The Use of Self-Sampling Devices via a Smartphone Application to Encourage Participation in Cervical Cancer Screening: A Pilot Study
Abstract
1. Introduction
- HPV DNA detection in a screen-and-treat approach, commencing at the age of 30 years, with regular screening every 5 to 10 years.
- HPV DNA detection in a screen, triage, and treat approach, also starting at the age of 30 years, with regular screening intervals of 5 to 10 years.
2. Materials and Methods
2.1. Study Design
2.2. Home Self-Sampling
2.3. HPV Testing
2.4. Statistical Analysis
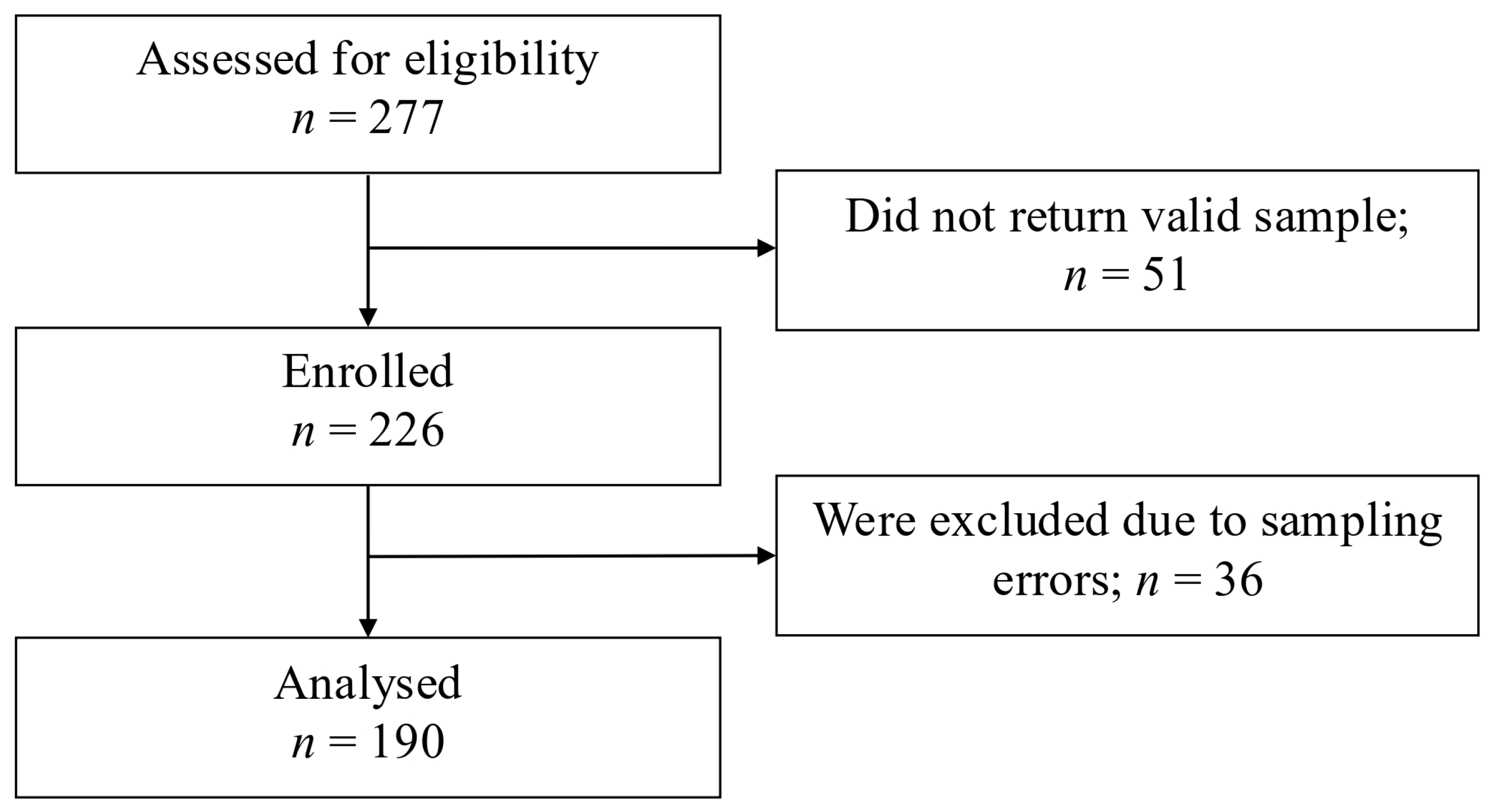
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Bhat, D. The ‘Why and How’ of Cervical Cancers and Genital HPV Infection. Cytojournal 2022, 19, 22. [Google Scholar] [CrossRef]
- Arbyn, M.; Castle, P.E. Offering Self-Sampling Kits for HPV Testing to Reach Women Who Do Not Attend in the Regular Cervical Cancer Screening Program. Cancer Epidemiol. Biomark. Prev. 2015, 24, 769–772. [Google Scholar] [CrossRef]
- Snijders, P.J.; Verhoef, V.M.; Arbyn, M.; Ogilvie, G.; Minozzi, S.; Banzi, R.; van Kemenade, F.J.; Heideman, D.A.; Meijer, C.J. High-risk HPV testing on self-sampled versus clinician-collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int. J. Cancer 2013, 132, 2223–2236. [Google Scholar] [CrossRef]
- Modibbo, F.; Iregbu, K.C.; Okuma, J.; Leeman, A.; Kasius, A.; de Koning, M.; Quint, W.; Adebamowo, C. Randomized trial evaluating self-sampling for HPV DNA based tests for cervical cancer screening in Nigeria. Infect. Agent Cancer 2017, 12, 11. [Google Scholar] [CrossRef]
- Avian, A.; Clemente, N.; Mauro, E.; Isidoro, E.; Di Napoli, M.; Dudine, S.; Del Fabro, A.; Morini, S.; Perin, T.; Giudici, F.; et al. Clinical validation of full HR-HPV genotyping HPV Selfy assay according to the international guidelines for HPV test requirements for cervical cancer screening on clinician-collected and self-collected samples. J. Transl. Med. 2023, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Kulasingam, S.L.; Whitham, H.K.; Hawes, S.E.; Lin, J.; Kiviat, N.B. Clinician and Patient Acceptability of Self-Collected Human Papillomavirus Testing for Cervical Cancer Screening. J. Womens Health (Larchmt) 2017, 26, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, K.; Kobetz, E.; Koru-Sengul, T.; Marcus, E.N.; Rodriguez, B.; Alonzo, Y.; Carrasquillo, O. Acceptability and Feasibility of Human Papilloma Virus Self-Sampling for Cervical Cancer Screening. J. Womens Health (Larchmt) 2016, 25, 944–951. [Google Scholar] [CrossRef]
