The Use of Larval Debridement Therapy and Negative-Pressure Wound Therapy for an Infected Wound After Thyroidectomy—A Case Report
Abstract
1. Introduction
2. Case Presentation
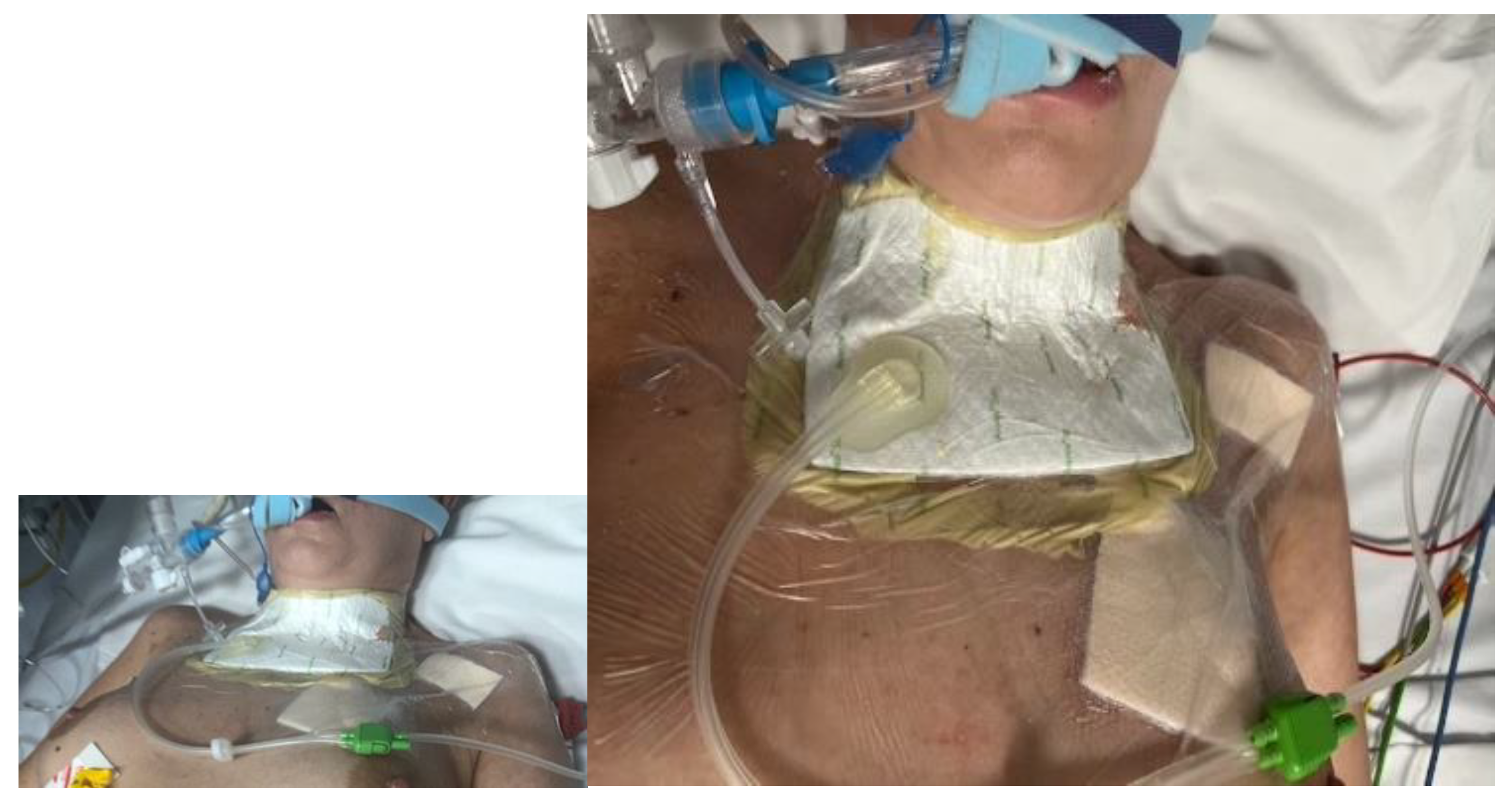
- Reintubation of the patient to protect the respiratory tract from the entry of larvae defensins and to enable the suctioning of secretions (a protective measure in the event of the unintentional entry of secretions during LDT). An endotracheal tube with a tightly filled balloon located below the fistula formed a barrier between the respiratory system and the atmospheric air and the wound environment. The pressure in the ventilation tube balloon was measured regularly to avoid excessive increases, which would be dangerous for tracheal function and could cause necrosis. During treatment, the patient was reintubated and under analgesic sedation. She did not experience any discomfort from the treatment.
