Prognostic Impact of Aspartate Aminotransferase-to-Platelet Ratio Index and Prognostic Nutrition Index in Hepatocellular Carcinoma Patients Undergoing Resection
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
- -
- PLR: 81.1% for ≤145.365, 61.3% for >145.365
- -
- ANRI: 79.3% for ≤13.09, 73.8% for >13.09
- -
- Fib-Alb: 84.7% for ≤71.03, 71.6% for >71.03
- -
- SII: 82.7% for ≤442.455, 65.3% for >442.455
- -
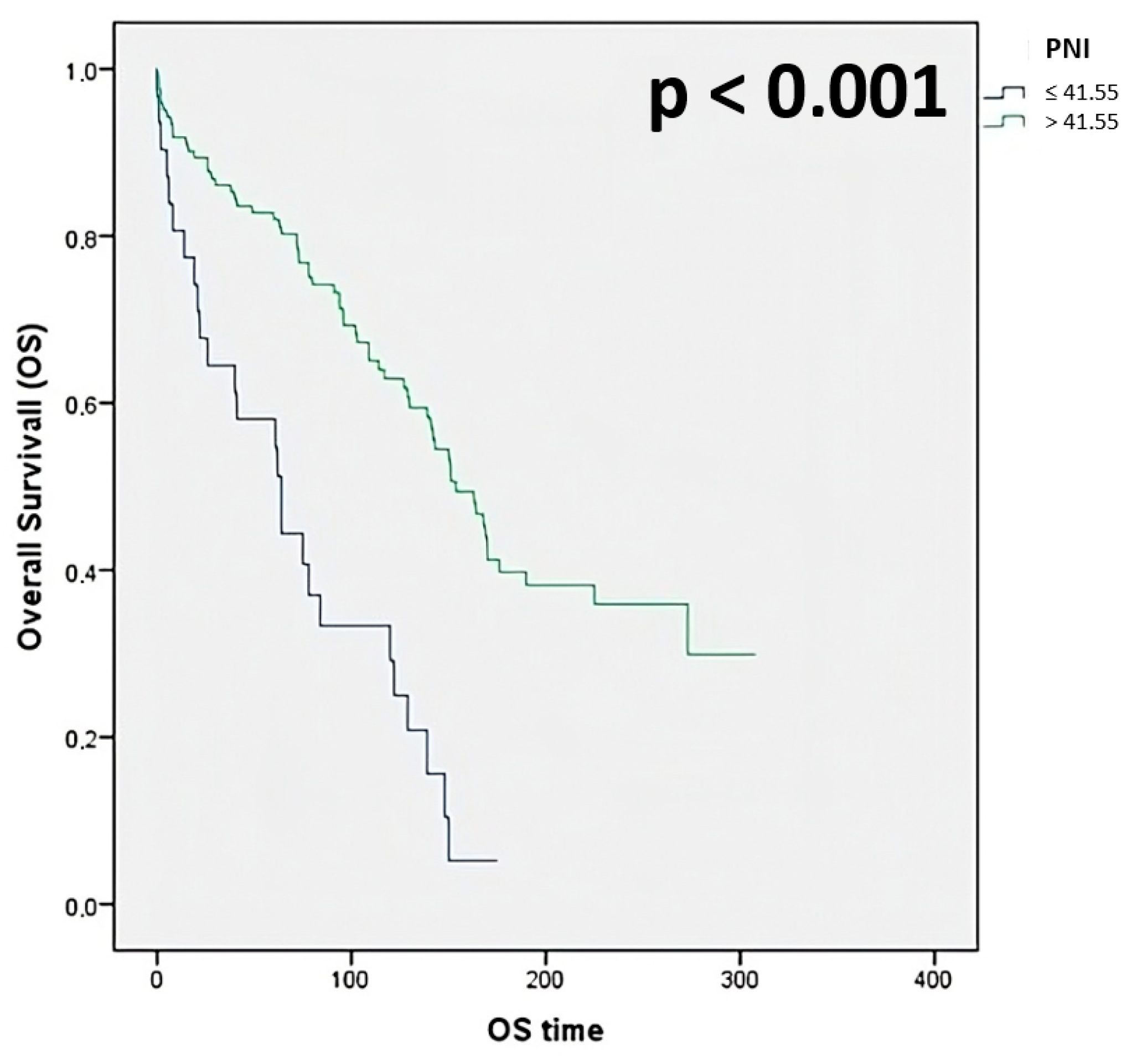
- PNI: 58.1% for ≤41.55, 81.9% for >41.55
- -
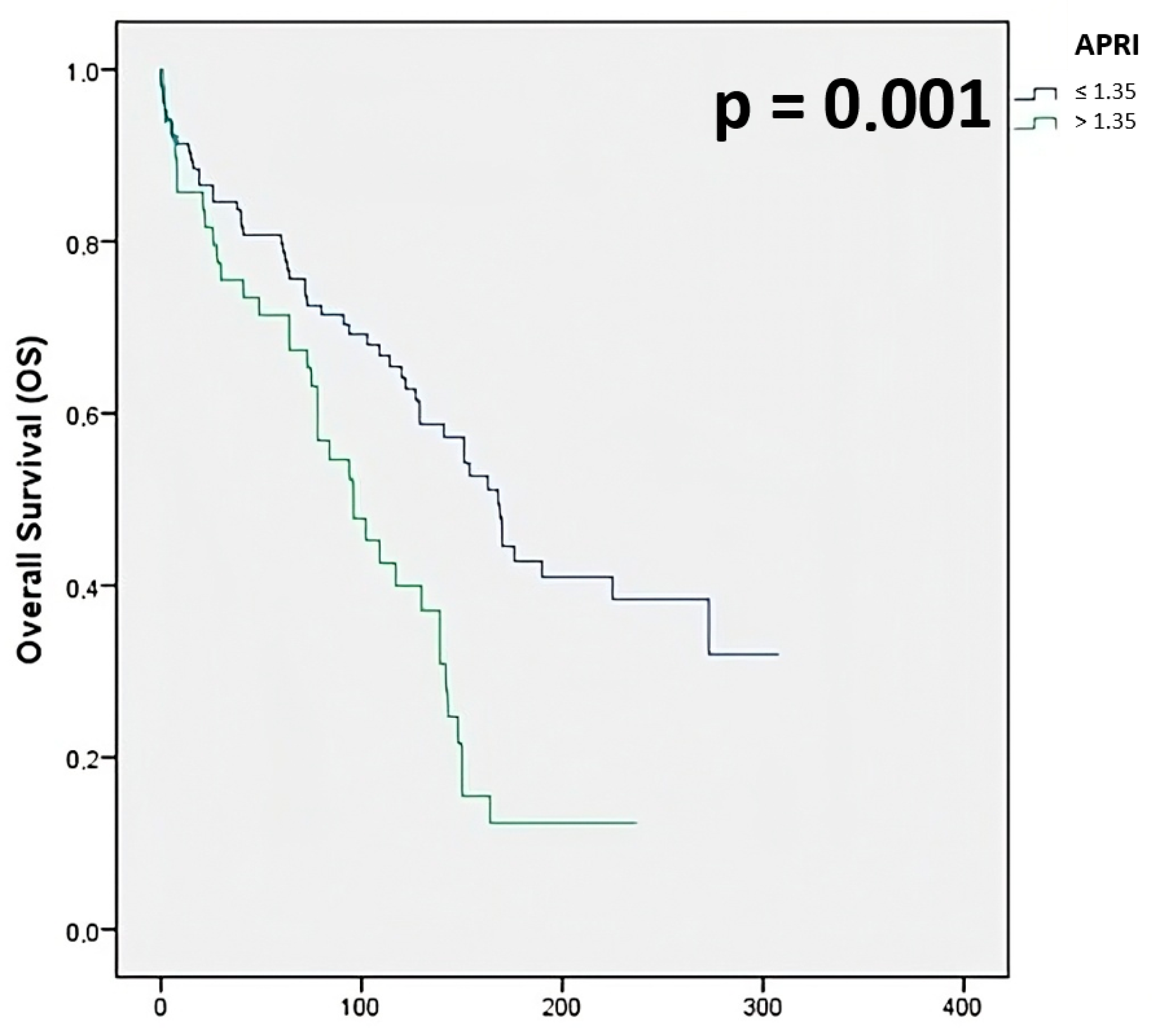
- APRI: 80.8% for ≤1.35, 71.4% for >1.35
- -
- ALBI: 85.7% for ≤−2.60, 68.1% for >−2.60 to ≤−1.39, 50.0% for >−1.39
- -
- PALBI: 85.6% for ≤−2.60, 62.4% for >−2.60 to ≤−1.39, 46.9% for >−1.39
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OS | Overall Survival |
| HCC | Hepatocellular carcinoma |
| NLR | Neutrophil-to-lymphocyte ratio |
| PLR | Platelet-to-lymphocyte ratio |
| LMR | Lymphocyte-to-monocyte ratio |
| ANRI | Aspartate aminotransferase-to-neutrophil ratio index |
| Fib-Alb | fibrinogen-to-albumin ratio |
| SII | Systemic immune-inflammation index |
| PNI | Prognostic nutritional index |
| APRI | Aspartate aminotransferase-to-platelet ratio index |
| LT | Liver transplantation |
| HR | Hepatic Resection |
| RFS | Recurrence-free survival |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| AFP | Alpha-fetoprotein |
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755. [Google Scholar] [CrossRef]
- Pinna, A.D.; Yang, T.; Mazzaferro, V.; De Carlis, L.; Zhou, J.; Roayaie, S.; Shen, F.; Sposito, C.; Cescon, M.; Di Sandro, S.; et al. Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma. Ann. Surg. 2018, 268, 868–875. [Google Scholar] [CrossRef]
- Grazi, G.L.; Ercolani, G.; Pierangeli, F.; Del Gaudio, M.; Cescon, M.; Cavallari, A.; Mazziotti, A. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann. Surg. 2001, 234, 71–78. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Otsuka, Y.; Kaneko, H.; Nagai, M.; Nomura, Y.; Yamamoto, M.; Otani, M.; Ohashi, Y.; Sugawara, K.; Koike, D.; et al. Comparisons of financial and short-term outcomes between laparoscopic and open hepatectomy: Benefits for patients and hospitals. Surg. Today 2016, 46, 535–542. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmache, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Chan, A.W.H.; Chan, A.K.C.; Jian, P.; Mo, F.; Chan, C.M.L.; Mok, K.; Liu, C.; Chong, C.C.; Chan, A.T.C.; et al. Systematic evaluation of circulating inflammatory markers for hepatocellular carcinoma. Liver Int. 2017, 37, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.H.; Chan, S.L.; Wong, G.L.H.; Wong, V.W.S.; Chong, C.C.N.; Lai, P.B.S.; Chan, H.L.Y.; To, K.-F. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Ann. Surg. Oncol. 2015, 22, 4138–4148. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Stebbing, J.; Ishizuka, M.; Khan, S.A.; Wasan, H.S.; North, B.V.; Kubota, K.; Sharma, R. A novel and validated prognostic index in hepatocellular carcinoma: The inflammation based index (IBI). J. Hepatol. 2012, 57, 1013–1020. [Google Scholar] [CrossRef]
- Zheng, H.; Li, P.; Kwok, J.G.; Korrapati, A.; Li, W.T.; Qu, Y.; Wang, X.Q.; Kisseleva, T.; Wang-Rodriguez, J.; Ongkeko, W.M. Alcohol and hepatitis virus-dysregulated lncRNAs as potential biomarkers for hepatocellular carcinoma. Oncotarget 2017, 9, 224–235. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chang, K.P.; Lin, Y.S.; Chang, T.S. Pretreatment combination of platelet counts and neutrophil-lymphocyte ratio predicts survival of nasopharyngeal cancer patients receiving intensity-modulated radiotherapy. Onco. Targets Ther. 2017, 10, 2751–2760. [Google Scholar] [CrossRef]
- Liu, W.; Ha, M.; Yin, N. Combination of platelet count and lymphocyte to monocyte ratio is a prognostic factor in patients undergoing surgery for non-small cell lung cancer. Oncotarget 2017, 8, 73198–73207. [Google Scholar] [CrossRef]