- Rosenbaum, A.J.; Gage, J.C.; Alfaro, K.M.; Ditzian, L.R.; Maza, M.; Scarinci, I.C.; Felix, J.C.; Castle, P.E.; Villalta, S.; Miranda, E.; et al. Acceptability of self-collected versus provider-collected sampling for HPV DNA testing among women in rural El Salvador. Int. J. Gynaecol. Obs. 2014, 126, 156–160. [Google Scholar] [CrossRef]
- Mbatha, J.N.; Galappaththi-Arachchige, H.N.; Mtshali, A.; Taylor, M.; Ndhlovu, P.D.; Kjetland, E.F.; Baay, M.F.D.; Mkhize-Kwitshana, Z.L. Self-sampling for human papillomavirus testing among rural young women of KwaZulu-Natal, South Africa. BMC Res. Notes 2017, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Trope, L.A.; Chumworathayi, B.; Blumenthal, P.D. Feasibility of community-based careHPV for cervical cancer prevention in rural Thailand. J. Low. Genit. Tract Dis. 2013, 17, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Gonzales, A.A.; Noonan, C.J.; Cherne, S.L.; Buchwald, D.S. Collaborative to Improve Native Cancer Outcomes (CINCO). Assessing Acceptability of Self-Sampling Kits, Prevalence, and Risk Factors for Human Papillomavirus Infection in American Indian Women. J. Community Health 2016, 41, 1049–1061. [Google Scholar] [CrossRef]
- Reisner, S.L.; Deutsch, M.B.; Peitzmeier, S.M.; White Hughto, J.M.; Cavanaugh, T.P.; Pardee, D.J.; McLean, S.A.; Panther, L.A.; Gelman, M.; Mimiaga, M.J.; et al. Test performance and acceptability of self- versus provider-collected swabs for high-risk HPV DNA testing in female-to-male trans masculine patients. PLoS ONE 2018, 13, e0190172. [Google Scholar] [CrossRef]
- Aiko, K.Y.; Yoko, M.; Saito, O.M.; Ryoko, A.; Yasuyo, M.; Mikiko, A.S.; Takeharu, Y.; Fumiki, H.; Etsuko, M. Accuracy of self-collected human papillomavirus samples from Japanese women with abnormal cervical cytology. J. Obs. Gynaecol. Res. 2017, 43, 710–717. [Google Scholar] [CrossRef]
- Hanley, S.J.; Fujita, H.; Yokoyama, S.; Kunisawa, S.; Tamakoshi, A.; Dong, P.; Kobayashi, N.; Watari, H.; Kudo, M.; Sakuragi, N. HPV self-sampling in Japanese women: A feasibility study in a population with limited experience of tampon use. J. Med. Screen. 2016, 23, 164–170. [Google Scholar] [CrossRef]