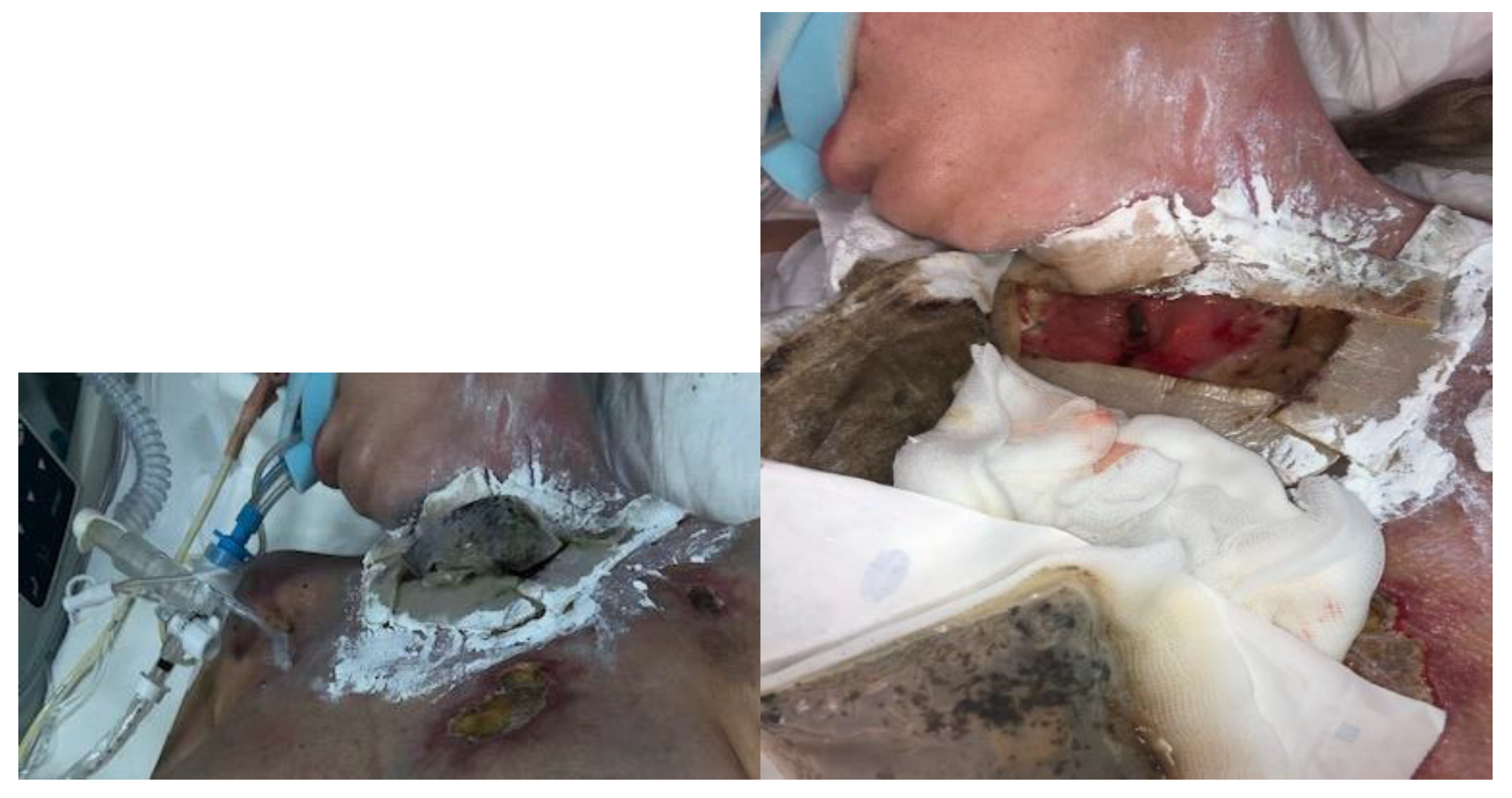
- The use of a BioBag in the area of the tracheocutaneous fistula (in larval therapy, this prevents the migration of larvae into the respiratory system).
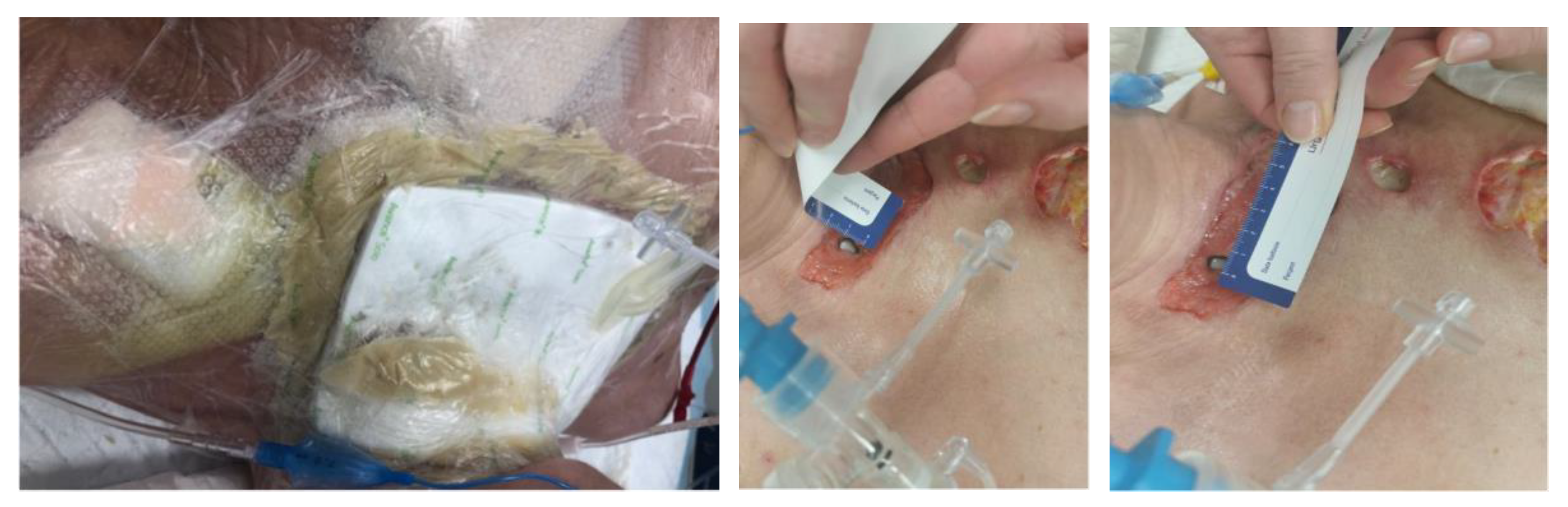
- The implementation of NPWT after LDT. Since the bottom of the fistula was partially missing and leakage was possible, three-quarters of the fistula surface was lined with a silicone dressing with a large layer of solid silicone and small holes in the mesh structure. The surface of the bottom itself was covered from the outside with a small layer of a thin hydrocolloid dressing, which was glued to the silicone mesh to form a bottom in the wound bed. In addition, the wound was filled to its depth with a sterile polyurethane foam, and an external dressing was placed in the last layer.
- Next, treatment with targeted dressings was applied.
- The trachea is a fairly rigid structure that is poorly supplied with blood, so there was a risk that it would become narrower after healing.
- In cases of infection, it is not possible to close the fistula (when the trachea is separated into two parts after purification via LDT)—consultation with an ENT oncologist ruled out the possibility of implanting a part of the trachea.
- If defensins flowed into the bronchi and lungs during LDT, the patient would require intensive monitoring.
- It is necessary to ensure the tightness of the NPWT when creating an artificial bottom.
- Wound treatment was considered according to the TIMERS strategy: tissue debridement (T), infection (I), moisture (M), epidermization stimulation (E), regeneration (R) and social and patient-related factors (S).
