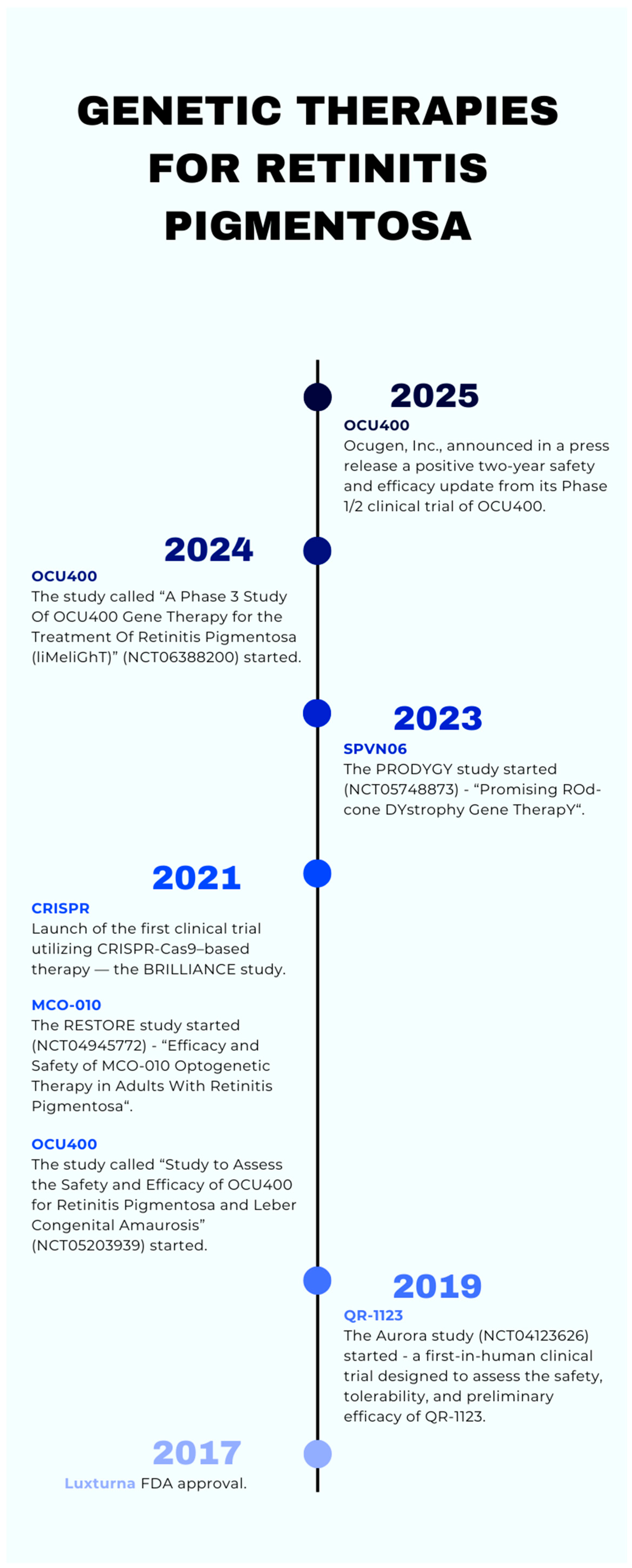
Genetic Therapies for Retinitis Pigmentosa: Current Breakthroughs and Future Directions