- Jones, H.E.; Wiegerinck, M.A.; Nieboer, T.E.; Mol, B.W.; Westhoff, C.L. Women in the Netherlands prefer self-sampling with a novel lavaging device to clinician collection of specimens for cervical cancer screening. Sex. Transm. Dis. 2008, 35, 916–917. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.P.; Alejandro, N.; Pérez, C.M.; Otero, Y.; Soto-Salgado, M.; Palefsky, J.M.; Tortolero-Luna, G.; Romaguera, J. Acceptability of cervical and anal HPV self-sampling in a sample of Hispanic women in Puerto Rico. P. R. Health Sci. J. 2012, 31, 205–212. [Google Scholar]
- Phoolcharoen, N.; Kantathavorn, N.; Krisorakun, W.; Taepisitpong, C.; Krongthong, W.; Saeloo, S. Acceptability of Self-Sample Human Papillomavirus Testing Among Thai Women Visiting a Colposcopy Clinic. J. Community Health 2018, 43, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, V.; Petignat, P.; Burton-Jeangros, C. To what extent will women accept HPV self-sampling for cervical cancer screening? A qualitative study conducted in Switzerland. Int. J. Womens Health 2015, 7, 883–888. [Google Scholar] [CrossRef]
- Howard, M.; Lytwyn, A.; Lohfeld, L.; Redwood-Campbell, L.; Fowler, N.; Karwalajtys, T. Barriers to acceptance of self-sampling for human papillomavirus across ethnolinguistic groups of women. Can. J. Public Health 2009, 100, 365–369. [Google Scholar] [CrossRef]
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; Kennedy, C.E.; Narasimhan, M. HPV self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef] [PubMed]
- Statista. Available online: https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/ (accessed on 28 April 2024).
- International Papillomavirus Society (IPVS). Available online: https://ipvsoc.org/news/fight-hpv-free-social-gaming-app/ (accessed on 28 April 2024).
- Real, F.J.; Rosen, B.L.; Bishop, J.M.; McDonald, S.; DeBlasio, D.; Kreps, G.L.; Klein, M.; Kahn, J.A. Usability Evaluation of the Novel Smartphone Application, HPV Vaccine: Same Way, Same Day, Among Pediatric Residents. Acad. Pediatr. 2021, 21, 742–749. [Google Scholar] [CrossRef]
- Woodall, W.G.; Zimet, G.; Kong, A.; Buller, D.; Reither, J.; Chilton, L.; Myers, V.; Starling, R. Vacteens.org: A Mobile Web app to Improve HPV Vaccine Uptake. Front. Digit. Health 2021, 3, 693688. [Google Scholar] [CrossRef]
- Genomica. Available online: https://genomica-cp701.wordpresstemporal.com/wp-content/uploads/2021/12/CLART-Hpv4-INGLES.pdf (accessed on 28 April 2024).