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDT | Larval debridement therapy |
| NPWT | Negative-pressure wound therapy |
| CTA | Computed tomography angiography |
| ICU | Intensive care unit |
| FDA | Food and Drug Administration |
| WBP | Wound bed preparation |
References
- Mrozikiewicz-Rakowska, B.; Tusiński, M.; Lipiński, P.; Bazaliński, D.; Dynarska, J.; Czwakiel, L.; Zymon, A.; Mospan, B.; Malinowska, K.; Sopata, M.; et al. Statement of the Polish Wound Management Association on larval therapyin wound management. Leczenie Ran 2023, 20, 89–95. [Google Scholar] [CrossRef]
- Nigam, Y.; Dudley, E.; Bexfield, A.; Bond, A.E.; Evans, J.; James, J. Chapter 2—The Physiology of Wound Healing by the Medicinal Maggot, Lucilia sericata. Adv. Insect Physiol. 2010, 39, 39–81. [Google Scholar]
- Cwajda-Białasik, J.; Mościcka, P.; Szewczyk, M. Wybrane metody leczenia ran przewlekłych. Pielęgniarstwo Chir. I Angiol. 2019, 1, 1–11. [Google Scholar]
- Pajarillo, C.; Sherman, R.A.; Sheridan, R.; Kazis, L.E. Health professionals’ perceptions of maggot debridement therapy. J. Wound Care 2021, 30, Sup9a. [Google Scholar] [CrossRef] [PubMed]
- Bazaliński, D.; Karnas, M.; Wołkowicz, M.; Kózka, M.; Więch, P. Zastosowanie larw Lucilla sericata w oczyszczaniu ran. Opis trzech przypadków. Leczenie Ran 2018, 15, 153–159. [Google Scholar] [CrossRef]
- Bartoszewicz, M.; Banasiewicz, T.; Bielecki, K.; Bigda, J.; Chrapusta, A.; Dydak, K.; Grzesiowski, P.; Junka, A.; Karoń, J.; Korzon-Burakowska, A.N.N.A.; et al. Zasady postępowania miejscowego i ogólnego w ranach/owrzodzeniach przewlekłych objętych procesem infekcji. Forum Zakażeń 2019, 10, 1–30. [Google Scholar] [CrossRef]
