Changes in Retinal Nerve Fiber and Ganglion Cell Layers After Chemical Injury: A Prospective Study
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akgun, Z.; Selver, O.B. Epidemiology and etiology of chemical ocular injury: A brief review. World J. Clin. Cases 2023, 11, 1245–1251. [Google Scholar] [CrossRef]
- Sharma, N.; Kaur, M.; Agarwal, T.; Sangwan, V.S.; Vajpayee, R.B. Treatment of acute ocular chemical burns. Surv. Ophthalmol. 2018, 63, 214–235. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, C.H.; Zhou, C.; Lei, F.; Cade, F.; Regatieri, C.V.; Črnej, A.; Paschalis, E.I. Glaucoma after corneal trauma or surgery—A rapid, inflammatory, IOP-independent pathway. Cornea 2019, 38, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.I.; Zhou, C.; Lei, F.; Scott, N.; Kapoulea, V.; Robert, M.-C.; Vavvas, D.; Dana, R.; Chodosh, J.; Dohlman, C.H. Mechanisms of retinal damage after ocular alkali burns. Am. J. Pathol. 2017, 187, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.I.; Lei, F.; Zhou, C.; Kapoulea, V.; Dana, R.; Chodosh, J.; Vavvas, D.G.; Dohlman, C.H. Permanent neuroglial remodeling of the retina following infiltration of CSF1R inhibition-resistant peripheral monocytes. Proc. Natl. Acad. Sci. USA 2018, 115, E11359–E11368. [Google Scholar] [CrossRef]
- Chen, X.; Lei, F.; Zhou, C.; Chodosh, J.; Wang, L.; Huang, Y.; Paschalis, E.I. Glaucoma after ocular surgery or trauma: The role of infiltrating monocytes and their response to cytokine inhibitors. Am. J. Pathol. 2020, 190, 2056–2066. [Google Scholar] [CrossRef]
- Zhou, C.; Lei, F.; Sharma, J.; Hui, P.-C.; Wolkow, N.; Dohlman, C.H.; Vavvas, D.G.; Chodosh, J.; Paschalis, E.I. Sustained inhibition of VEGF and TNF-α achieves multi-ocular protection and prevents formation of blood vessels after severe ocular trauma. Pharmaceutics 2023, 15, 2059. [Google Scholar] [CrossRef]
- Zhou, C.; Robert, M.-C.; Kapoulea, V.; Lei, F.; Stagner, A.M.; Jakobiec, F.A.; Dohlman, C.H.; Paschalis, E.I. Sustained subconjunctival delivery of infliximab protects the cornea and retina following alkali burn to the eye. Investig. Opthalmol. Vis. Sci. 2017, 58, 96–105. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Durbin, M.K.; Budenz, D.L.; Cirrus OCT Normative Database Study Group. Interocular symmetry in peripapillary retinal nerve fiber layer thickness measured with the Cirrus HD-OCT in healthy eyes. Am. J. Ophthalmol. 2011, 151, 514–521.e1. [Google Scholar] [CrossRef]