Abstract
1. Introduction
2. Methods Section
3. Brief Characteristics of Selected Genes Causing RP
3.1. Rhodopsin Gene (RHO)
3.2. PRPF31
3.3. RPE65
3.4. NR2E3
4. Current Methods of Prevention and Treatment
4.1. Diet (Vitamin A; Omega-3 Fatty Acid; Lutein; Zeaxanthin)
4.2. Pharmacological Approach in Cystoid Macular Edema (CME)
4.3. Surgical Treatment—ARGUS II
4.4. Hyperbaric Oxygen
4.5. Stem Cells
5. Emerging Genetic Therapies for RP
5.1. Gene Therapy and Delivery Methods
5.2. Luxturna
5.2.1. What Exactly Is Luxturna?
5.2.2. Safety Profile and Adverse Effects of Luxturna
5.2.3. Key Clinical Trials: Phase 3 Data
5.2.4. Vision Testing and Functional Outcomes
5.2.5. Impact and Future Directions of Luxturna
5.2.6. Limitations of Luxturna
5.3. OCU400
5.3.1. What Exactly Is OCU400?
5.3.2. Safety Profile, Adverse Effects, and Visual Function Improvement
5.3.3. Future Development and Direction of OCU400
5.4. CRISPR
5.4.1. Modern Classification of CRISPR–Cas Systems
5.4.2. Clinical Trials: Phases 1, 2, and 3
5.4.3. Future Development and Direction of CRISPR
5.4.4. Off-Side Effects and Challenges of CRISPR
5.5. MCO-010
5.5.1. What Exactly Is MCO-010?
5.5.2. Preclinical and Clinical Studies Phase 1 and 2
5.5.3. Future Development and Direction of MCO-010
5.5.4. Side Effects of MCO-010
5.6. QR-1123
5.7. SPVN06
| (a) | |||
| Feature | Luxturna | OCU400 | MCO-010 |
| Therapy Type | Gene replacement (RPE65-specific) [53] | Gene-agnostic (NR2E3 network modulator) [55,56] | Optogenetic (mutation-independent) [64] |
| Target Gene/Mechanism | RPE65 (biallelic mutations) [46] | NR2E3 (network reprogramming) [55] | MCO gene (multi-characteristic opsin) [64] |
| Delivery Method | Subretinal injection [46] | Subretinal injection [55] | Intravitreal injection [65] |
| Eligibility | Only for patients with confirmed RPE65 biallelic mutations [46] | Broad RP patients’ population [56] | All RP genotypes [64] |
| Clinical Trial Phase | Completed Phase 3 + approved [50] | Phase 1/2 completed, Phase 3 ongoing [57,58] | Phase 2b completed (RESTORE study) [64] |
| Efficacy Highlights | Improvement in functional vision (MLMT); enhanced light sensitivity (FST); better real-life mobility; durable efficacy; greater gains in younger patients [50,51,52] | Visual function preserved/improved in 100% of treated eyes; enhanced low-light visual acuity; stabilization/improvement in mobility performance (MLMT) [58] | Significant visual improvement in mobility tests in varying light conditions [64]. |
| Adverse Effects | Retinal detachment; cataract; inflammation; ↑ intraocular pressure [48] | Mild events; surgery-related SAEs resolved without lasting effects [57] | Mild intraocular pressure ↑, anterior chamber cells [66] |
| Long-term Data | Available (up to 2.3 years, PERCEIVE) [49] | Available (2 years) [58] | Limited (short-term follow-up) |
| Regulatory Status | FDA + EMA approved [46] | EMA: ATMP status, FDA: EAP granted [59,60] | BLA planned H2 2025 [66] |
| Limitations | High cost; limited to RPE65; subretinal injection risk [48] | Still under investigation (not yet approved); limited long-time data; uncertain response across all genotypes; subretinal injection risk [58,59,60] | Awaiting approval; limited long-term data |
| (b) | |||
| Feature | CRISPR (general) | QR-1123 | SPVN06 |
| Therapy Type | Gene editing (precise mutation correction) [44] | AON; allele-specific; RNase H1-activating [67] | Mutation-agnostic AAV-based gene therapy delivering two functional proteins encoded by the NXNL1 gene [69,71] |
| Target Gene/ Mechanism | RHO, CEP290, etc. (mutation-specific) [63] | RHO gene with P23H mutation (c.68 C > A) [66] | Targets cone survival through delivery of RdCVF and RdCVFL [69,70] |
| Delivery Method | Viral/non-viral [44] | IVT injection (unilateral); single or repeated every 3 months [68] | Subretinal injection of AAV vector carrying RdCVF and RdCVFL genes [69] |