- Kozłowska, E.; Popow, A.; Kwiatkowska, O. Zastosowanie terapii podciśnieniowej w medycynie w medycynie. Leczenie Ran 2019, 16, 79–83. [Google Scholar] [CrossRef]
- Gupta, A.; Kundal, A.; Mani, R.; Gajula, B.; Sindhuri, B.G.; Chennat, J.J.; Kumar, U.; Rajput, D. Negative pressure wound therapy in surgical practice: An Institutional experience from a tertiary centre of North India. Pol. Przegl. Chir. 2023, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ściskała, K.; Niedzielska, I.; Buliński, M.; Bąk, M.; Niedzielski, D. Rola Terapii Podciśnieniowej w Gojeniu Ran u Pacjentów Oddziału Chirurgii Szczękowo-Twarzowej. Stomatologia News.pl 2019; dostęp. Available online: https://stomatologianews.pl/rola-terapii-podcisnieniowej-gojeniu-ran-pacjentow-oddzialu-chirurgii-szczekowo-twarzowej-etap-wstepny/ (accessed on 17 June 2025).
- Romeyke, T. Use of biosurgery for the treatment of foot ulcers infected with therapy-resistant bacteria: A case report. J. Wound Care 2024, 33, Sup4a. [Google Scholar] [CrossRef] [PubMed]
- Moya-López, J.; Costela-Ruiz, V.; García-Recio, E.; Sherman, R.A.; De Luna-Bertos, E. Advantages of Maggot Debridement Therapy for Chronic Wounds: A Bibliographic Review. Adv. Ski. Wound Care 2020, 33, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Trottier, C.; Anwar, S.; Baxter, L.; Nierenberg, N. Maggots: Antimicrobial Stewards and Life Savers. Ann. Intern. Med. Clin. Cases 2024, 3, e230693. [Google Scholar] [CrossRef]
- Bazaliński, D.; Sieńczak, K.; Pytlak, K.; Przybek-Mita, J.; Pelczar, K.; Leppert, W.; Więch, P. Pain Assessment in Patients Undergoing Maggot Debridement Therapy in the Process of Local Treatment of Chronic Wounds. J. Clin. Med. 2024, 13, 884. [Google Scholar] [CrossRef] [PubMed]
