Comparison of Artificial Intelligence–Derived Heart Age with Chronological Age Using Normal Sinus Electrocardiograms in Patients with No Evidence of Cardiac Disease
Abstract
1. Introduction
2. Materials and Methods
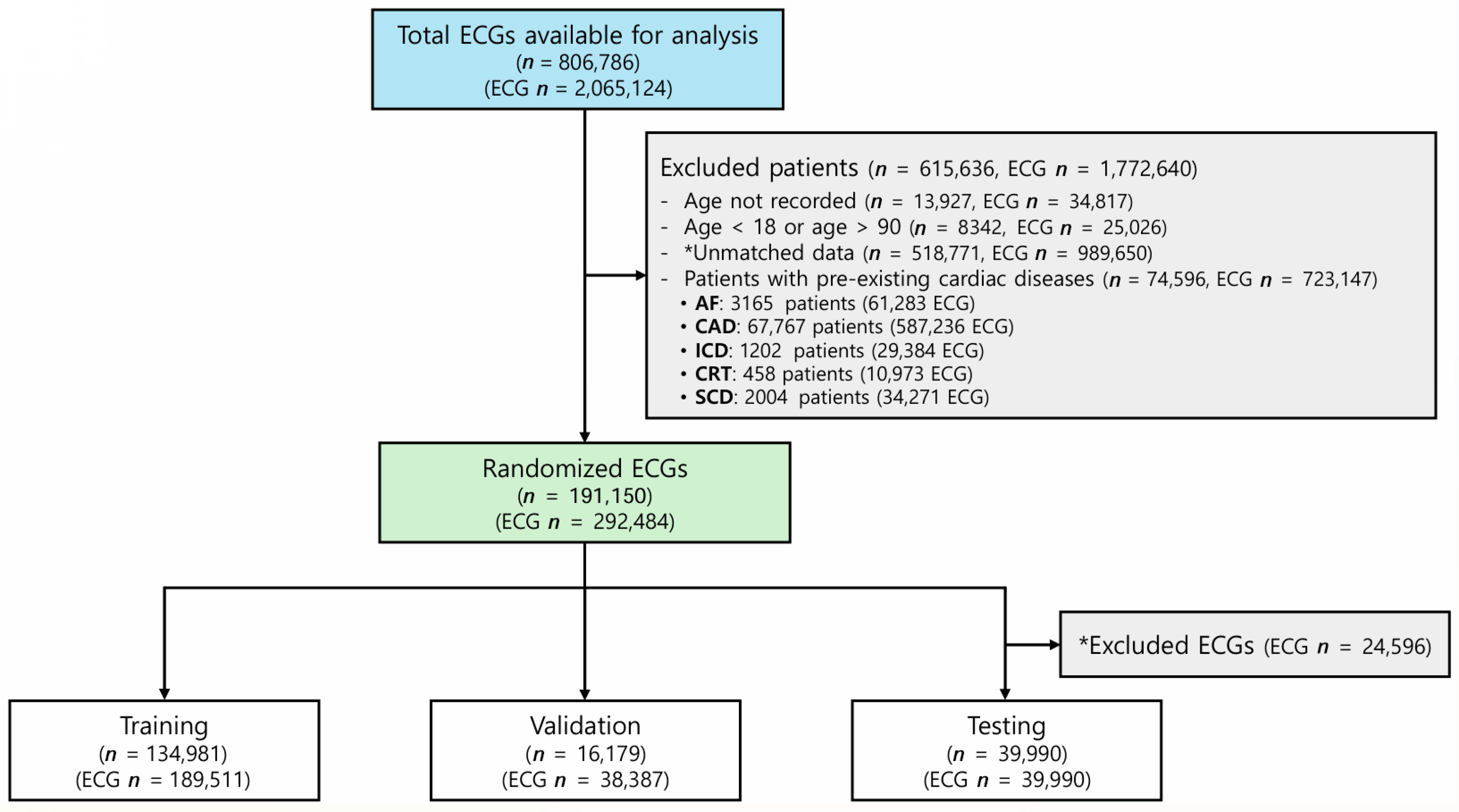
2.1. Study Population
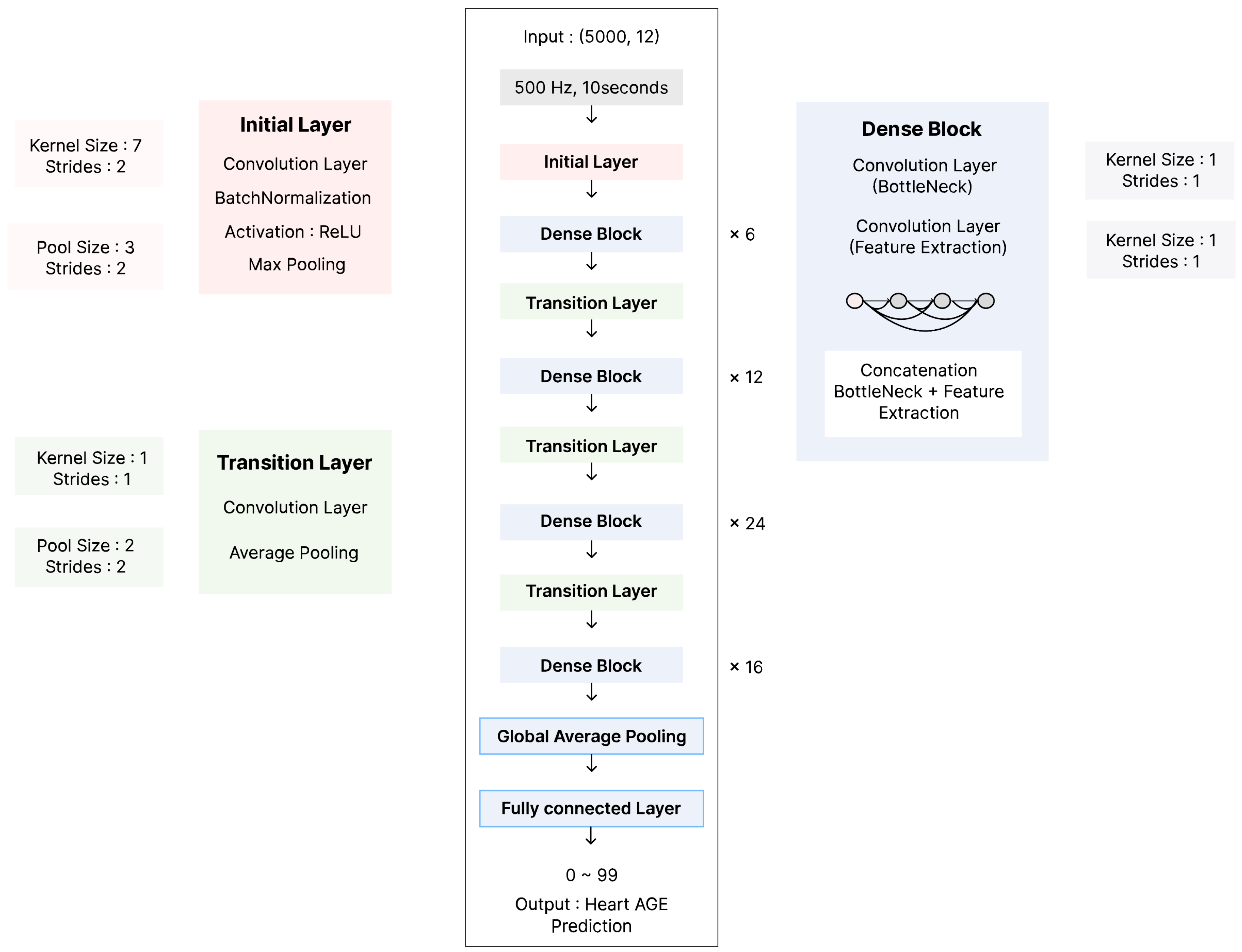
2.2. AI Model Development and Training
2.3. Statistical Analysis
3. Results
3.1. Study Population and Data Characteristics
3.2. Performance of the ECG-Heart Age Prediction Model
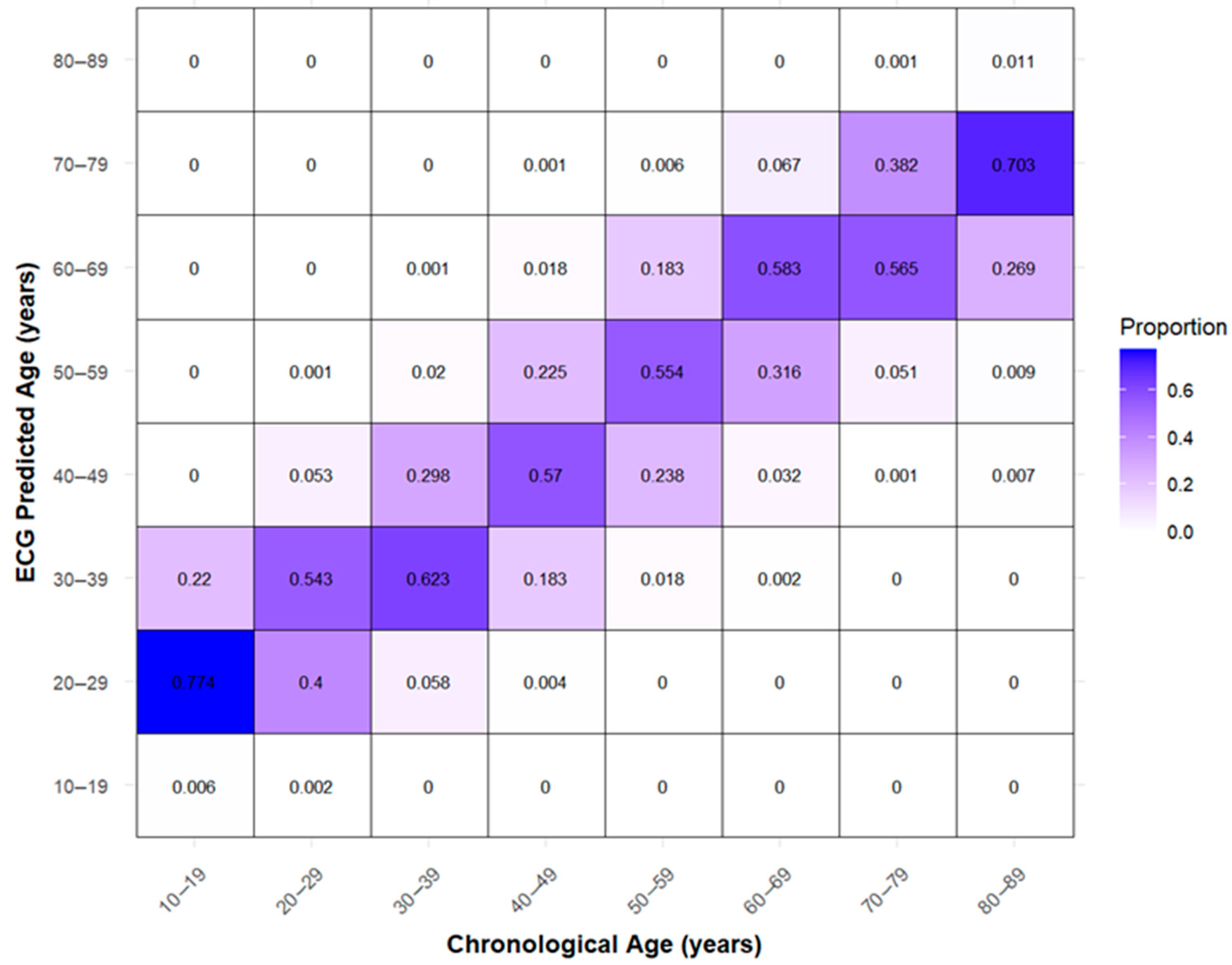
3.3. Differences Between AI-Predicted Heart Age and Actual Age
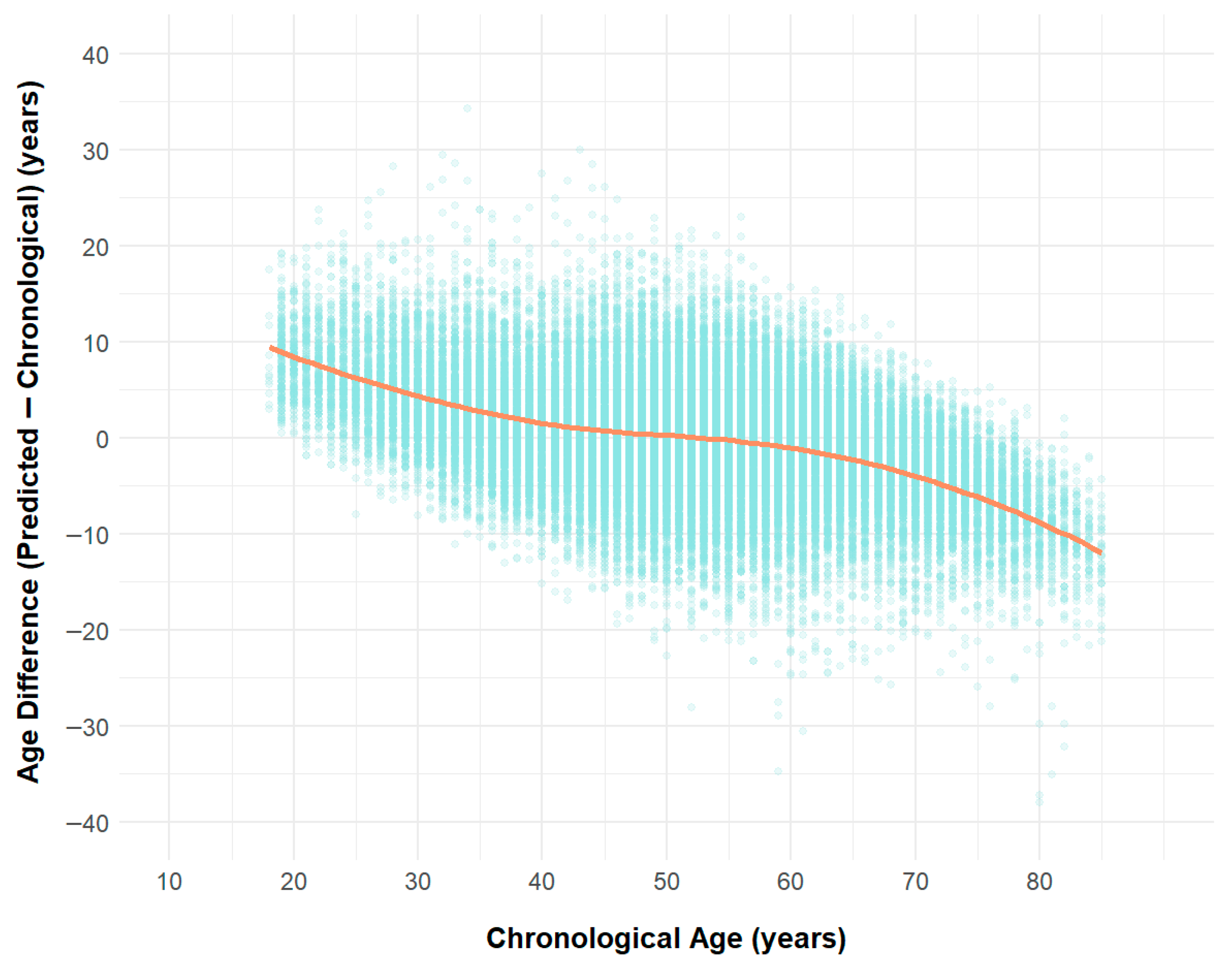
3.4. External Validation and Model Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| AI | Artificial Intelligence |
| BA | Biological Age |
| BMI | Body Mass Index |
| CA | Chronological Age |
| CAD | Coronary Artery Disease |
| CKD | Chronic Kidney Disease |
| CNN | Convolutional Neural Network |
| CRT | Cardiac Resynchronization Therapy |
| CVA | Cerebrovascular Accident |
| DM | Diabetes Mellitus |
| ECG | Electrocardiogram |
| HR | Heart Rate |
| HTN | Hypertension |
| ICD | Implantable Cardioverter Defibrillator |
| IQR | Interquartile Range |
| IVSd | Interventricular Septal Wall Thickness |
| LAVI | Left Atrial Volume Index |
| LOESS | Locally Estimated Scatterplot Smoothing |
| LVEF | Left Ventricular Ejection Fraction |