- Budenz, D.L. Symmetry between the right and left eyes of the normal retinal nerve fiber layer measured with optical coherence tomography (an AOS thesis). Trans. Am. Ophthalmol. Soc. 2008, 106, 252–275. [Google Scholar]
- Pawar, N.; Maheshwari, D.; Ravindran, M.; Ramakrishnan, R. Interocular symmetry of retinal nerve fiber layer and optic nerve head parameters measured by Cirrus high-definition optical coherence tomography in a normal pediatric population. Indian. J. Ophthalmol. 2017, 65, 955–962. [Google Scholar] [CrossRef]
- Zhou, M.; Lu, B.; Zhao, J.; Wang, Q.; Zhang, P.; Sun, X. Interocular symmetry of macular ganglion cell complex thickness in young Chinese subjects. PLoS ONE 2016, 11, e0159583. [Google Scholar] [CrossRef]
- Tan, C.S.; Sadda, S.R. Normative database of retinal nerve fiber layer and macular retinal thickness in a Thai population. J. Glaucoma. 2014, 23, 661–666. [Google Scholar]
- Kang, J.W.; Lee, Y.; Park, Y.H. Establishment of normative retinal nerve fiber layer thickness in healthy Koreans using Huvitz optical coherence tomography and comparison with Cirrus OCT. Curr. Eye Res. 2021, 46, 877–883. [Google Scholar]
- Bizrah, M.; Yusuf, A.; Ahmad, S. An update on chemical eye burns. Eye 2019, 33, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Ting, D.S.J.; Al Saadi, A.; Said, D.G. Chemical eye injury: Pathophysiology, assessment and management. Eye 2020, 34, 2001–2019. [Google Scholar] [CrossRef]
- Satue, M.; Castro, L.; Vilades, E.; Cordon, B.; Errea, J.M.; Pueyo, A.; Chueca, E.P.; Garcia-Martin, E. Ability of Swept-source OCT and OCT-angiography to detect neuroretinal and vasculature changes in patients with Parkinson disease and essential tremor. Eye 2023, 37, 1314–1319. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Ghasemi Falavarjani, K.; Akil, H.; Sadda, S.R. Impact of image quality on OCT angiography based quantitative measurements. Int. J. Retin. Vitr. 2017, 3, 13. [Google Scholar] [CrossRef]
- Allen, N.E.; Crawford, A.Z.; McGhee, C.N.J.; Meyer, J.J. Chemical eye injuries: A 10 year retrospective review of acute presentations and clinical outcomes in Auckland, New Zealand. Sci. Rep. 2024, 14, e8264. [Google Scholar] [CrossRef]
- Hong, J.; Qiu, T.; Wei, A.; Sun, X.; Xu, J. Clinical characteristics and visual outcome of severe ocular chemical injuries in Shanghai. Ophthalmology 2010, 117, 2268–2272. [Google Scholar] [CrossRef]