- Chilimoniuk, Z.; Borkowska, A.; Dobosz, M.; Chałupnik, A.; Sobstyl, A. Przegląd dostępnych terapii w leczeniu zespołu stopy cukrzycowej. In Postępy w Diagnostyce Medycznej; Monografia Naukowa: Lublin, Poland, 2020; pp. 79–89. [Google Scholar]
- Phang, Z.H.; Khoo, S.S.; Gunasagaran, J.; Tunku Ahmad, T.S. Clinical outcome of Maggot Debridement Therapy followed by Negative Pressure Wound Therapy for chronic hand wound with Multi-Drug Resistant Organism infection: Two cases and review of the literature. J. Orthop. Surg. 2021, 29, 23094990211067302. [Google Scholar] [CrossRef] [PubMed]
- Funes, A.L. Maggot Debridement Therapy vs. Negative Pressure Wound Therapy and Their Effectiveness on Tissue Healing on Older Adults. Soar. J. Undergrad. Res. 2024, 2024, 8. [Google Scholar]
- Seidel, D.; Lefering, R. NPWT resource use compared with conventional wound treatment in subcutaneous abdominal wounds with healing impairment after surgery: SAWHI randomized clinical trial results. Ann. Surg. 2022, 275, e290–e298. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, A. Zastosowanie podciśnieniowej metody leczenia ran (NPWT) w hospicjum domowym w hospicjum domowym. Psychoonkologia 2015, 1, 27–33. [Google Scholar]


| Contraindications to LDT |
|---|
| Lack of knowledge of the etiology of the wound |
| Necrosis and dry, scab-covered wounds |
| Wounds penetrating into body cavities, very deep wounds that may potentially communicate with body cavities |
| Severe coagulation disorders, active bleeding from the wound, wounds in the immediate vicinity of large vessels |
| Anemia requiring transfusion of blood products |
| Ischemic wounds without revascularization treatment |
| Presence of a tumor at the bottom or edges of the wound |