| LVIDd | Left Ventricular Internal Diameter in Diastole |
| LVIDs | Left Ventricular Internal Diameter in Systole |
| LVMI | Left Ventricular Mass Index |
| MAE | Mean Absolute Error |
| NT-proBNP | N-terminal pro B-type Natriuretic Peptide |
| PAOD | Peripheral Arterial Occlusive Disease |
| PCC | Pearson Correlation Coefficient |
| R2 | Coefficient of Determination |
| RAP | Right Atrial Pressure |
| RMSE | Root Mean Square Error |
| RVSP | Right Ventricular Systolic Pressure |
| SD | Standard Deviation |
References
- Brant, L.C.C.; Ribeiro, A.H.; Pinto-Filho, M.M.; Kornej, J.; Preis, S.R.; Fetterman, J.L.; Eromosele, O.B.; Magnani, J.W.; Murabito, J.M.; Larson, M.G.; et al. Association Between Electrocardiographic Age and Cardiovascular Events in Community Settings: The Framingham Heart Study. Circ. Cardiovasc. Qual. Outcomes 2023, 16, e009821. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, F.; Kapa, S.; Friedman, P.A.; LeBrasseur, N.K.; Klavetter, E.; Mangold, K.E.; Attia, Z.I. Assessing Biological Age: The Potential of ECG Evaluation Using Artificial Intelligence: JACC Family Series. JACC Clin. Electrophysiol. 2024, 10, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.S.; Lee, D.H.; Jo, Y.; Lee, S.C.; Choi, W.; Kim, D.H. Artificial intelligence-estimated biological heart age using a 12-lead electrocardiogram predicts mortality and cardiovascular outcomes. Front. Cardiovasc. Med. 2023, 10, 1137892. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Suzuki, S.; Motogi, J.; Nakai, H.; Matsuzawa, W.; Takayanagi, T.; Umemoto, T.; Hyodo, A.; Satoh, K.; Arita, T.; et al. Cardiovascular events and artificial intelligence-predicted age using 12-lead electrocardiograms. Int. J. Cardiol. Heart Vasc. 2023, 44, 101172. [Google Scholar] [CrossRef] [PubMed]
- Lindow, T.; Palencia-Lamela, I.; Schlegel, T.T.; Ugander, M. Heart age estimated using explainable advanced electrocardiography. Sci. Rep. 2022, 12, 9840. [Google Scholar] [CrossRef]
- Ladejobi, A.O.; Medina-Inojosa, J.R.; Shelly Cohen, M.; Attia, Z.I.; Scott, C.G.; LeBrasseur, N.K.; Gersh, B.J.; Noseworthy, P.A.; Friedman, P.A.; Kapa, S.; et al. The 12-lead electrocardiogram as a biomarker of biological age. Eur. Heart J. Digit. Health 2021, 2, 379–389. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, C.S.; Luo, Y.S.; Lee, Y.T.; Lin, C. Electrocardiogram-Based Heart Age Estimation by a Deep Learning Model Provides More Information on the Incidence of Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 754909. [Google Scholar] [CrossRef] [PubMed]
- Kligfield, P. The centennial of the Einthoven electrocardiogram. J. Electrocardiol. 