- Solomon, D.; Davey, D.; Kurman, R.; Moriarty, A.; O’Connor, D.; Prey, M.; Raab, S.; Sherman, M.; Wilbur, D.; Wright, T., Jr.; et al. Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA 2002, 287, 2114–2119. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017, 8, CD008587. [Google Scholar] [CrossRef]
- Chrysostomou, A.C.; Stylianou, D.C.; Constantinidou, A.; Kostrikis, L.G. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses 2018, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Gök, M.; Heideman, D.A.; van Kemenade, F.J.; de Vries, A.L.; Berkhof, J.; Rozendaal, L.; Beliën, J.A.; Overbeek, L.; Babović, M.; Snijders, P.J.; et al. Offering self-sampling for human papillomavirus testing to non-attendees of the cervical screening programme: Characteristics of the responders. Eur. J. Cancer 2012, 48, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Tranberg, M.; Bech, B.H.; Blaakær, J.; Jensen, J.S.; Svanholm, H.; Andersen, B. Preventing cervical cancer using HPV self-sampling: Direct mailing of test-kits increases screening participation more than timely opt-in procedures—A randomized controlled trial. BMC Cancer 2018, 18, 273. [Google Scholar] [CrossRef]
- Haguenoer, K.; Sengchanh, S.; Gaudy-Graffin, C.; Boyard, J.; Fontenay, R.; Marret, H.; Goudeau, A.; Pigneaux de Laroche, N.; Rusch, E.; Giraudeau, B. Vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: A randomised trial. Br. J. Cancer 2014, 111, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.; Bartoszek, M.; Marlow, L.; Wardle, J. Barriers to cervical cancer screening attendance in England: A population-based survey. J. Med. Screen. 2009, 16, 199–204. [Google Scholar] [CrossRef]
- Bos, A.B.; Rebolj, M.; Habbema, J.D.; van Ballegooijen, M. Nonattendance is still the main limitation for the effectiveness of screening for cervical cancer in the Netherlands. Int. J. Cancer 2006, 119, 2372–2375. [Google Scholar] [CrossRef]
- Sultana, F.; Mullins, R.; English, D.R.; Simpson, J.A.; Drennan, K.T.; Heley, S.; Wrede, C.D.; Brotherton, J.M.; Saville, M.; Gertig, D.M. Women’s experience with home-based self-sampling for human papillomavirus testing. BMC Cancer 2015, 15, 849. [Google Scholar] [CrossRef] [PubMed]
- Madzima, T.R.; Vahabi, M.; Lofters, A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: Focused literature review. Can. Fam. Physician 2017, 63, 597–601. [Google Scholar]
- Crofts, V.; Flahault, E.; Tebeu, P.M.; Untiet, S.; Fosso, G.K.; Boulvain, M.; Vassilakos, P.; Petignat, P. Education efforts may contribute to wider acceptance of human papillomavirus self-sampling. Int. J. Womens Health 2015, 7, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sauvaget, C.; Fayette, J.M.; Muwonge, R.; Wesley, R.; Sankaranarayanan, R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int. J. Gynaecol Obs. 2011, 113, 14–24. [Google Scholar] [CrossRef]