| Fever, infection spreading beyond the wound bed, systemic infection |
| Lack of patient acceptance of LDT as a treatment method, lack of patient consent for LDT, lack of cooperation from the patient |
| Allergic reactions to insect chitin, eggs, soy, or fly larvae |
| Pregnancy |
| Indications | Contraindications |
|---|---|
| Chronic wounds (bedsores, shin ulcers) | Dry and hard necrosis |
| Postoperative and posttraumatic wounds | Uncontrolled systemic infections |
| Infected and heavily exuding wounds | Fistulas leading to the cavernous organs |
| Skin and organ fistulas | Presence of tumor tissue in the wound |
| Diabetic foot disease | Uncontrolled bleeding |
| Date | Medical Incident |
|---|---|
| 5 January 2023 | Operated on a scheduled basis—total strumectomy due to ca. papillare. |
| 6–7 January 2023 | Dynamic deterioration in general condition, hypotension, tachycardia and desaturation, requiring passive oxygen therapy. Dynamic septic shock after prior revision of the postoperative wound, treatment in the intensive care unit, broad-spectrum empirical antibiotic therapy: meronem + vancomycin + clindamycin + metronidazole. |
| 9 January 2023 | Right pleural drainage—approximately 400 mL of dark brown fluid was evacuated. Microbiological result: positive culture from the wound—S. pyogenes). Antibiotic therapy was modified (vancomycin and metronidazole were discontinued; ampicillin was added). |
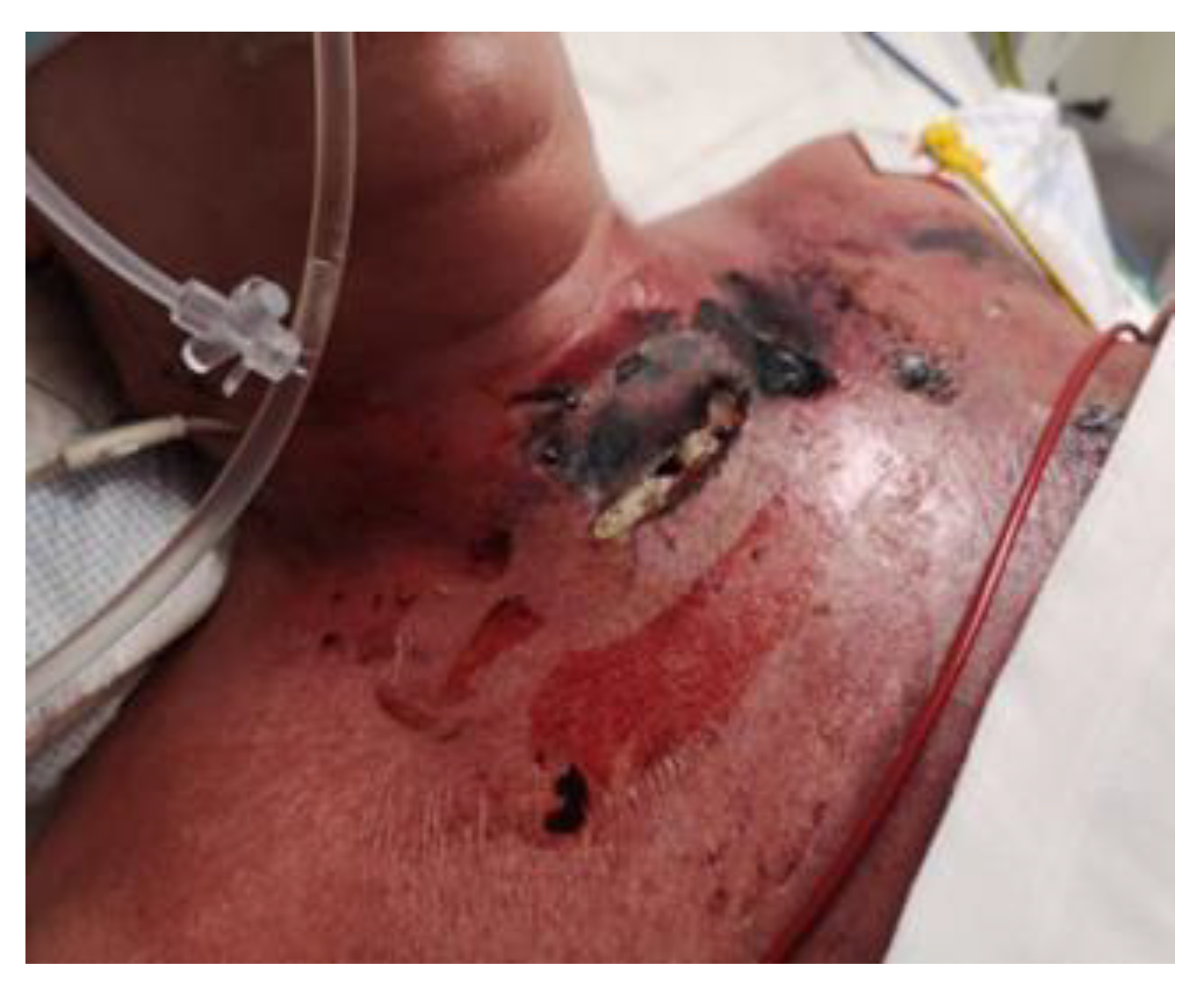
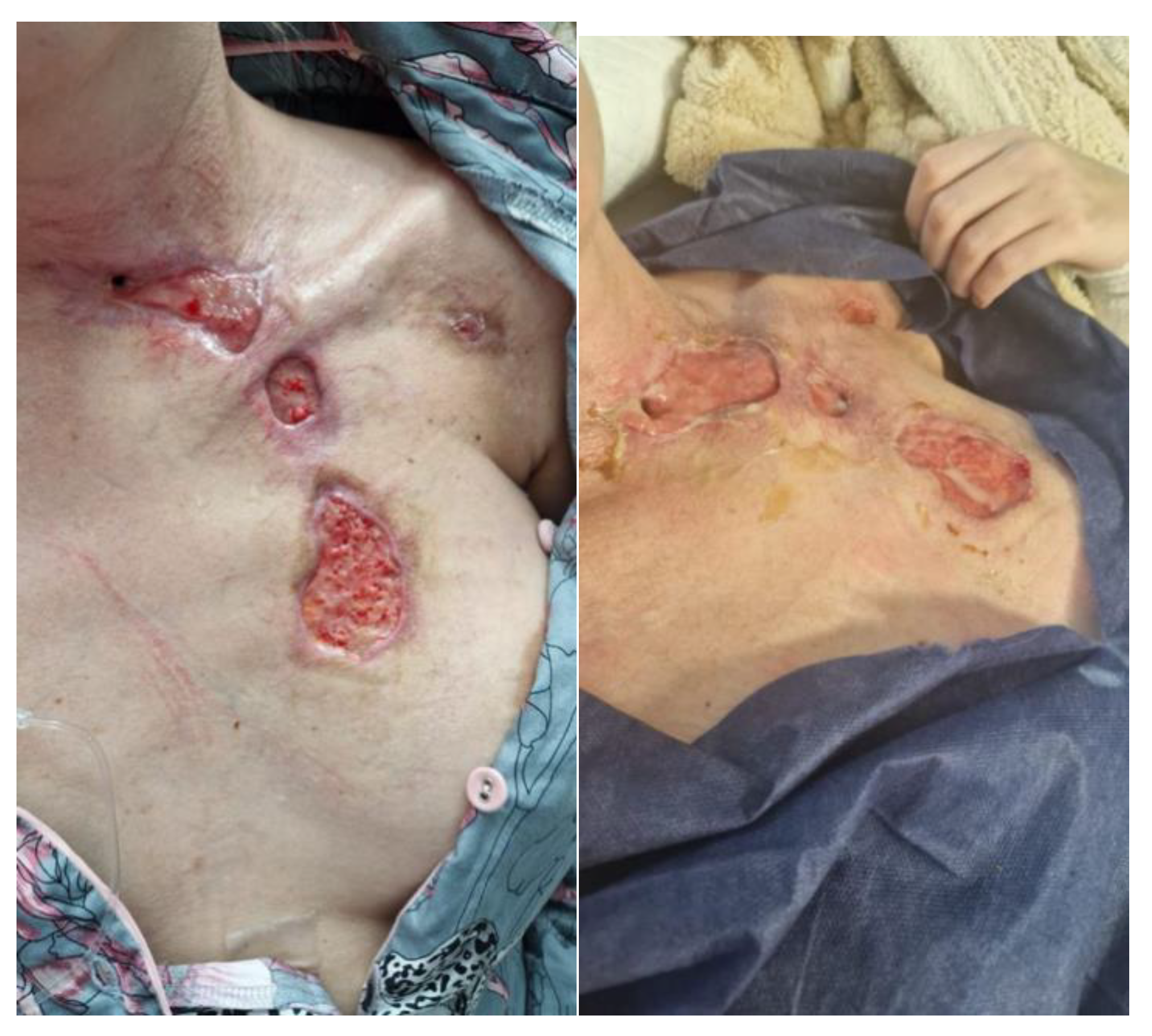
| 11 January 2023 | The skin was entirely covered with papular eruptions, with erythema and exfoliation of the epidermis in the course of streptococcal toxemia, and with localized necrotic lesions with epidermolysis and blisters on the chest and neck. |
