14 | 15 |
| Weight at presentation (grams) | 13 | 14 |
| Type of delivery (vaginal or caesarean) | 13 | 14 |
| Abdominal distension (presenting symptom) | 13 | 14 |
| Trisomy 21 | 13 | 14 |
| Malrotation of intestine | 13 | 14 |
| Anorectal malformation | 12 | 13 |
| Age at admission (days) | 10 | 11 |
| Maternal age | 10 | 11 |
| Apgar 5 min | 9 | 10 |
| Syndrome | 9 | 10 |
| Volvulus (presenting symptom) | 9 | 10 |
| Venous malformation | 9 | 10 |
| Renal anomalies | 9 | 10 |
| Oesophageal atresia/Tracheoesophageal fistula | 9 | 10 |
| Failure to pass meconium (presenting symptom) | 8 | 9 |
| Chromosomal abnormalities | 8 | 9 |
| Cystic fibrosis | 8 | 9 |
| Annular pancreas | 8 | 9 |
| Race (Caucasian, African-American, Asian, other) | 7 | 7 |
| Apgar 1 min | 7 | 7 |
| ASA class | 7 | 7 |
| Ventricular septal defect | 7 | 7 |
| Gastroschisis | 7 | 7 |
| Location of delivery | 6 | 6 |
| Patent ductus arteriosus | 6 | 6 |
| Meckel diverticulum | 6 | 6 |
| Small for gestational age | 5 | 5 |
| Age at diagnosis (days) | 5 | 5 |
| Primipara vs. multipara | 5 | 5 |
| Duodenal atresia | 5 | 5 |
| VACTERL association | 5 | 5 |
| Structure and Process Variables | n | % |
|---|---|---|
| Structure | ||
| Hospital level | 25 | 27 |
| Treatment at a tertiary referral center | 20 | 21 |
| Presence of a neonatal intensive care unit | 11 | 12 |
| Operation performed by (licensed specialized) pediatric surgeon | 10 | 11 |
| Facilities for pediatric ventilation | 8 | 9 |
| Hospital transfer (patient transfer status) | 6 | 6 |
| Location of delivery | 6 | 6 |
| Facilities for total parenteral nutrition | 5 | 5 |
| Process variables | n | % |
| Performance of a resection | 41 | 44 |
| Primary anastomosis | 39 | 41 |
| Placement of a stoma | 32 | 34 |
| Prenatal diagnosis | 27 | 29 |
| Total duration of parenteral nutrition (in days) | 27 | 29 |
| Duration of procedure | 24 | 26 |
| Time to full enteral nutrition (in days, since operation) | 23 | 24 |
| Duration of follow up | 22 | 23 |
| Type of procedure (laparotomy or laparoscopy) | 21 | 22 |
| Performance of an abdominal X-ray for diagnosis | 19 | 20 |
| Description of the type of resection | 19 | 20 |
| Performance of tapering enteroplasty | 18 | 19 |
| Time to start enteral feeding (in days, since operation) | 15 | 16 |
| Time to initial oral feeding (in days, since operation) | 15 | 16 |
| Description of the surgical technique | 15 | 16 |
| Type of anastomosis (end/end, end/side, side/side) | 15 | 16 |
| Prenatal ultrasound | 14 | 15 |
| Prenatal diagnosis of polyhydramnios | 13 | 14 |
| Type of sutures | 12 | 13 |
| Discrepancy proximal and distal diameter | 11 | 12 |
| Time to establishment of full oral intake | 10 | 11 |
| Time to bowel continuity | 10 | 11 |
| Performance of intravenous fluid replacement at and during admission | 10 | 11 |
| Nasogastric tube placement | 10 | 11 |
| Technique of the anastomosis (e.g., hand sewn, stapler) | 9 | 10 |
| Suturing technique used for the anastomosis | 9 | 10 |
| Intestinal perforation found during operation (intraoperative finding) | 9 | 10 |
| Provision of preoperative antibiotics | 9 | 10 |
| Type of incision | 8 | 9 |
| Malnutrition at admission or during hospital stay | 8 | 9 |
| Performance of laboratory investigations | 8 | 9 |
| Placement of a central line/peripherally inserted central catheter | 8 | 9 |
| Performance of the bishop-Koop procedure | 8 | 9 |
| Performance of an abdominal ultrasound for diagnosis | 8 | 9 |
| Performance of 1-year follow-up | 8 | 9 |
| Duration of mechanical ventilation | 7 | 7 |
| Breast milk use | 7 | 7 |
| Timing of start of parenteral nutrition (age of start, pre- or postoperatively) | 6 | 6 |
| Measurement of serum electrolytes | 6 | 6 |
| Measurement of direct bilirubin | 6 | 6 |
| Location of anastomosis | 6 | 6 |