| Eligibility | Mutation-specific; under development [63] | Patients with adRP caused by the P23H mutation in the RHO gene [66] | Patients with rod–cone dystrophies, especially RP in intermediate stage [70] |
| Clinical Trial Phase | Preclinical and early clinical trials [63] | Phase I/II (Aurora Study, initiated in 2019); currently active but not recruiting [68] | Phase I/II clinical trial (PRODYGY) [70] |
| Efficacy Highlights | Some restored visual function in trials [45] Depends on gene; early results promising | Preclinical studies in adRP animal and human models demonstrated selective reduction of P23H rhodopsin and prevention of photoreceptor degeneration [67] | Preclinical studies in P23H rhodopsin transgenic pigs showed preservation of cone structure and function [69] |
| Adverse Effects | Off-target effects; immune response; DSB risks [44,45,63] | Not fully disclosed; ocular and non-ocular adverse events monitored over 12 months as primary outcome measures [68] | Phase I (PRODYGY) demonstrated good tolerability [71] |
| Long-term Data | Not available yet | Long-term human data not yet available; trial includes a 12-month follow-up period [68] | Not yet available; trial ongoing |
| Regulatory Status | Experimental | Investigational drug; not approved for clinical use yet [68] | Investigational drug; not approved for clinical use yet |
| Limitations | Precision/safety concerns, regulatory delay [44,45] | Mutation-specific therapy (effective only for P23H variant); mutation is rare and primarily found in the U.S. population (~2500–3000 individuals) [67] | Still in early clinical development; long-term efficacy and safety data pending; requires subretinal surgery |
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | Adeno-Associated Virus |
| adRP | Autosomal dominant retinitis pigmentosa |
| AMD | Age-related macular degeneration |
| Anti-VEGF | Anti-Vascular Endothelial Growth Factor |
| AON | Antisense oligonucleotide |
| ATMP | Advanced Therapy Medicinal Product |
| BCVA | Best-corrected visual acuity |
| BLA | Biologics License Application |
| CME | Cystoid Macular Edema |
| CPC | Chromatic pupillometry |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CRISPR–Cas9 | CRISPR-associated protein 9 |
| crRNA | CRISPR RNA |
| DSBs | Double-strand breaks |
| EAP | Expanded Access Program |
| EMA | European Medicines Agency |
| ERG | Electroretinogram |
| FAF | Fundus autofluorescence |
| FDA | Food and Drug Administration |
| FST | Full-field light sensitivity threshold testing |
| gRNA | Guide RNA |
| IOL | Intraocular lens |
| IRD | Inherited retinal disease |
| IVT | Intravitreal |
| LCA | Leber congenital amaurosis |
| MLMT | Multi-luminance mobility test |
| NR2E3 | Nuclear Receptor Subfamily 2 Group E Member 3 |
| pre-mRNA | Precursor mRNA |
| PRPF31 | Pre-mRNA processing factor 31 |
| RdCVF | Rod-derived Cone Viability Factor |
| RdCVFL | Rod-derived Cone Viability Factor Long Form |
| RHO | Rhodopsin Gene |
| ROS | Rod outer segment |
| RP | Retinitis pigmentosa |
| RPE | Retinal pigment epithelium |
| RPE65 | Retinal pigment epithelium-specific 65 kDa |
| SAEs | Serious adverse events |
| sgRNA | Single-guide RNA |
| TEAEs | Treatment-emergent adverse events |
| tri-snRNP | Tri-small nuclear ribonucleoprotein |
| TWIN-PE | Twin prime editing |
| USH1 | Usher syndrome type I |
| USH2A | Usher syndrome type IIA |
| USH3 | Usher syndrome type III |
References