- Cuzick, J.; Arbyn, M.; Sankaranarayanan, R.; Tsu, V.; Ronco, G.; Mayrand, M.H.; Dillner, J.; Meijer, C.J. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine 2008, 26 (Suppl. 10), K29–K41. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Simon, M.; de Sanjosé, S.; Clarke, M.A.; Poljak, M.; Rezhake, R.; Berkhof, J.; Nyaga, V.; Gultekin, M.; Canfell, K.; et al. Accuracy and effectiveness of HPV mRNA testing in cervical cancer screening: A systematic review and meta-analysis. Lancet Oncol. 2022, 23, 950–960. [Google Scholar] [CrossRef]
- Tranberg, M.; Jensen, J.S.; Bech, B.H.; Blaakær, J.; Svanholm, H.; Andersen, B. Good concordance of HPV detection between cervico-vaginal self-samples and general practitioner-collected samples using the Cobas 4800 HPV DNA test. BMC Infect. Dis. 2018, 18, 348. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Ouh, Y.T.; Hong, J.H.; Min, K.J.; So, K.A.; Kim, T.J.; Paik, E.S.; Lee, J.W.; Moon, J.H.; Lee, J.K. Comparison of urine, self-collected vaginal swab, and cervical swab samples for detecting human papillomavirus (HPV) with Roche Cobas HPV, Anyplex II HPV, and RealTime HR-S HPV assay. J. Virol. Methods 2019, 269, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.W.; Khurshed, F.; van Niekerk, D.J.; Krajden, M.; Greene, S.B.; Hobbs, S.; Coldman, A.J.; Franco, E.L.; Ogilvie, G.S. Women’s intentions to self-collect samples for human papillomavirus testing in an organized cervical cancer screening program. BMC Public Health 2014, 14, 1060. [Google Scholar] [CrossRef]
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; Herrero, R.; Franceschi, S.; Meijer, C.J.; Snijders, P.; Bosch, F.X.; de Sanjosé, S.; Muñoz, N. IARC Multi-centre Cervical Cancer Study Group. Smoking and cervical cancer: Pooled analysis of the IARC multi-centric case--control study. Cancer Causes Control 2003, 14, 805–814. [Google Scholar] [CrossRef] [PubMed]
| Patients | N (%) |
|---|---|
| Age (Mean ± SD) | 36.89 ± 13.22 |
| BMI | |
| <18.5, n (%) | 16 (7.1) |
| 18.5–24.9, n (%) | 146 (64.6) |
| >25, n (%) | 50 (22.1) |
| N.A., n (%) | 14 (6.2) |
| Number of pregnancies, (Mean ± SD) | 1.10 ± 1.34 |
| Menopause, n (%) | 20 (8.8) |
| Oral contraceptive, (Mean ± SD) | 39 (17.3) |
| Smoking | |
| -Smoker, n (%) | 14 (6.2) |
| -Non-Smoker, n (%) | 212 (93.8) |
| HPV Vaccination | |
| -Not vaccinated | 190 (84.1) |
| -Vaccinated | 36 (15.9) |
| -Cervarix® | 7 (19.4) |
| -Gardasil-4® | 26 (72.2) |
| -Gardasil-9® | 2 (5.6) |
| Education level | |
| -Middle school certificate, n (%) | 23 (8.3) |
| -Higher education, n (%) | 111 (40.1) |
| -University degree, n (%) | 121 (43.7) |
| -Not indicated, n (%) | 22 (7.9) |
| Sample sent back for analysis: | |
| -unsuccessful, n (%) | 51 (18.4) |
| -successful, n (%) | 226 (81.6) |
| -Traditional mailbox | 196 (86.7) |
| -Courier company | 30 (13.3) |
| Digital activation of Kit | |
| -Unsuccessful activation, n (%) | 62 (22.4) |
| -Successful activation, n (%) | 215 (77.6) |
| -Immediately | 165 (77) |
| -Within 7 days | 39 (18) |
| -Between 8 and 18 days | 11 (5) |
| Self-Sampling Vaginal Swab (N = 190) | Medical Sampling Vaginal Swab (N = 190) | CLART (N = 190) | |
|---|---|---|---|
| HPV-HR positivity | 33 (17.4) | 38 (20) | 36 (18.9) |