| 16–18 January 2023 | Candida albicans was identified in bronchial washings, and fluconazole and clotrimazole were administered topically. Thoracentesis was performed, evacuating 800 mL of fluid. |
| 2–4 February 2023 | A tracheal fistula was suspected, confirmed using a neck CT scan. In microbiology, Klebsiella pneumoniae sp. was identified on a vascular catheter and punctures were listed. Tazocin was administered. Swab from the wound: Pseudomonas aeruginosa MDR. Avibactam administered intravenously + colistin in nebulization. |
| 11 February 2023 | Consulted with a specialist in dressings and wound treatment. The patient was qualified for larval therapy on February 15, after prior preparation of the skin with the recommended dressings and preparations (Granudacyn liquid and gel antiseptics, Iruxol Mono, Granuflex). |
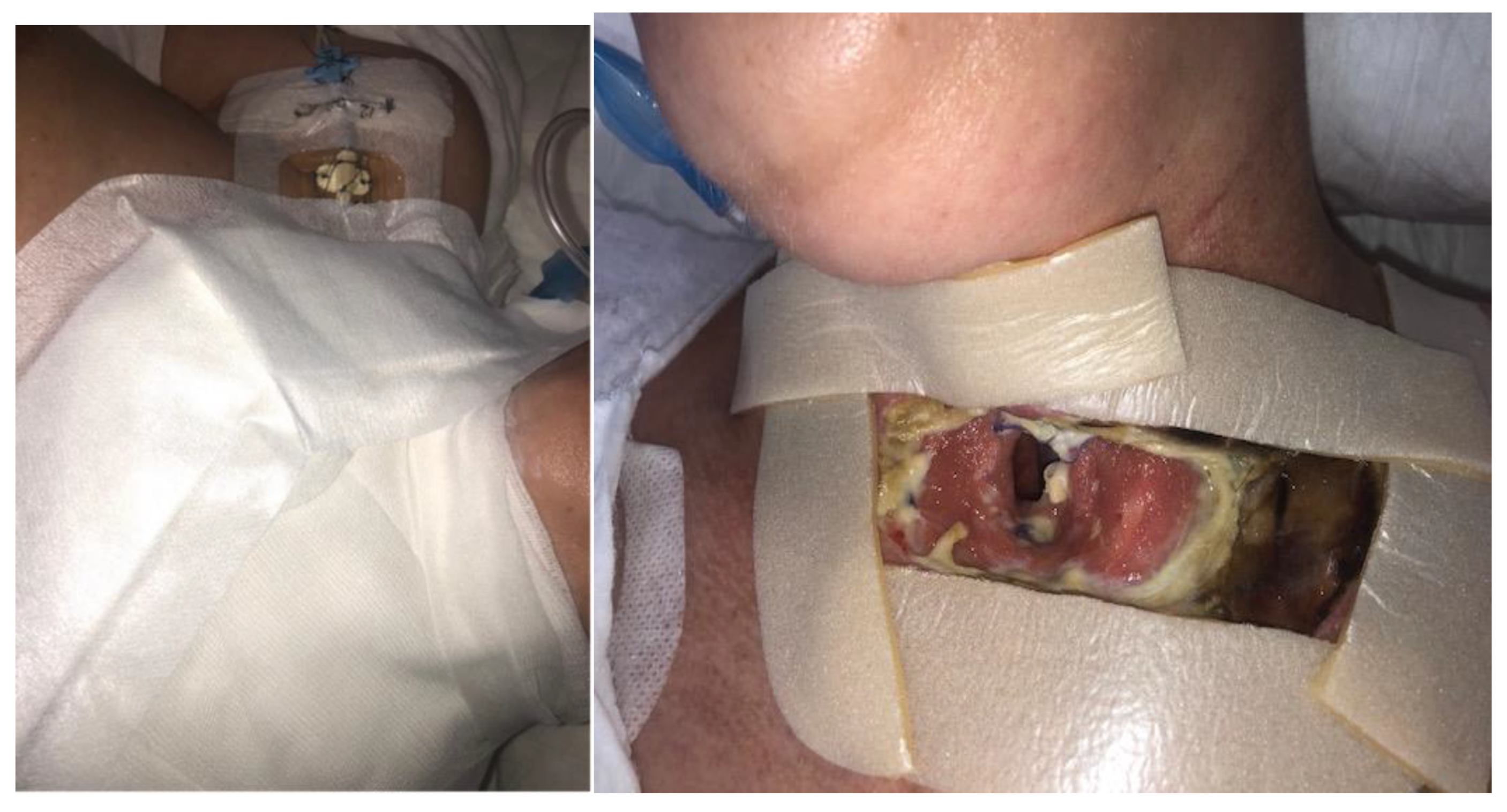
| 15 February 2023 | The patient was intubated. Preliminary treatment was performed: wound necrectomy and a dressing with 200 larvae was applied. |
| 18 February 2023 | Larval therapy was completed and NPWT was applied (−150 mmHg). |
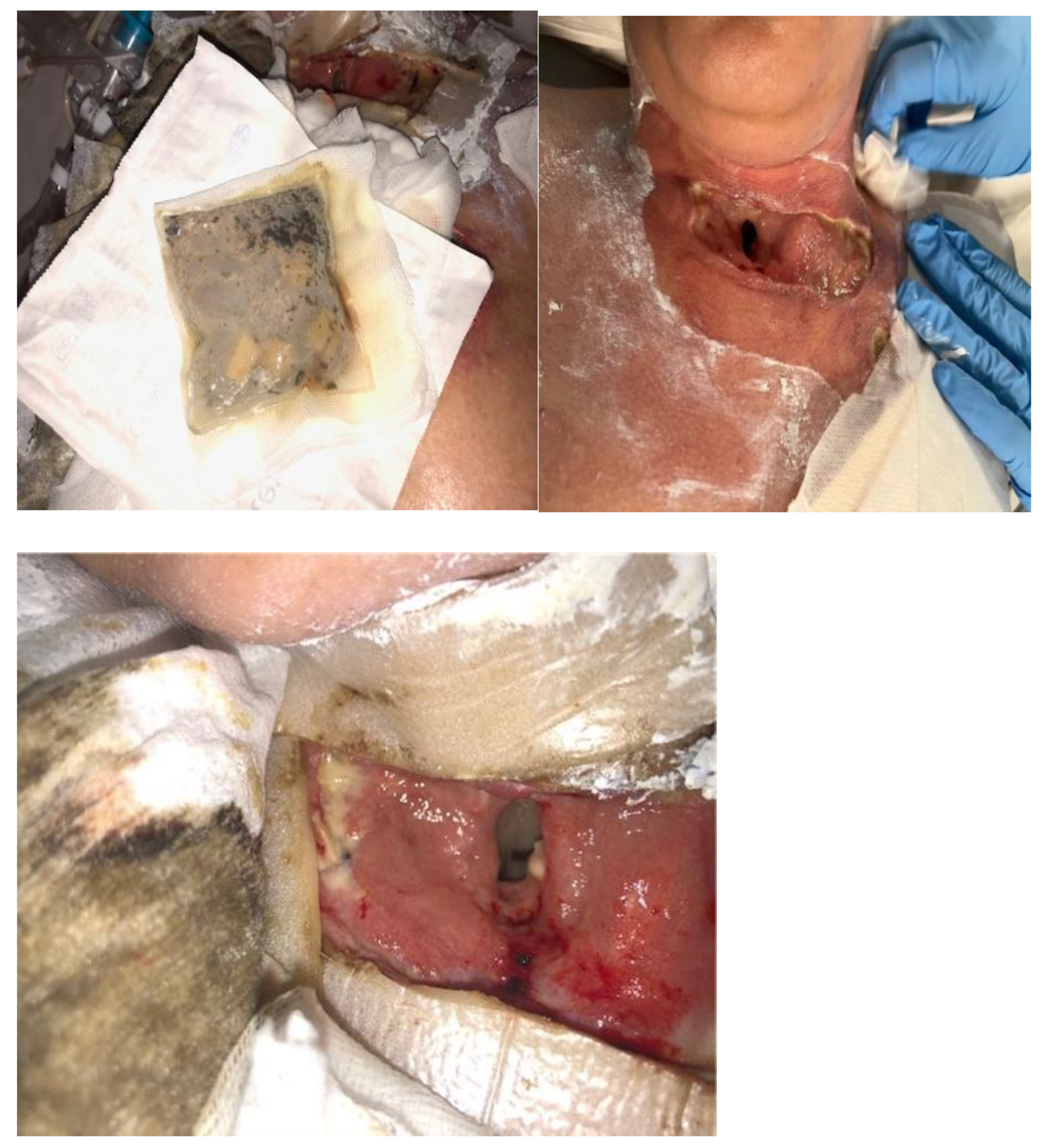
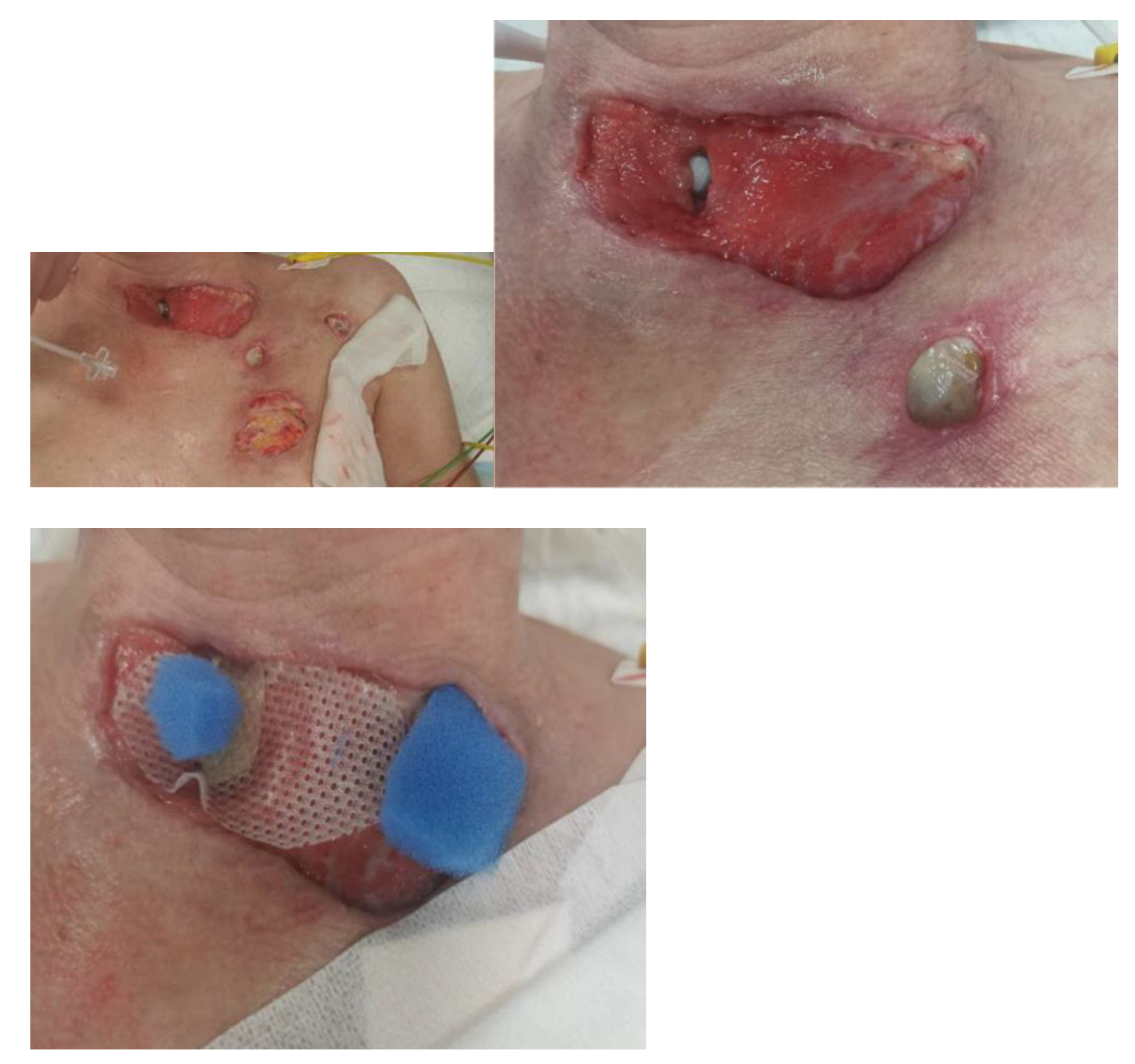
| 8 March 2023 | NPWT therapy was completed, mechanical and chemical cleansing was applied to the wound, and dressings containing silver ions were applied. The patient was extubated and left on spontaneous breathing with slight oxygen supplementation. |
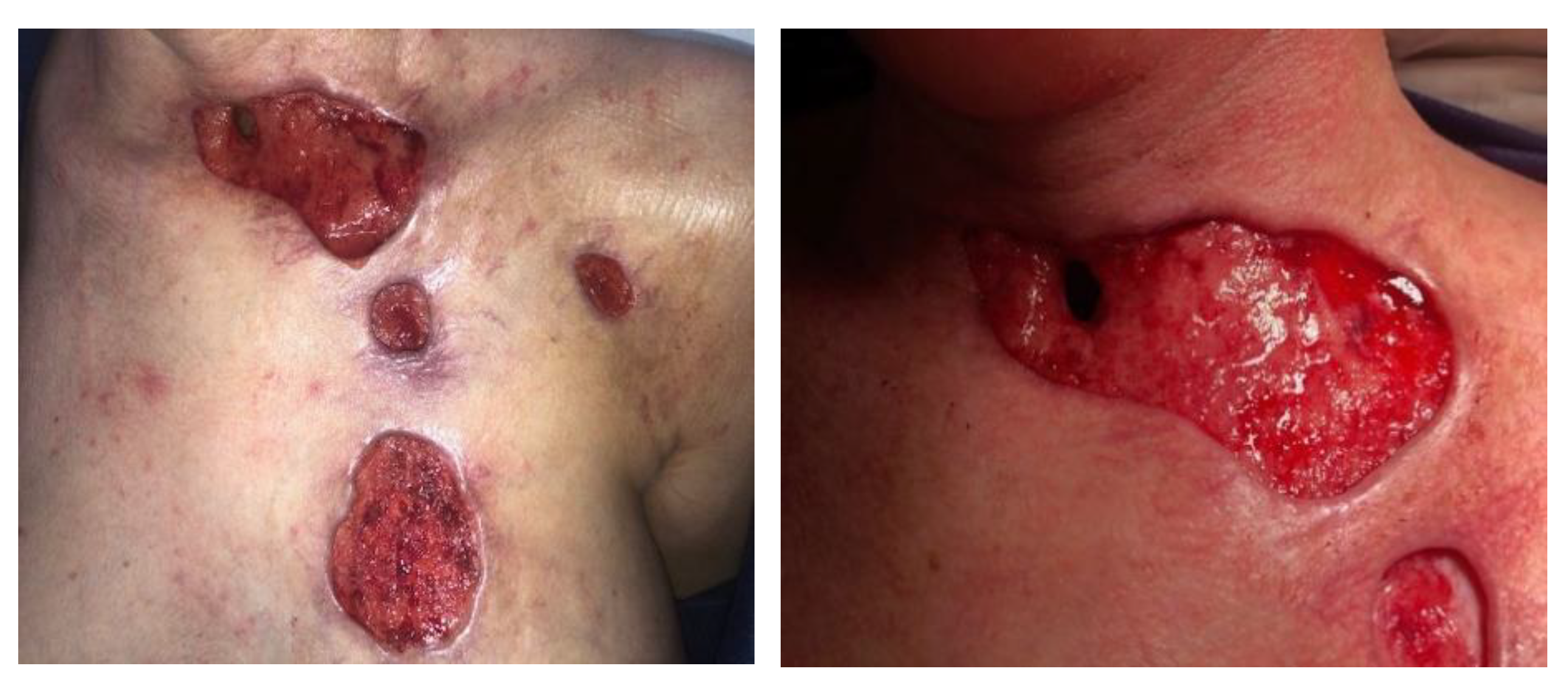
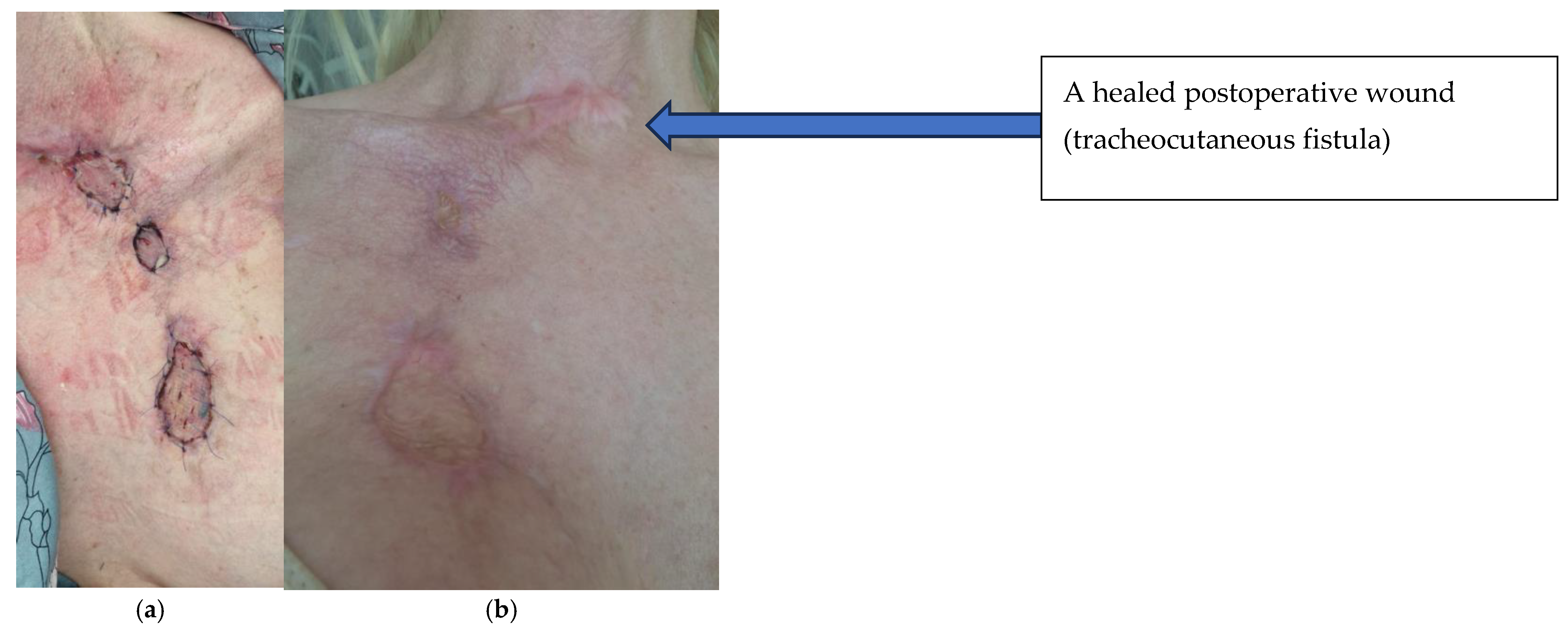
| 7 April 2023 | Planned skin autotransplantation procedure for granulation of wounds, leaving a free field above the tracheal fistula. Local wounds after strumectomy and necrectomy with secondary skin autotransplantation secured with an occlusive dressing. |