2002, 35, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Feigenbaum, H. Echocardiographic examination of the left ventricle. Circulation 1975, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, V.K.; Sengupta, P.P.; Gentile, F.; Khandheria, B.K. History of echocardiography and its future applications in medicine. Crit. Care Med. 2007, 35, S309–S313. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Marina-Breysse, M. Current and Future Use of Artificial Intelligence in Electrocardiography. J. Cardiovasc. Dev. Dis. 2023, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.M.; Ribeiro, A.H.; Paixão, G.M.M.; Ribeiro, M.H.; Pinto-Filho, M.M.; Gomes, P.R.; Oliveira, D.M.; Sabino, E.C.; Duncan, B.B.; Giatti, L.; et al. Deep neural network-estimated electrocardiographic age as a mortality predictor. Nat. Commun. 2021, 12, 5117. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Howson, S.A.; Booth, A.E.C.; Shahmohamadi, E.; Lim, M.; Bacchi, S.; Roberts-Thomson, R.L.; Middeldorp, M.E.; Emami, M.; Psaltis, P.J.; et al. Artificial intelligence age prediction using electrocardiogram data: Exploring biological age differences. Heart Rhythm. 2025, 22, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Lindow, T.; Maanja, M.; Schelbert, E.B.; Ribeiro, A.H.; Ribeiro, A.L.P.; Schlegel, T.T.; Ugander, M. Heart age gap estimated by explainable advanced electrocardiography is associated with cardiovascular risk factors and survival. Eur. Heart J. Digit. Health 2023, 4, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Johnson, K.W.; Rosenson, R.S.; Wang, Z.; Aydar, M.; Baber, U.; Min, J.K.; Tang, W.H.W.; Halperin, J.L.; Narayan, S.M. Deep learning for cardiovascular medicine: A practical primer. Eur. Heart J. 2019, 40, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, A.; Zito, E.; Pierucci, N.; Matteucci, A.; La Fazia, V.M. A Talk with ChatGPT: The Role of Artificial Intelligence in Shaping the Future of Cardiology and Electrophysiology. J. Pers. Med. 2025, 15, 205. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total (n = 191,150) |
|---|---|
| Demographics | |
| Age, years | 51.4 ± 13.8 |
| Male, n (%) | 80,808 (42.3) |
| BMI (kg/m2) | 16.7 ± 11.4 |
| HR (bpm) | 77.9 ± 13.7 |
| Echocardiographic Measures | |
| LVEF (%) | 64.9 ± 5.7 |
| LVIDd (mm) | 47.9 ± 4.5 |
| LVIDs (mm) | 28.3 ± 3.6 |
| IVSd (mm) | 8.6 ± 1.2 |
| LAVI (mL/m2) | 29.3 ± 8.0 |
| E/A ratio | 1.0 ± 0.4 |
| E/e′ ratio | 8.1 ± 2.5 |
| RAP (mmHg) | 5.2 ± 1.1 |
| RVSP (mmHg) | 26.4 ± 7.3 |
| LVMI (g/m2) | 85.0 ± 21.0 |
| Laboratory Data | |
| NT-proBNP (pg/mL) | 88.3 ± 110.1 |
| Medical History | |
| DM, n (%) | 7352 (3.8) |
| HTN, n (%) | 21,948 (11.5) |
| CKD, n (%) | 4359 (2.3) |
| CAD, n (%) | 406 (0.2) |
| PAOD, n (%) | 1228 (0.6) |
| CVA, n (%) | 1743 (0.9) |
| R2 | MAE | RMSE | Pearson Correlation | Difference Age Mean | Difference Age SD | n |
|---|---|---|---|---|---|---|