- O’Neal, T.B.; Tripathy, K.; Luther, E.E. Retinitis Pigmentosa. In StatPearls, Internet; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S. Retinitis pigmentosa: A brief overview. Indian J. Ophthalmol. 2011, 59, 343–346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q. Retinitis Pigmentosa: Progress and Perspective. Asia-Pacific J. Ophthalmol. 2016, 5, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Vaccarella, L.; Olatunji, S.; Cebulla, C.; Christoforidis, J. Diagnostic challenges in retinitis pigmentosa: Genotypic multiplicity and phenotypic variability. Curr. Genom. 2011, 12, 267–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chao, D.L.; Burr, A.; Pennesi, M. RPE65-Related Leber Congenital Amaurosis/Early-Onset Severe Retinal Dystrophy. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2019; pp. 1993–2025. [Google Scholar]
- Ali, M.U.; Rahman, M.S.U.; Cao, J.; Yuan, P.X. Genetic characterization and disease mechanism of retinitis pigmentosa; current scenario. 3 Biotech 2017, 7, 251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, M.Y.; Liu, J.; Mehrotra, D.; Liu, Y.; Guo, Y.; Baldera-Aguayo, P.A.; Mooney, V.L.; Nour, A.M.; Yan, E.C. Thermal stability of rhodopsin and progression of retinitis pigmentosa: Comparison of S186W and D190N rhodopsin mutants. J. Biol. Chem. 2013, 288, 17698–17712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Luo, H.; Xiao, X.; Li, S.; Sun, W.; Yi, Z.; Wang, P.; Zhang, Q. Spectrum-frequency and genotype-phenotype analysis of rhodopsin variants. Exp. Eye Res. 2021, 203, 108405. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Xu, K.; Zhang, X.; Xie, Y.; Li, Y. Sector Retinitis Pigmentosa caused by mutations of the RHO gene. Eye 2019, 33, 592–599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.; Chen, L.; Tam, O.S.; Huang, X.F.; Pang, C.P.; Jin, Z.B. Whole exome sequencing reveals genetic predisposition in a large family with retinitis pigmentosa. BioMed Res. Int. 2014, 2014, 302487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kremmer, S.; Eckstein, A.; Gal, A.; Apfelstedt-Sylla, E.; Wedemann, H.; Rüther, K.; Zrenner, E. Ocular findings in patients with autosomal dominant retinitis pigmentosa and Cys110Phe, Arg135Gly, and Gln344stop mutations of rhodopsin. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Kiser, K.; Webb-Jones, K.D.; Bowne, S.J.; Sullivan, L.S.; Daiger, S.P.; Birch, D.G. Time Course of Disease Progression of PRPF31-mediated Retinitis Pigmentosa. Am. J. Ophthalmol. 2019, 200, 76–84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azizzadeh Pormehr, L.; Ahmadian, S.; Daftarian, N.; Mousavi, S.A.; Shafiezadeh, M. PRPF31 reduction causes mis-splicing of the phototransduction genes in human organotypic retinal culture. Eur. J. Hum. Genet. 2020, 28, 491–498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rose, A.M.; Bhattacharya, S.S. Variant haploinsufficiency and phenotypic non-penetrance in PRPF31-associated retinitis pigmentosa. Clin. Genet. 2016, 90, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Mordes, D.; Luo, X.; Kar, A.; Kuo, D.; Xu, L.; Fushimi, K.; Yu, G.; Sternberg, P., Jr.; Wu, J.Y. Pre-mRNA splicing and retinitis pigmentosa. Mol. Vis. 2006, 12, 1259–1271. [Google Scholar] [PubMed] [PubMed Central]
- Wheway, G.; Douglas, A.; Baralle, D.; Guillot, E. Mutation spectrum of PRPF31, genotype-phenotype correlation in retinitis pigmentosa, and opportunities for therapy. Exp. Eye Res. 2020, 192, 107950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrari, S.; Di Iorio, E.; Barbaro, V.; Ponzin, D.; Sorrentino, F.S.; Parmeggiani, F. Retinitis pigmentosa: Genes and disease mechanisms. Curr. Genom. 2011, 12, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, X.; Li, Y.; Guo, T.; Yang, L. Correlation between Genotype and Phenotype in 69 Chinese Patients with USH2A Mutations: A comparative study of the patients with Usher Syndrome and Nonsyndromic Retinitis Pigmentosa. Acta Ophthalmol. 2021, 99, e447–e460. [Google Scholar] [CrossRef] [PubMed]
- Znoiko, S.L.; Crouch, R.K.; Moiseyev, G.; Ma, J.X. Identification of the RPE65 protein in mammalian cone photoreceptors. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1604–1609. [Google Scholar] [PubMed]