| HPV-negativity | 157 (82.6) | 152 (80) | 154 (81.1) |
| Coinfected HPV | 10 | 11 | 18 |
| Genotype of HPV-HR | |||
| -Type 16 | 6 (3.1) | 8 (4.2) | 5 (2.6) |
| -Type 18 | 0 (0) | 1 (0.5) | 0 (0) |
| -Type 31 | 8 (4.2) | 10 (5.2) | 7 (3.7) |
| -Type 33 | 0 (0) | 0 (0) | 1 (0.5) |
| -Type 35 | 0 (0) | 0 (0) | 0 (0) |
| -Type 39 | 5 (2.6) | 4 (2.1) | 1 (0.5) |
| -Type 45 | 1 (0.5) | 1 (0.5) | 1 (0.5) |
| -Type 51 | 4 (2.1) | 5 (2.6) | 5 (2.6) |
| -Type 52 | 3 (1.6) | 2 (1.0) | 3 (1.6) |
| -Type 56 | 3 (1.6) | 3 (1.6) | 1 (0.5) |
| -Type 58 | 8 (4.2) | 7 (3.7) | 8 (4.2) |
| -Type 59 | 2 (1.0) | 2 (1.0) | 2 (1.0) |
| -Type 66 | 4 (2.1) | 5 (2.6) | 9 (4.7) |
| -Type 68 | 2 (1.0) | 3 (1.6) | 0 (0) |
| CLART 1 vs. HPV Selfy 1 | CLART 1 vs. HPV Selfy 2 | HPV 2 vs. HPV Selfy 1 | |
|---|---|---|---|
| Sensitivity | 86.1% (95%CI: 70.50–95.33%) | 77.8% (95%CI: 60.85–89.88%) | 81.5% (95%CI: 65.67–92.26%) |
| Specificity | 95.4% (95%CI: 90.86–98.15%) | 96.8% (95%CI: 92.59–98.94%) | 98.7% (95%CI: 95.33–99.84%) |
| Accuracy | 93.7% (95%CI: 89.23–96.69%) | 93.2% (95%CI: 88.58–96.31%) | 95.3% (95%CI: 91.20–97.81%) |
| PPV | 81.6% (95%CI: 67.97–90.24%) | 84.8% (95%CI: 69.91–93.10%) | 93.9% (95%CI: 79.51–98.41%) |
| NPV | 96.7% (95%CI: 92.87–98.52%) | 94.9% (95%CI: 90.99–97.17%) | 95.5% (95%CI: 91.65–97.67%) |
| K Cohen | 79.9% (95%CI: 68.90–90.80%) | 77.0% (95%CI: 65.10–88.90%) | 84.4% (95%CI: 74.60–94.30%) |
| OA agrement | 93.7% | 93.2% | 95.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotti, F.; Ficarola, F.; Fais, G.; De Cicco Nardone, C.; Montera, R.; Luvero, D.; Cundari, G.B.; Avian, A.; Riva, E.; Castriciano, S.; et al. The Use of Self-Sampling Devices via a Smartphone Application to Encourage Participation in Cervical Cancer Screening: A Pilot Study. J. Clin. Med. 2025, 14, 5569. https://doi.org/10.3390/jcm14155569
Plotti F, Ficarola F, Fais G, De Cicco Nardone C, Montera R, Luvero D, Cundari GB, Avian A, Riva E, Castriciano S, et al. The Use of Self-Sampling Devices via a Smartphone Application to Encourage Participation in Cervical Cancer Screening: A Pilot Study. Journal of Clinical Medicine. 2025; 14(15):5569. https://doi.org/10.3390/jcm14155569
Chicago/Turabian StylePlotti, Francesco, Fernando Ficarola, Giuseppina Fais, Carlo De Cicco Nardone, Roberto Montera, Daniela Luvero, Gianna Barbara Cundari, Alice Avian, Elisabetta Riva, Santina Castriciano, and et al. 2025. "The Use of Self-Sampling Devices via a Smartphone Application to Encourage Participation in Cervical Cancer Screening: A Pilot Study" Journal of Clinical Medicine 14, no. 15: 5569. https://doi.org/10.3390/jcm14155569
APA StylePlotti, F., Ficarola, F., Fais, G., De Cicco Nardone, C., Montera, R., Luvero, D., Cundari, G. B., Avian, A., Riva, E., Castriciano, S., Angeletti, S., Ciccozzi, M., Angioli, R., & Terranova, C. (2025). The Use of Self-Sampling Devices via a Smartphone Application to Encourage Participation in Cervical Cancer Screening: A Pilot Study. Journal of Clinical Medicine, 14(15), 5569. https://doi.org/10.3390/jcm14155569