| 0.783 | 5.023 | 6.389 | 0.885 | −0.057 | 6.389 | 39,990 |
| Age Difference (ECG Age − Chronological Age) | Chronological Age | ECG Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | Min | Max | Mean ± SD | Median | IQR | Q1 (25%) | Q3 (75%) | Mean ± SD | Median | Mean ± SD | Median | n |
| 10–19 | 0.49 | 19.3 | 8.3 ± 3.96 | 8.5 | 5.32 | 5.34 | 10.7 | 18.9 ± 0.25 | 19 | 27.2 ± 3.98 | 27.5 | 177 |
| 20–29 | −7.96 | 28.2 | 6.2 ± 4.67 | 5.81 | 6.21 | 2.99 | 9.2 | 25.4 ± 2.81 | 26 | 31.6 ± 4.88 | 31.2 | 2486 |
| 30–39 | −13.1 | 34.3 | 2.81 ± 5.2 | 2.61 | 6.63 | −0.71 | 5.92 | 35.0 ± 2.8 | 35 | 37.8 ± 5.46 | 37.4 | 5665 |
| 40–49 | −21.1 | 30 | 0.69 ± 5.97 | 0.58 | 7.65 | −3.22 | 4.43 | 45.0 ± 2.87 | 45 | 45.7 ± 6.45 | 45.4 | 8961 |
| 50–59 | −34.7 | 23 | −0.19 ± 6.17 | −0.02 | 8.14 | −4.17 | 3.97 | 54.4 ± 2.83 | 54 | 54.2 ± 6.61 | 54.3 | 11,569 |
| 60–69 | −30.6 | 15.6 | −2.02 ± 5.63 | −1.68 | 7.29 | −5.49 | 1.8 | 63.9 ± 2.78 | 64 | 61.8 ± 5.94 | 62.3 | 7135 |
| 70–79 | −28 | 7.77 | −5.54 ± 4.67 | −5.25 | 6.06 | −8.38 | −2.32 | 73.7 ± 2.74 | 73 | 68.2 ± 4.75 | 68.7 | 3461 |
| 80–89 | −38 | 1.99 | −10.3 ± 4.75 | −9.86 | 4.94 | −12.4 | −7.48 | 81.8 ± 1.58 | 82 | 71.5 ± 4.76 | 71.8 | 536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Song, S.-H.; Park, Y.J.; Lee, Y.-H.; Kim, J.; Jeon, J.; Woo, K.; Kim, J.; Kim, J.Y.; Park, S.-J.; et al. Comparison of Artificial Intelligence–Derived Heart Age with Chronological Age Using Normal Sinus Electrocardiograms in Patients with No Evidence of Cardiac Disease. J. Clin. Med. 2025, 14, 5548. https://doi.org/10.3390/jcm14155548