- Ahmmed, A.A.; Ting, D.S.J.; Figueiredo, F.C. Epidemiology, economic and humanistic burdens of ocular surface chemical injury: A narrative review. Ocul. Surf. 2021, 20, 199–211. [Google Scholar] [CrossRef]
- Kate, A.; Sharma, S.; Yathish, S.; Das, A.V.; Malepati, N.; Donthineni, P.R.; Basu, S.; D’sOuza, S.; Shanbhag, S.S. Demographic profile and clinical characteristics of patients presenting with acute ocular burns. Indian. J. Ophthalmol. 2023, 71, 2694–2703. [Google Scholar] [CrossRef]
- Quesada, J.M.-A.; Lloves, J.M.; Delgado, D.V. Ocular chemical burns in the workplace: Epidemiological characteristics. Burns 2020, 46, 1212–1218. [Google Scholar] [CrossRef]
- Lu, Z.; Chu, T.; Yang, Z.-H.; Xia, X.; Shen, Y.-H.; Chen, J.-H.; Wang, J.-H. Epidemiological features and management of eye burn patients in Wuxi, China. BMJ Open Ophthalmol. 2023, 8, e001171. [Google Scholar] [CrossRef] [PubMed]
- Said, D.G.; Dua, H.S. Chemical burns acid or alkali, what’s the difference? Eye 2020, 34, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Cade, F.; Paschalis, E.I.; Regatieri, C.V.; Vavvas, D.G.; Dana, R.; Dohlman, C.H. Alkali burn to the eye: Protection using TNF-α inhibition. Cornea 2014, 33, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Saunders, L.J.; Zangwill, L.M.; Daga, F.B.; Crowston, J.G.; Medeiros, F.A. Impact of normal aging and progression definitions on the specificity of detecting retinal nerve fiber layer thinning. Am. J. Ophthalmol. 2017, 181, 106–113. [Google Scholar] [CrossRef]
- Lee, W.J.; Baek, S.U.; Kim, Y.K.; Park, K.H.; Jeoung, J.W. Rates of ganglion cell-inner plexiform layer thinning in normal, open-angle glaucoma and pseudoexfoliation glaucoma eyes: A trend-based analysis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 599–604. [Google Scholar] [CrossRef]
- Soh, Z.; Yu, M.; Chen, Y.; Thakur, S.; Lavanya, R.; Tham, Y.C.; Koh, V.; Aung, T.; Cheng, C. Physiological change in ganglion cell inner plexiform layer and nerve fibre layer thickness over six years. Clin. Exp. Ophthalmol. 2025, 53, 391–401. [Google Scholar] [CrossRef]
- Vanclooster, A.; De Zaeytijd, J.; Roels, D. Expanding the spectrum of alkali retinopathy: Maculopathy following alkali burn. Case Rep. Ophthalmol. 2022, 13, 657–662. [Google Scholar] [CrossRef]
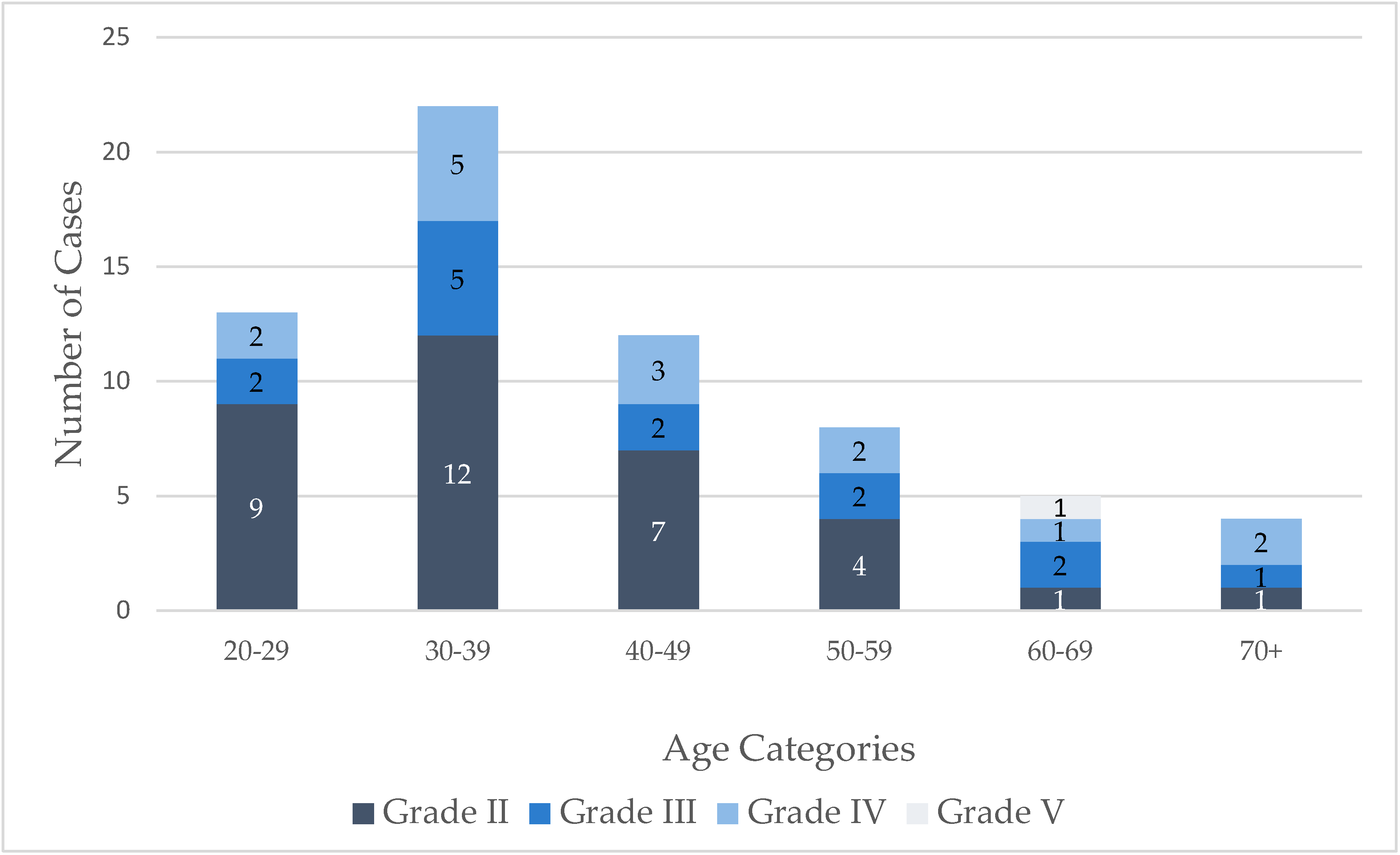
| Number (n) | Percentage (%) | |
|---|---|---|
| Demographics and Clinical Characteristics | ||
| Sex | ||
| Male | 23 | 43.4 |
| Female | 30 | 56.6 |
| Age (years) | 41.84 ± 14.65 | |
| Hospitalization | ||
| Yes | 10 | 18.9 |
| No | 43 | 81.1 |