- Seeliger, M.W.; Grimm, C.; Ståhlberg, F.; Friedburg, C.; Jaissle, G.; Zrenner, E.; Guo, H.; Remé, C.E.; Humphries, P.; Hofmann, F.; et al. New views on RPE65 deficiency: The rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat. Genet. 2001, 29, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Bennasir, H.; Sridhar, S.; Abdel-Razek, T.T. Vitamin A from physiology to disease prevention. Research in Autism Spectrum Disorders. Int. J. Pharm. Sci. Rev. 2010, 1, 68–73. [Google Scholar]
- Milam, A.H.; Rose, L.; Cideciyan, A.V.; Barakat, M.R.; Tang, W.X.; Gupta, N.; Aleman, T.S.; Wright, A.F.; Stone, E.M.; Sheffield, V.C.; et al. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 473–478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escher, P.; Gouras, P.; Roduit, R.; Tiab, L.; Bolay, S.; Delarive, T.; Chen, S.; Tsai, C.C.; Hayashi, M.; Zernant, J.; et al. Mutations in NR2E3 can cause dominant or recessive retinal degenerations in the same family. Hum. Mutat. 2009, 30, 342–351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blanco-Kelly, F.; García Hoyos, M.; Lopez Martinez, M.A.; Lopez-Molina, M.I.; Riveiro-Alvarez, R.; Fernandez-San Jose, P.; Avila-Fernandez, A.; Corton, M.; Millan, J.M.; García Sandoval, B.; et al. Dominant Retinitis Pigmentosa, p.Gly56Arg Mutation in NR2E3: Phenotype in a Large Cohort of 24 Cases. PLoS ONE 2016, 11, e0149473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berson, E.L.; Rosner, B.; Sandberg, M.A.; Hayes, K.C.; Nicholson, B.W.; Weigel-DiFranco, C.; Willett, W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch. Ophthalmol. 1993, 111, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Berson, E.L.; Rosner, B.; Sandberg, M.A.; Hayes, K.C.; Nicholson, B.W.; Wegel-DiFranco, C.; Willett, W. Vitamin A supplementation for retinitis pigmentosa. Arch. Ophthalmol. 1993, 111, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Maslim, J.; Valter, K.; Mervin, K. The influence of oxygen levels on the death and survival of photoreceptors. In Retinal Degeneration in Degenerative Diseases of the Retina; Anderson, R.E., Ed.; Plenum Press: New York, NY, USA, 1996; pp. 371–377. [Google Scholar]
- Hajali, M.; Fishman, G.A.; Anderson, R.J. The prevalence of cystoid macular oedema in retinitis pigmentosa patients determined by optical coherence tomography. Br. J. Ophthalmol. 2008, 92, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Bakthavatchalam, M.; Lai, F.H.P.; Rong, S.S.; Ng, D.S.; Brelen, M.E. Treatment of cystoid macular edema secondary to retinitis pigmentosa: A systematic review. Surv. Ophthalmol. 2018, 63, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Pechnikova, N.A.; Poimenidou, M.; Iliadis, I.; Zafeiriou-Chatziefraimidou, M.; Iaremenko, A.V.; Yaremenko, T.V.; Domvri, K.; Yaremenko, A.V. Pre-Clinical and Clinical Advances in Gene Therapy of X-Linked Retinitis Pigmentosa: Hope on the Horizon. J. Clin. Med. 2025, 14, 898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- da Cruz, L.; Dorn, J.D.; Humayun, M.S.; Dagnelie, G.; Handa, J.; Barale, P.O.; Sahel, J.A.; Stanga, P.E.; Hafezi, F.; Safran, A.B.; et al. Five-Year Safety and Performance Results from the Argus II Retinal Prosthesis System Clinical Trial. Ophthalmology 2016, 123, 2248–2254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mears, A.J.; Kondo, M.; Swain, P.K.; Takada, Y.; Bush, R.A.; Saunders, T.L.; Sieving, P.A.; Swaroop, A. Nrl is required for rod photoreceptor development. Nat. Genet. 2001, 29, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Vingolo, E.M.; Pelaia, P.; Forte, R.; Rocco, M.; Giusti, C.; Rispoli, E. Does hyperbaric oxygen (HBO) delivery rescue retinal photoreceptors in retinitis pigmentosa? Doc. Ophthalmol. 