Kim MJ, Song S-H, Park YJ, Lee Y-H, Kim J, Jeon J, Woo K, Kim J, Kim JY, Park S-J, et al. Comparison of Artificial Intelligence–Derived Heart Age with Chronological Age Using Normal Sinus Electrocardiograms in Patients with No Evidence of Cardiac Disease. Journal of Clinical Medicine. 2025; 14(15):5548. https://doi.org/10.3390/jcm14155548
Chicago/Turabian StyleKim, Myoung Jung, Sung-Hee Song, Young Jun Park, Young-Hyun Lee, Jongwoo Kim, JaeHu Jeon, KyungChang Woo, Juwon Kim, Ju Youn Kim, Seung-Jung Park, and et al. 2025. "Comparison of Artificial Intelligence–Derived Heart Age with Chronological Age Using Normal Sinus Electrocardiograms in Patients with No Evidence of Cardiac Disease" Journal of Clinical Medicine 14, no. 15: 5548. https://doi.org/10.3390/jcm14155548
APA StyleKim, M. J., Song, S.-H., Park, Y. J., Lee, Y.-H., Kim, J., Jeon, J., Woo, K., Kim, J., Kim, J. Y., Park, S.-J., On, Y. K., & Park, K.-M. (2025). Comparison of Artificial Intelligence–Derived Heart Age with Chronological Age Using Normal Sinus Electrocardiograms in Patients with No Evidence of Cardiac Disease. Journal of Clinical Medicine, 14(15), 5548. https://doi.org/10.3390/jcm14155548