- Nomelini, R.S.; Chiovato, A.F.C.; Abdulmassih, F.B.F.; da Silva, R.C.; Tavares-Murta, B.M.; Murta, E.F.C. Neutrophil-to-lymphocyte ratio and platelet count as prognostic factors in ovarian malignancies. J. Cancer Res. Ther. 2019, 15, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Bosonnet, L.; Raraty, M.; Sutton, R.; Neoptolemos, J.P.; Campbell, F.; Ghaneh, P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am. J. Surg. 2009, 197, 466–472. [Google Scholar] [CrossRef]
- Wang, J.; Qu, J.; Li, Z.; Che, X.; Liu, J.; Teng, Y.; Jin, B.; Zhao, M.; Zhang, L.; Liu, Y.; et al. Combination of platelet count and neutrophil-lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival in metastatic advanced gastric cancer. Biomark. Med. 2017, 11, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Couinaud, C. Liver anatomy: Portal (and suprahepatic) or biliary segmentation. Dig. Surg. 1999, 16, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Roayaie, P.S.; Jibara, G.; Berhane, S.; Tabrizian, P.; Park, J.W.; Yang, J.; Yan, L.; Johnson, P. PALBI-An Objective Score Based on Plate- lets, Albumin & Bilirubin Stratifies HCC Patients Undergoing Resection & Ablation Better than Child’s Classification. In AASLD LiverLearning®; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Lu, L.-H.; Zhang, Y.-F.; Mu-Yan, C.; Kan, A.; Zhong, X.-P.; Mei, J.; Ling, Y.-H.; Li, S.-H.; Shi, M.; Wei, W.; et al. Platelet-albumin-bilirubin grade: Risk stratification of liver failure, prognosis after resection for hepatocellular carcinoma. Dig. Liver Dis. 2019, 51, 1430–1437. [Google Scholar] [CrossRef]
- Sun, X.-D.; Shi, X.-J.; Chen, Y.-G.; Wang, C.-L.; Ma, Q.; Lv, G.-Y. Elevated Preoperative Neutrophil-Lymphocyte Ratio Is Associated with Poor Prognosis in Hepatocellular Carcinoma Patients Treated with Liver Transplantation: A Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 4743808. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Yugawa, K.; Shimokawa, M.; Yoshiya, S.; Mano, Y.; Takeishi, K.; Toshima, T.; Maehara, Y.; Mori, M.; Yoshizumi, T. Prognostic significance of inflammatory biomarkers in hepatocellular carcinoma following hepatic resection. BJS Open 2019, 3, 500–508. [Google Scholar] [CrossRef]