1998, 97, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Song, D.J.; Bao, X.L.; Fan, B.; Li, G.Y. Mechanism of Cone Degeneration in Retinitis Pigmentosa. Cell Mol. Neurobiol. 2023, 43, 1037–1048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mcmonnies, C.W. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin. Exp. Optom. 2015, 98, 122–125. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Y.; Liu, X.; Ghazaryan, E.; Li, Y.; Xie, J.; Su, G. Recent advances of stem cell therapy for retinitis pigmentosa. Int. J. Mol. Sci. 2014, 15, 14456–14474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Florido, A.; Vingolo, E.M.; Limoli, P.; Contento, L. Mesenchymal Stem Cells for Treatment of Retinitis Pigmentosa: Short Review. J. Stem Cells Res. Dev. Ther. 2021, 7, 066. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Messias, A.; Voltarelli, J.C.; Scott, I.U.; Jorge, R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: A phase I trial. Retina 2011, 31, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Tuekprakhon, A.; Sangkitporn, S.; Trinavarat, A.; Pawestri, A.R.; Vamvanij, V.; Ruangchainikom, M.; Luksanapruksa, P.; Pongpaksupasin, P.; Khorchai, A.; Dambua, A.; et al. Intravitreal autologous mesenchymal stem cell transplantation: A non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Res. Ther. 2021, 12, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satarian, L.; Nourinia, R.; Safi, S.; Kanavi, M.R.; Jarughi, N.; Daftarian, N.; Arab, L.; Aghdami, N.; Ahmadieh, H.; Baharvand, H. Intravitreal Injection of Bone Marrow Mesenchymal Stem Cells in Patients with Advanced Retinitis Pigmentosa; a Safety Study. J. Ophthalmic Vis. Res. 2017, 12, 58–64. [Google Scholar] [CrossRef]
- Mansouri, V. X-Linked Retinitis Pigmentosa Gene Therapy: Preclinical Aspects. Ophthalmol. Ther. 2023, 12, 7–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bairqdar, A.; Karitskaya, P.E.; Stepanov, G.A. Expanding Horizons of CRISPR/Cas Technology: Clinical Advancements, Therapeutic Applications, and Challenges in Gene Therapy. Int. J. Mol. Sci. 2024, 25, 13321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.F.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- U.S. Food and Drug Administration. Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products; U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/media/109487/download (accessed on 30 May 2025).
- European Medicines Agency. Luxturna: EPAR—Public Assessment Report; European Medicines Agency: London, UK, 2019; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/luxturna (accessed on 30 May 2025).
- EyeWiki. Voretigene Neparvovec-Rzyl (Luxturna™). Available online: https://eyewiki.org/Voretigene_neparvovec-rzyl_ (accessed on 30 May 2025).
- Sobh, M.; Lagali, P.S.; Ghiasi, M.; Montroy, J.; Dollin, M.; Hurley, B.; Leonard, B.C.; Dimopoulos, I.; Lafreniere, M.; Fergusson, D.A. Safety and efficacy of adeno-associated viral gene therapy in patients with retinal degeneration: A systematic review and meta-analysis. Transl. Vis. Sci. Technol. 2023, 12, 24. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Identifier NCT00999609, A Safety and Efficacy Study in Subjects with Leber Congenital Amaurosis (LCA) Using Adeno-Associated Viral Vector to Deliver the Gene for Human RPE65 to the Retinal Pigment Epithelium (RPE); National Library of Medicine (US): Bethesda, MD, USA, 2009. Available online: https://clinicaltrials.gov/study/NCT00999609 (accessed on 30 May 2025).
- ClinicalTrials.gov. Identifier NCT04516369, A Safety and Efficacy Study in Japanese Subjects with Leber Congenital Amaurosis (LCA) Using Adeno-Associated Viral Vector to Deliver the Gene for Human RPE65 to the Retinal Pigment Epithelium (RPE); National Library of Medicine (US): Bethesda, MD, USA, 2020. Available online: https://clinicaltrials.gov/study/NCT04516369 (accessed on 30 May 2025).
