Revisiting Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma: Real-World Evidence of Survival Benefit with First-Line Immunotherapy and Targeted Therapy Regimens
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Temporal Trends in CRN from 2005 to 2024
3.2. Baseline Characteristics
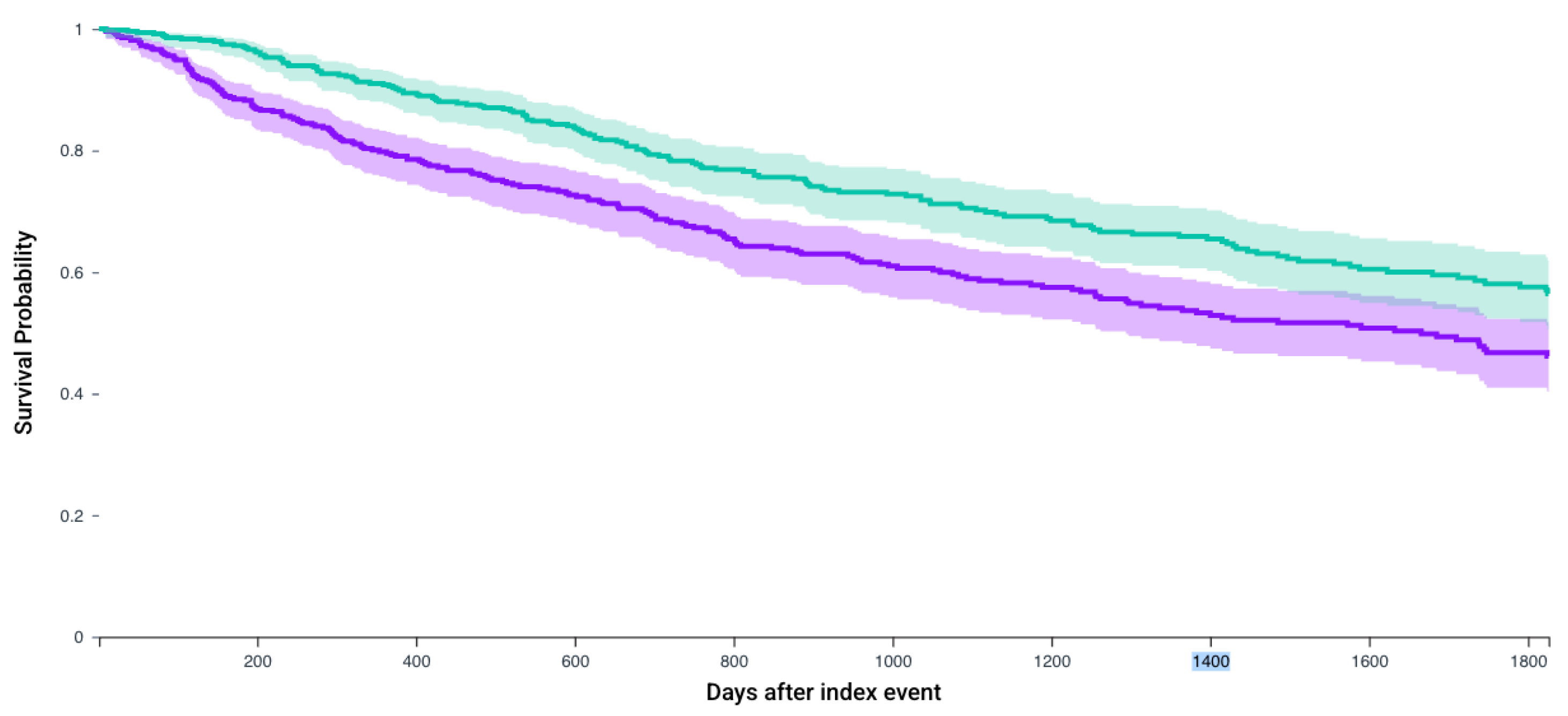
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCC | Renal Cell Carcinoma |
| mRCC | Metastatic Renal Cell Carcinoma |
| ICI | Immune Checkpoint Inhibitors |
| CRN | Cytoreductive Nephrectomy |
| CARMENA | Cancer du Rein Metastatique Nephrectomie et Antiangiogéniques |
| SURTIME | Immediate Surgery or Surgery After Sunitinib Malate in Treating Patients With Metastatic Kidney Cancer |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 and PD-L2 | Programmed cell death protein ligand 1 and 2 |
| CTLA4 | Cytotoxic T-lymphocyte associated protein 4 |
| NCCN | National Comprehensive Cancer Network |
| TKI | Tyrosine Kinase Inhibitors |
| VEGFR | Vascular Endothelial Growth Factor Receptor Inhibitors |
| IMDC | International Metastatic RCC Database Consortium |
| BMI | Body Mass Index |
| HTN | Hypertension |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Gudbjartsson, T.; Thoroddsen, A.; Petursdottir, V.; Hardarson, S.; Magnusson, J.; Einarsson, G.V. Effect of incidental detection for survival of patients with renal cell carcinoma: Results of population-based study of 701 patients. Urology 2005, 66, 1186–1191. [Google Scholar] [CrossRef]
- Palsdottir, H.B.; Hardarson, S.; Petursdottir, V.; Jonsson, A.; Jonsson, E.; Sigurdsson, M.I.; Einarsson, G.V.; Gudbjartsson, T. Incidental detection of renal cell carcinoma is an independent prognostic marker: Results of a long-term, whole population study. J. Urol. 2012, 187, 48–53. [Google Scholar] [CrossRef]
- Patel, H.D.; Gupta, M.; Joice, G.A.; Srivastava, A.; Alam, R.; Allaf, M.E.; Pierorazio, P.M. Clinical Stage Migration and Survival for Renal Cell Carcinoma in the United States. Eur. Urol. Oncol. 2019, 2, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Graves, A.; Hessamodini, H.; Wong, G.; Lim, W.H. Metastatic renal cell carcinoma: Update on epidemiology, genetics, and therapeutic modalities. Immunotargets Ther. 2013, 2, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Dariane, C.; Timsit, M.O.; Mejean, A. Position of cytoreductive nephrectomy in the setting of metastatic renal cell carcinoma patients: Does the CARMENA trial lead to a paradigm shift? Bull. Cancer 2018, 105 (Suppl. S3), S229–S234. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Mulders, P.; Jewett, M.; Wagstaff, J.; van Thienen, J.V.; Blank, C.U.; van Velthoven, R.; del Pilar Laguna, M.; Wood, L.; van Melick, H.H.E.; et al. Comparison of Immediate vs. Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019, 5, 164–170. [Google Scholar] [CrossRef]
- Mejean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.B.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef]
- Rose, T.L.; Kim, W.Y. Renal Cell Carcinoma: A Review. JAMA 2024, 332, 1001–1010. [Google Scholar] [CrossRef]
- Ernst, M.S.; Navani, V.; Wells, J.C.; Donskov, F.; Basappa, N.; Labaki, C.; Pal, S.K.; Meza, L.; Wood, L.A.; Ernst, D.S.; et al. Outcomes for International Metastatic Renal Cell Carcinoma Database Consortium Prognostic Groups in Contemporary First-line Combination Therapies for Metastatic Renal Cell Carcinoma. Eur. Urol. 2023, 84, 109–116. [Google Scholar] [CrossRef]
- Psutka, S.P.; Kim, S.P.; Gross, C.P.; Van Houten, H.; Thompson, R.H.; Abouassaly, R.; Weight, C.; Boorjian, S.A.; Leibovich, B.C.; Shah, N.D. The impact of targeted therapy on management of metastatic renal cell carcinoma: Trends in systemic therapy and cytoreductive nephrectomy utilization. Urology 2015, 85, 442–450. [Google Scholar] [CrossRef]
- Rappold, P.M.; Silagy, A.W.; Kotecha, R.R.; Hakimi, A.A. Immune checkpoint blockade in renal cell carcinoma. J. Surg. Oncol. 2021, 123, 739–750. [Google Scholar] [CrossRef]
- Lee, L.; Cheung, W.Y.; Atkinson, E.; Krzyzanowska, M.K. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J. Clin. Oncol. 2011, 29, 106–117. [Google Scholar] [CrossRef]
- Flanigan, R.C.; Salmon, S.E.; Blumenstein, B.A.; Bearman, S.I.; Roy, V.; McGrath, P.C.; Caton, J.R.J.; Munshi, N.; Crawford, E.D. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 2001, 345, 1655–1659. [Google Scholar] [CrossRef]
- Mickisch, G.H.; Garin, A.; van Poppel, H.; de Prijck, L.; Sylvester, R.; European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet 2001, 358, 966–970. [Google Scholar] [CrossRef]
- Van Praet, C.; Slots, C.; Vasdev, N.; Rottey, S.; Fonteyne, V.; Andras, I.; Albersen, M.; De Meerleer, G.; Bex, A.; Decaestecker, K. Current role of cytoreductive nephrectomy in metastatic renal cell carcinoma. Turk. J. Urol. 2021, 47 (Suppl. S1), S79–S84. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef]