| Hospitalization days | 5.10 ± 2.38 | |
| Time from injury to ED arrival (hours) | 5.74 (0.25–48) | |
| Occupation | ||
| 11 | 20.8 |
| 2 | 3.8 |
| 26 | 49.1 |
| 10 | 18.8 |
| 4 | 7.5 |
| Season distribution | ||
| Summer | 8 | 15.1 |
| Autumn | 17 | 32.1 |
| Winter | 7 | 13.2 |
| Spring | 21 | 39.6 |
| Place of injury | ||
| Household | 43 | 81.1 |
| Workplace | 10 | 18.9 |
| Irrigation status before ED arrival | ||
| Performed | 48 | 90.6 |
| Not performed | 5 | 9.4 |
| Part | ||
| 42 | 79.2 |
| 11 | 20.8 |
| Chemical agent | ||
| Alkali | 31 | 58.5 |
| 20 | 37.7 |
| 2 | 3.8 |
| Acute management of ocular burn | ||
| Oral doxycycline | 10 | 18.9 |
| Oral vitamin C | 20 | 37.7 |
| Topical corticosteroids | 52 | 98.1 |
| Therapeutic contact lens | 5 | 9.4 |
| Control Group (n = 87, eyes = 87) | Chemical Burn Group (n = 53, eyes = 64) | p Value | Corrected p Value * | |
|---|---|---|---|---|
| Patient Characteristics | ||||
| Sex Male Female | 41 (47.1%) 46 (52.9%) | 23 (43.4%) 30 (56.6%) | χ2 = 0.061, p = 0.805 | |
| Age (years) | 45.49 ± 13.35 (21–87) | 41.84 ± 14.65 (21–84) | z = −1.867, p = 0.062 | |
| OCT Characteristics | ||||
| RNFL total (µm) | 103.34 ± 7.81 (85–130) | 102.20 ± 8.87 (78–123) | t = −0.838, p = 0.404 | |
| RNFL superior (µm) | 125.47 ± 12.41 (96–153) | 124.94 ± 14.21 (78–159) | t = −0.245, p = 0.806 | |
| RNFL nasal (µm) | 82.08 ± 11.02 (59–104) | 77.59 ± 10.97 (57–107) | t = −2.477, p = 0.014 | 0.056 |
| RNFL inferior (µm) | 134.59 ± 12.66 (104–173) | 132.55 ± 14.34 (100–159) | t = −0.925, p = 0.357 | |
| RNFL temporal (µm) | 75.33 ± 12.37 (56–108) | 72.33 ± 10.50 (52–97) | t = −1.571, p = 0.118 | |
| GCL ++ total (µm) | 105.03 ± 6.34 (91–126) | 104.06 ± 8.14 (87–122) | t = −0.825, p = 0.411 | |
| GCL ++ superior (µm) | 104.01 ± 6.47 (91–123) | 102.94 ± 8.14 (84–118) | t = −0.903, p = 0.368 | |
| GCL ++ inferior (µm) | 105.89 ± 6.63 (92–129) | 105.12 ± 8.58 (89–125) | t = −0.614, p = 0.540 | |
| 3 Months | 6 Months | p Value | |
|---|---|---|---|
| OCT Characteristics | |||
| RNFL total (µm) | 98.11 ± 10.17 | 96.22 ± 8.33 | t = 1.681, p = 0.131 |
| RNFL superior (µm) | 121.11 ± 13.89 | 119.89 ± 11.51 | t = 0.753, p = 0.473 |
| RNFL nasal (µm) | 76.33 ± 13.14 | 73.44 ± 9.54 | t = 1.702, p = 0.127 |
| RNFL inferior (µm) | 126.78 ± 15.18 | 122.4 ± 14.99 | t = 2.102, p = 0.069 |
| RNFL temporal (µm) | 67.78 ± 8.91 | 68.67 ± 8.20 | t = −1.018, p = 0.338 |
| GCL ++ total (µm) | 101.56 ± 5.56 | 101.44 ± 4.12 | t = 0.286, p = 0.782 |