| Procedure priority (emergency, elective, semi-elective) | 6 | 6 |
| Resuscitation | 6 | 6 |
| Length of resected intestine (cm) | 6 | 6 |
| Age at diagnosis (days) | 5 | 5 |
| Feeding intolerance (postoperative) | 5 | 5 |
| Tripple bubble sign diagnosed (on X-ray) | 5 | 5 |
| Measurement of albumin | 5 | 5 |
| Double bubble sign diagnosed | 5 | 5 |
| Estimated blood loss | 5 | 5 |
| Time between presentation and surgery | 5 | 5 |
| Dilated bowel loops visible (on X-ray) | 5 | 5 |
| Number of anastomoses | 5 | 5 |
| Outcomes | n | % |
|---|---|---|
| Survival rate/mortality | 66 | 70 |
| Complications | 56 | 60 |
| Length of hospital stay | 52 | 55 |
| Sepsis | 48 | 51 |
| Unplanned reoperation | 40 | 43 |
| Anastomotic leakage | 36 | 38 |
| Wound infection | 28 | 30 |
| Bowel obstruction | 26 | 28 |
| Anastomotic stricture/stenosis | 19 | 20 |
| Short bowel syndrome | 17 | 18 |
| Remaining small bowel length | 17 | 18 |
| Respiratory infection | 17 | 18 |
| Necrotizing enterocolitis (NEC) | 16 | 17 |
| In-hospital mortality (death before discharge) | 13 | 14 |
| Wound dehiscence | 12 | 13 |
| Episodes of meconium peritonitis | 11 | 12 |
| Central line-associated bloodstream infection (CLABSI) | 10 | 11 |
| Hepatic cholestasis | 10 | 11 |
| Infection (general) | 9 | 10 |
| Liver failure | 9 | 10 |
| Stoma related complications | 9 | 10 |
| Number of operations | 8 | 9 |
| Pneumonia | 7 | 7 |
| Intestinal necrosis | 7 | 7 |
| Readmission | 7 | 7 |
| Short term complications (<30 days) | 7 | 7 |
| Ileus | 7 | 7 |
| Growth | 7 | 7 |
| Postoperative intestinal perforation | 6 | 6 |
| 30-day mortality | 6 | 6 |
| Development | 6 | 6 |
| Growth in weight | 6 | 6 |
| Growth in height | 6 | 6 |
| Preservation of ileocecal valve | 6 | 6 |
| Re-anastomosis | 6 | 6 |
| Meconium ileus | 6 | 6 |
| Aspiration pneumonitis | 6 | 6 |
| Stomal prolapse | 6 | 6 |
| Gastrostomy or gastrojejunostomy dependent | 5 | 5 |
| Incisional hernia | 5 | 5 |
| High output stoma | 5 | 5 |
| Development of parenteral nutrition associated liver dysfunction (PNALD) | 5 | 5 |
| Surgical re-exploration and enterostomy | 5 | 5 |
| Urinary tract infection | 5 | 5 |
| Respiratory failure | 5 | 5 |
| Ischemia (intraoperative, primary surgery) | 5 | 5 |
| Postoperative length of hospital stay | 5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Kamp, L.M.; Moglia, C.; La Pergola, E.; Rossi, D.; Teunissen, N.M.; Migliazza, L.; Wijnen, R.M.H. Building the Foundation for Standardized Care Metrics in Jejunoileal Atresia: A Systematic Review of Reported Baseline Characteristics, Treatment Variables and Outcomes. J. Clin. Med. 2025, 14, 5693. https://doi.org/10.3390/jcm14165693
van der Kamp LM, Moglia C, La Pergola E, Rossi D, Teunissen NM, Migliazza L, Wijnen RMH. Building the Foundation for Standardized Care Metrics in Jejunoileal Atresia: A Systematic Review of Reported Baseline Characteristics, Treatment Variables and Outcomes. Journal of Clinical Medicine. 2025; 14(16):5693. https://doi.org/10.3390/jcm14165693
Chicago/Turabian Stylevan der Kamp, Linde Margriet, Cristina Moglia, Enrico La Pergola, Daniel Rossi, Nadine Maria Teunissen, Lucia Migliazza, and René Maria Henricus Wijnen. 2025. "Building the Foundation for Standardized Care Metrics in Jejunoileal Atresia: A Systematic Review of Reported Baseline Characteristics, Treatment Variables and Outcomes" Journal of Clinical Medicine 14, no. 16: 5693. https://doi.org/10.3390/jcm14165693
APA Stylevan der Kamp, L. M., Moglia, C., La Pergola, E., Rossi, D., Teunissen, N. M., Migliazza, L., & Wijnen, R. M. H. (2025). Building the Foundation for Standardized Care Metrics in Jejunoileal Atresia: A Systematic Review of Reported Baseline Characteristics, Treatment Variables and Outcomes. Journal of Clinical Medicine, 14(16), 5693. https://doi.org/10.3390/jcm14165693