- Wang, Y.; Attar, B.M.; Fuentes, H.E.; Jaiswal, P.; Tafur, A.J. Evaluation of the prognostic value of platelet to lymphocyte ratio in patients with hepatocellular carcinoma. J. Gastrointest. Oncol. 2017, 8, 1065–1071. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hung, H.H.; Su, C.W.; Lai, C.R.; Chau, G.Y.; Chan, C.C.; Huang, Y.H.; Huo, T.I.; Lee, P.C.; Kao, W.Y.; Lee, S.D.; et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol. Int. 2010, 4, 691–699. [Google Scholar] [CrossRef]
- Shen, S.L.; Fu, S.J.; Chen, B.; Kuang, M.; Li, S.Q.; Hua, Y.P.; Liang, L.J.; Guo, P.; Hao, Y.; Peng, B.G. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann. Surg. Oncol. 2014, 21, 3802–3809. [Google Scholar] [CrossRef]
- Yugawa, K.; Maeda, T.; Nagata, S.; Sakai, A.; Edagawa, M.; Omine, T.; Kometani, T.; Yamaguchi, S.; Konishi, K.; Hashimoto, K. A novel combined prognostic nutritional index and aspartate aminotransferase-to-platelet ratio index-based score can predict the survival of patients with hepatocellular carcinoma who undergo hepatic resection. Surg. Today 2022, 52, 1096–1108. [Google Scholar] [CrossRef]
- Kanda, M.; Fujii, T.; Kodera, Y.; Nagai, S.; Takeda, S.; Nakao, A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br. J. Surg. 2011, 98, 268–274. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Yang, Z.Y.; Zhang, L.D.; Ping-Bie Wang, S.G.; Ma, K.S.; Li, X.W.; Dong, J.H. Increased liver-infiltrating CD8+FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Hum. Immunol. 2010, 71, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.M.; Zhao, Q.; Wu, Y.; Peng, C.; Wang, J.; Xu, Z.; Yin, X.Y.; Zheng, L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol. 2011, 54, 948–955. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Okuda, M.; Li, K.; Beard, M.R.; Showalter, L.A.; Scholle, F.; Lemon, S.M.; Weinman, S.A. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 2002, 122, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Jiang, C.P.; Cao, Y.; Zhang, G.; Chen, W.B.; Ding, Y.T. Preoperative serum liver enzyme markers for predicting early recurrence after curative resection of hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Hafner, M.; Echtenacher, B.; Mannel, D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999, 59, 1295–1300. [Google Scholar]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berardi, G.; Muttillo, E.M.; Colasanti, M.; Mariano, G.; Meniconi, R.L.; Ferretti, S.; Guglielmo, N.; Angrisani, M.; Lucarini, A.; Garofalo, E.; et al. Challenging Scenarios and Debated Indications for Laparoscopic Liver Resections for Hepatocellular Carcinoma. Cancers 2023, 15, 1493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| NLR | N (absolute neutrophil count)/L (absolute lymphocyte count) |
| PLR | P (absolute platelet count)/L (lymphocyte count) |
| LMR | L (absolute lymphocyte count)/M (absolute monocyte count) |
| PNI | 10 × serum albumin (g/dL) + 0.005 × L (lymphocyte count) |
| SII | P (absolute platelet count) × N (absolute neutrophil count)/L (absolute lymphocyte count) |
| ANRI | AST (Aspartate Aminotransferase count)/N (absolute neutrophil count) |
| APRI | [(AST Level IU/L/AST Upper Limit of Normal IU/L)/P (absolute platelet count)] × 100 |
| Fib-Alb | Fibrinogen/Albumin (g/dL) × 100 |
| ALBI | ([log10 bilirubin (μmol/L) × 0.66] + [albumin (g/L) × −0.085] |
| PALBI | 2.02 × log10 bilirubin (μmol/L) − 0.37 × (log10 bilirubin)2 − 0.04 × albumin (g/L) − 3.48 × log10 platelets + 1.01 × (log10 platelets)2 |
| Variable | n (%) | 5 yrs OS (%) | Exp (B) | 95% CI | p |
|---|---|---|---|---|---|
| Sex Male Female | 114 (74.5) 39 (25.5) | 78.9 71.5 | 0.83 | 0.53–1.31 | 0.424 |
| Age ≤65 yrs >65 yrs | 54 (35.3) 99 (64.7) | 77.8 76.7 | 0.86 | 0.55–1.34 | 0.502 |
| ASA score 2 3 + 4 | 53 (34.6) 100 (65.4) | 81.0 74.5 | 0.36 | 0.09–1.52 | 0.163 |
| BMI † Kg/m2 ≤25 >25 < 30 ≥30 | 78 (51.0) 47 (30.7) 28 (18.3) | 74.4 76.6 85.4 | 0.98 | 0.74–1.29 | 0.865 |
| Cirrhosis No Yes | 26 (17.0) 127 (83.0) | 69.2 78.7 | 1.10 | 0.65–1.85 | 0.720 |
| Portal hypertension No Yes | 121 (79.1) 32 (20.9) | 75.2 84.4 | 0.82 | 0.50–1.36 | 0.444 |
| MELD score <10 ≥10 | 146 (95.4) 7 (4.6) | 78.6 68.2 | 0.60 | 0.32–1.12 | 0.106 |