| July 2023/October 2023 | No complications. Planned skin autotransplantation procedure for granulation of wounds, leaving a free field above the tracheal fistula. Local wounds after strumectomy and necrectomy with secondary skin autotransplantation secured with an occlusive dressing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dynarska, J.; Zagrodnik, E.; Kisielska, A.; Jurczak, A.; Machałowski, T.; Wieder-Huszla, S. The Use of Larval Debridement Therapy and Negative-Pressure Wound Therapy for an Infected Wound After Thyroidectomy—A Case Report. J. Clin. Med. 2025, 14, 5634. https://doi.org/10.3390/jcm14165634
Dynarska J, Zagrodnik E, Kisielska A, Jurczak A, Machałowski T, Wieder-Huszla S. The Use of Larval Debridement Therapy and Negative-Pressure Wound Therapy for an Infected Wound After Thyroidectomy—A Case Report. Journal of Clinical Medicine. 2025; 14(16):5634. https://doi.org/10.3390/jcm14165634
Chicago/Turabian StyleDynarska, Jolanta, Edyta Zagrodnik, Agnieszka Kisielska, Anna Jurczak, Tomasz Machałowski, and Sylwia Wieder-Huszla. 2025. "The Use of Larval Debridement Therapy and Negative-Pressure Wound Therapy for an Infected Wound After Thyroidectomy—A Case Report" Journal of Clinical Medicine 14, no. 16: 5634. https://doi.org/10.3390/jcm14165634
APA StyleDynarska, J., Zagrodnik, E., Kisielska, A., Jurczak, A., Machałowski, T., & Wieder-Huszla, S. (2025). The Use of Larval Debridement Therapy and Negative-Pressure Wound Therapy for an Infected Wound After Thyroidectomy—A Case Report. Journal of Clinical Medicine, 14(16), 5634. https://doi.org/10.3390/jcm14165634