- Stingl, K.; Kempf, M.; Jung, R.; Kortüm, F.; Righetti, G.; Reith, M.; Dimopoulos, S.; Ott, S.; Kohl, S.; Stingl, K. Therapy with voretigene neparvovec. How to measure success? Prog. Retin. Eye Res. 2023, 92, 101115. [Google Scholar] [CrossRef] [PubMed]
- Nuzbrokh, Y.; Kassotis, A.S.; Ragi, S.D.; Jauregui, R.; Tsang, S.H. Treatment-emergent adverse events in gene therapy trials for inherited retinal diseases: A narrative review. Ophthalmol. Ther. 2020, 9, 709–724. [Google Scholar] [CrossRef]
- Igoe, J.M.; Lam, B.L.; Gregori, N.Z. Update on Clinical Trial Endpoints in Gene Therapy Trials for Inherited Retinal Diseases. J. Clin. Med. 2024, 13, 5512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Retina UK. Phase 3 Clinical Trial has been Given Approval by FDA; Buckingham: Retina, UK, 2024; Available online: https://retinauk.org.uk/news/phase-3-clinical-trial-has-been-given-approval-by-fda/ (accessed on 30 May 2025).
- John, M.C.; Quinn, J.; Hu, M.L.; Cehajic-Kapetanovic, J.; Xue, K. Gene-agnostic therapeutic approaches for inherited retinal degenerations. Front. Mol. Neurosci. 2023, 15, 1068185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Upadhyay, A.K.; Qamar, H.; Matloob, S.; Chavali, M. Safety and efficacy results from a phase 1/2 clinical trial of OCU400 modifier gene therapy for treatment of retinitis pigmentosa. Cytotherapy 2024, 26, S18. [Google Scholar] [CrossRef]
- Ocugen, Inc. Ocugen, Inc. Announces Positive 2-Year Data Across Multiple Mutations from Phase 1/2 Clinical Trial of OCU400—A Novel Modifier Gene Therapy for Retinitis Pigmentosa. GlobeNewswire. 2025. Available online: https://www.globenewswire.com/news-release/2025/01/13/3008252/0/en/Ocugen-Inc-Announc-es-Positive-2-Year-Data-Across-Multiple-Mutations-from-Phase-1-2-Clinical-Trial-of-OCU400-A-Novel-Modifier-Gene-Therapy-for-Retinitis-Pigmentosa.html (accessed on 30 May 2025).
- Johnson, V. Ocugen’s Retinitis Pigmentosa Gene Therapy Available in EAP Ahead of BLA Submission. CGTLive®. 2024. Available online: https://www.cgtlive.com/view/ocugen-retinitis-pigmentosa-gene-therapy-eap (accessed on 30 May 2025).
- Koennecke, G. Ocugen, Inc. Receives European Medicines Agency ATMP Designation for New Gene Therapy OCU400. 2025. Available online: https://europe.ophthalmologytimes.com/view/ocugen-inc-receives-european-medicines-agency-atmp-designation-for-new-gene-therapy (accessed on 30 May 2025). Ophthalmology Times Europe.
- Open-Label, Single Ascending Dose Study to Evaluate the Safety, Tolerability, and Efficacy of EDIT-101 in Adult and Pediatric Participants With Leber Congenital Amaurosis Type 10 (LCA10), With CEP290-Related Retinal Degeneration (BRILLIANCE). ClinicalTrials.gov, NCT03872479. Available online: https://www.clinicaltrials.gov/study/NCT03872479 (accessed on 18 July 2025).