| Child-Pugh classification A B | 147 (96.1) 6 (3.9) | 78.9 33.3 | 0.26 | 0.11–0.61 | 0.002 |
| BCLC stage 0 + A B + C | 79 (51.6) 74 (48.4) | 88.1 66.2 | 0.40 | 0.26–0.61 | <0.001 |
| Mini-invasive surgery No Yes | 127 (83.0) 26 (17.0) | 74.8 88.5 | 1.45 | 0.72–2.90 | 0.297 |
| Type of resection Anatomical Non-anatomical | 61 (39.9) 92 (60.1) | 65.6 84.8 | 1.57 | 1.05–2.42 | 0.028 |
| Type of hepatectomy Minor (<3 segments) Major (≥3 segments) | 132 (86.3) 21 (13.7) | 81.8 47.6 | 0.40 | 0.24–0.67 | <0.001 |
| Largest tumor size ≤5 cm >5 cm | 96 (62.7) 57 (37.3) | 85.4 63.2 | 0.47 | 0.30–0.69 | <0.001 |
| Edmondson grading G1–2 G3–4 | 78 (51.0) 75 (49.0) | 85.6 67.1 | 0.36 | 0.08–1.64 | 0.204 |
| Microvascular invasion No Yes | 88 (57.5) 65 (42.5) | 84.1 67.7 | 0.79 | 0.47–1.08 | 0.106 |
| Capsuled tumor No Yes | 99 (64.7) 54 (35.3) | 73.7 83.3 | 1.12 | 0.72–1.76 | 0.624 |
| Satellite lesions Negative Positive | 139 (90.8) 14 (9.2) | 77.0 85.7 | 0.99 | 0.50–1.97 | 0.975 |
| Degree of radicality R0 R1 | 146 (95.4) 7 (4.6) | 78.7 42.9 | 0.30 | 0.12–0.77 | 0.012 |
| Transfusion of RBCs ‡ No Yes | 116 (75.8) 37 (24.2) | 84.3 54.1 | 0.37 | 0.24–0.58 | <0.001 |
| Transfusion of FFP § No Yes | 137 (89.5) 16 (10.5) | 79.4 56.3 | 0.44 | 0.24–0.80 | 0.007 |
| Pringle maneuver No Yes | 107 (70.0) 46 (30.0) | 77.5 76.1 | 0.91 | 0.58–1.42 | 0.673 |
| Postoperative complication No Yes | 79 (51.6) 74 (48.4) | 88.6 64.9 | 0.34 | 0.22–0.53 | <0.001 |
| AFP || (ng/mL) ≤200 >200 | 63 (41.2) 90 (58.8) | 87.7 57.7 | 0.53 | 0.31–0.93 | 0.026 |
| Variable | n (%) | 5 yrs OS (%) | Exp (B) | 95% CI | p |
|---|---|---|---|---|---|
| NLR <2.555 ≥2.555 | 90 (58.8) 63 (41.2) | 80.0 73.0 | 0.74 | 0.49–1.12 | 0.158 |
| PLR ≤145.365 >145.365 | 122 (79.7) 31 (20.3) | 81.1 61.3 | 0.59 | 0.37–0.96 | 0.035 |
| LMR ≤2.96 >2.96 | 80 (52.3) 73 (47.7) | 72.5 82.2 | 1.10 | 0.72–1.67 | 0.688 |
| ANRI ≤13.09 >13.09 | 92 (60.1) 61 (39.9) | 79.3 73.8 | 0.65 | 0.43–0.99 | 0.043 |
| Fib-Alb ≤71.03 >71.03 | 72 (47.1) 81 (52.9) | 84.7 71.6 | 0.57 | 0.36–0.85 | 0.007 |
| SII ≤442.455 >442.455 | 104 (68.0) 49 (32.0) | 82.7 65.3 | 0.65 | 0.42–0.99 | 0.046 |