| GCL ++ superior (µm) | 100.33 ± 4.89 | 99.78 ± 4.06 | t = 0.743, p = 0.479 |
| GCL ++ inferior (µm) | 102.78 ± 5.17 | 102.33 ± 4.21 | t = 0.645, p = 0.537 |
| Odds Ratio | Confidence Interval (95%) | p Value | |
|---|---|---|---|
| Demographics and clinical characteristics | |||
| Sex | 0.285 | ||
| Age | 0.595 | ||
| Irrigation status before ED arrival (performed or not performed) | 0.786 | ||
| Insulting agent (acid or alkali) | 0.813 | ||
| Dua grade (I–VI) | 4.816 | 1.103–21.030 | 0.037 |
| Odds Ratio | Confidence Interval (95%) | p Value | |
|---|---|---|---|
| Demographics and clinical characteristics | |||
| Sex | 0.488 | ||
| Age | 0.085 | ||
| Irrigation status before ED arrival (performed or not performed) | 0.581 | ||
| Insulting agent (acid or alkali) | 0.186 | ||
| Dua’s grade (I–VI) | 0.280 | ||
| Control Group (n = 87) | Chemical Burn Group Dua grade ≥ 3 (n = 30) | p Value | Corrected p Value * | |
|---|---|---|---|---|
| RNFL total (µm) | 103.34 ± 7.81 (85–130) | 100.17 ± 9.57 (78–121) | t = −1.811, p = 0.073 | |
| RNFL superior (µm) | 125.47 ± 12.41 (96–153) | 120.20 ± 16.49 (78–159) | t = −1.836, p = 0.069 | |
| RNFL nasal (µm) | 82.08 ± 11.02 (59–104) | 75.73 ± 10.88 (57–107) | t = −2.729, p = 0.007 | 0.035 |
| RNFL inferior (µm) | 134.59 ± 12.66 (104–173) | 129.53 ± 15.54 (100–156) | t = −1.775, p = 0.078 | |
| RNFL temporal (µm) | 75.33 ± 12.37 (56–108) | 72.20 ± 10.47 (52–95) | t = −1.241, p = 0.217 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skruodyte, J.; Olechnovic, J.; Serpytis, P. Changes in Retinal Nerve Fiber and Ganglion Cell Layers After Chemical Injury: A Prospective Study. J. Clin. Med. 2025, 14, 5601. https://doi.org/10.3390/jcm14155601
Skruodyte J, Olechnovic J, Serpytis P. Changes in Retinal Nerve Fiber and Ganglion Cell Layers After Chemical Injury: A Prospective Study. Journal of Clinical Medicine. 2025; 14(15):5601. https://doi.org/10.3390/jcm14155601
Chicago/Turabian StyleSkruodyte, Justina, Justina Olechnovic, and Pranas Serpytis. 2025. "Changes in Retinal Nerve Fiber and Ganglion Cell Layers After Chemical Injury: A Prospective Study" Journal of Clinical Medicine 14, no. 15: 5601. https://doi.org/10.3390/jcm14155601
APA StyleSkruodyte, J., Olechnovic, J., & Serpytis, P. (2025). Changes in Retinal Nerve Fiber and Ganglion Cell Layers After Chemical Injury: A Prospective Study. Journal of Clinical Medicine, 14(15), 5601. https://doi.org/10.3390/jcm14155601