- Khurana, A.; Sayed, N.; Singh, V.; Khurana, I.; Allawadhi, P.; Rawat, P.S.; Navik, U.; Pasumarthi, S.K.; Bharani, K.K.; Weiskirchen, R. A comprehensive overview of CRISPR/Cas 9 technology and application thereof in drug discovery. J. Cell Biochem. 2022, 123, 1674–1698. [Google Scholar] [CrossRef] [PubMed]
- McClements, M.E.; Elsayed, M.E.A.A.; Major, L.; de la Camara, C.M.; MacLaren, R.E. Gene Therapies in Clinical Development to Treat Retinal Disorders. Mol. Diagn. Ther. 2024, 28, 575–591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ClinicalTrials.gov. Efficacy and Safety of MCO-010 Optogenetic Therapy in Adults with Retinitis Pigmentosa [RESTORE]. Nanoscope Therapeutics Inc. NCT04945772, 30 June 2021. [Google Scholar]
- Sullivan, L.S.; Bowne, S.J.; Birch, D.G.; Hughbanks-Wheaton, D.; Heckenlively, J.R.; Lewis, R.A.; Garcia, C.A.; Ruiz, R.S.; Blanton, S.H.; Northrup, H.; et al. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: A screen of known genes in 200 families. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3052–3064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyer, D.S.; Bergstrom, L.; Emanuelli, A.; Gonzalez, V.H.; Wykoff, C.C.; Gupta, S.; Liao, D.S.; Zak, V.; Chavala, S.H.; Mohanty, S.; et al. Efficacy and safety of MCO-010 optogenetic therapy for vision restoration in patients with severe vision loss due to retinitis pigmentosa: A phase 2b randomized, sham-controlled, multi-center, multi-dose, double-masked clinical trial (RESTORE). Investig. Ophthalmol. Vis. Sci. 2023, 64, 5443. Available online: https://www.prnewswire.com/news-releases/nanoscope-therapeutics-announces-positive-top-line-results-from-randomized-controlled-trial-of-mco-010-for-retinitis-pigmentosa-302098510.html (accessed on 18 July 2025).
- Girach, A.; Audo, I.; Birch, D.G.; Huckfeldt, R.M.; Lam, B.L.; Leroy, B.P.; Michaelides, M.; Russell, S.R.; Sallum, J.M.F.; Stingl, K.; et al. RNA-based therapies in inherited retinal diseases. Ther. Adv. Ophthalmol. 2022, 14, 25158414221134602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ProQR Therapeutics. A Prospective First-In-Human Study to Evaluate the Safety and Tolerability of QR-1123 in Subjects with Autosomal Dominant Retinitis Pigmentosa Due to the P23H Mutation in the RHO Gene (AURORA). ClinicalTrials.gov Identifier NCT04123626, 11 October 2019. [Google Scholar]
- Noel, J.; Jalligampala, A.; Marussig, M.; Vinot, P.-A.; Marie, M.; Butler, M.; Lorget, F.; Boissel, S.; Leveillard, T.D.; Sahel, J.A.; et al. SPVN06, a Novel Mutation-Independent AAV-based Gene Therapy, Protects Cone Degeneration in a Pig Model of Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1189. [Google Scholar]
- SparingVision. SparingVision Advances PRODYGY Trial into Phase II Following Positive DSMB Recommendation. Available online: https://sparingvision.com/sparingvision-advances-prodygy-trial-into-phase-ii-following-positive-dsmb-recommendation/ (accessed on 21 January 2025).
- SparingVision. Promising ROd-cone DYstrophy Gene TherapY (PRODYGY). ClinicalTrials.gov Identifier: NCT05748873. Available online: https://clinicaltrials.gov/study/NCT05748873 (accessed on 20 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pniakowska, Z.; Dzieża, N.; Kustosik, N.; Przybylak, A.; Jurowski, P. Genetic Therapies for Retinitis Pigmentosa: Current Breakthroughs and Future Directions. J. Clin. Med. 2025, 14, 5661. https://doi.org/10.3390/jcm14165661
Pniakowska Z, Dzieża N, Kustosik N, Przybylak A, Jurowski P. Genetic Therapies for Retinitis Pigmentosa: Current Breakthroughs and Future Directions. Journal of Clinical Medicine. 2025; 14(16):5661. https://doi.org/10.3390/jcm14165661
Chicago/Turabian StylePniakowska, Zofia, Natasza Dzieża, Natalia Kustosik, Aleksandra Przybylak, and Piotr Jurowski. 2025. "Genetic Therapies for Retinitis Pigmentosa: Current Breakthroughs and Future Directions" Journal of Clinical Medicine 14, no. 16: 5661. https://doi.org/10.3390/jcm14165661
APA StylePniakowska, Z., Dzieża, N., Kustosik, N., Przybylak, A., & Jurowski, P. (2025). Genetic Therapies for Retinitis Pigmentosa: Current Breakthroughs and Future Directions. Journal of Clinical Medicine, 14(16), 5661. https://doi.org/10.3390/jcm14165661