| PNI ≤41.55 >41.55 | 31 (20.3) 122 (79.7) | 58.1 81.9 | 3.02 | 1.89–4.58 | <0.001 |
| APRI ≤1.35 >1.35 | 104 (68.0) 49 (32.0) | 80.8 71.4 | 0.49 | 0.32–0.75 | 0.001 |
| ALBI ≤−2.60 >−2.60 ≤ −1.39 >−1.39 | 77 (50.3) 72 (47.1) 4 (2.6) | 85.7 68.1 50.0 | 0.48 | 0.31–0.73 | 0.001 |
| PALBI ≤−2.60 >−2.60 ≤ −1.39 >−1.39 | 97 (51.6) 48 (48.4) 8 (48.4) | 85.6 62.4 46.9 | 0.65 | 0.42–0.99 | 0.049 |
| HR | 95% CI | p | |
|---|---|---|---|
| BCLC stage | 0.38 | 0.22–0.66 | 0.001 |
| Postoperative complications | 0.48 | 0.28–0.82 | 0.007 |
| PNI | 2.47 | 1.33–4.59 | 0.004 |
| APRI | 0.48 | 0.28–0.81 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risaliti, M.; De Peppo, V.; Bartolini, I.; Tirloni, L.; Scarinci, A.; Terrenato, I.; Grazi, G.L. Prognostic Impact of Aspartate Aminotransferase-to-Platelet Ratio Index and Prognostic Nutrition Index in Hepatocellular Carcinoma Patients Undergoing Resection. J. Clin. Med. 2025, 14, 5665. https://doi.org/10.3390/jcm14165665
Risaliti M, De Peppo V, Bartolini I, Tirloni L, Scarinci A, Terrenato I, Grazi GL. Prognostic Impact of Aspartate Aminotransferase-to-Platelet Ratio Index and Prognostic Nutrition Index in Hepatocellular Carcinoma Patients Undergoing Resection. Journal of Clinical Medicine. 2025; 14(16):5665. https://doi.org/10.3390/jcm14165665
Chicago/Turabian StyleRisaliti, Matteo, Valerio De Peppo, Ilenia Bartolini, Luca Tirloni, Andrea Scarinci, Irene Terrenato, and Gian Luca Grazi. 2025. "Prognostic Impact of Aspartate Aminotransferase-to-Platelet Ratio Index and Prognostic Nutrition Index in Hepatocellular Carcinoma Patients Undergoing Resection" Journal of Clinical Medicine 14, no. 16: 5665. https://doi.org/10.3390/jcm14165665
APA StyleRisaliti, M., De Peppo, V., Bartolini, I., Tirloni, L., Scarinci, A., Terrenato, I., & Grazi, G. L. (2025). Prognostic Impact of Aspartate Aminotransferase-to-Platelet Ratio Index and Prognostic Nutrition Index in Hepatocellular Carcinoma Patients Undergoing Resection. Journal of Clinical Medicine, 14(16), 5665. https://doi.org/10.3390/jcm14165665