- Zhu, Z.; Jin, Y.; Zhou, J.; Chen, F.; Chen, M.; Gao, Z.; Hu, L.; Xuan, J.; Li, X.; Song, Z.; et al. PD1/PD-L1 blockade in clear cell renal cell carcinoma: Mechanistic insights, clinical efficacy, and future perspectives. Mol. Cancer 2024, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Iisager, L.; Ahrenfeldt, J.; Donskov, F.; Ljungberg, B.; Bex, A.; Lund, L.; Lyskjær, I.; Fristrup, N. Multicenter randomized trial of deferred cytoreductive nephrectomy in synchronous metastatic renal cell carcinoma receiving checkpoint inhibitors: The NORDIC-SUN-Trial. BMC Cancer 2024, 24, 260. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, U.N.; Tangen, C.; Tripathi, A.; Shuch, B.M.; Pal, S.K.; Barata, P.C.; Tan, A.; Zuckerman, P.; Mayerson, E.; Lara, P.L.N.; et al. SWOG S1931 (PROBE): Phase III randomized trial of immune checkpoint inhibitor (ICI) combination regimen with or without cytoreductive nephrectomy (CN) in advanced renal cancer. J. Clin. Oncol. 2022, 40, TPS402. [Google Scholar] [CrossRef]
- Hanna, N.; Sun, M.; Meyer, C.P.; Nguyen, P.L.; Pal, S.K.; Chang, S.L.; de Velasco, G.; Trinh, Q.-D.; Choueiri, T.K. Survival Analyses of Patients With Metastatic Renal Cancer Treated With Targeted Therapy With or Without Cytoreductive Nephrectomy: A National Cancer Data Base Study. J. Clin. Oncol. 2016, 34, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Bakouny, Z.; El Zarif, T.; Dudani, S.; Wells, J.C.; Gan, C.L.; Donskov, F.; Shapiro, J.; Davis, I.D.; Parnis, F.; Ravi, P.; et al. Upfront Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors or Targeted Therapy: An Observational Study from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. 2023, 83, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Hutchinson, R.C.; Ghandour, R.A.; Freifeld, Y.; Fang, D.; Sagalowsky, A.I.; Lotan, Y.; Bagrodia, A.; Margulis, V.; Hammers, H.J.; et al. Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: An analysis of the National Cancer Database. Urol. Oncol. 2020, 38, 604.e9–604.e17. [Google Scholar] [CrossRef]
- Zerdan, M.B.; Niforatos, S.; Arunachalam, S.; Jamaspishvili, T.; Wong, R.; Bratslavsky, G.; Jacob, J.; Ross, J.; Shapiro, O.; Goldberg, H.; et al. Role of Cytoreductive Nephrectomy in Metastatic Clear Cell Renal cell Carcinoma in the Era of immunotherapy: An Analysis of the National Cancer Database. Clin. Genitourin. Cancer 2024, 22, 102193. [Google Scholar] [CrossRef]
- Tannir, N.M.; Albiges, L.; McDermott, D.F.; Burotto, M.; Choueiri, T.K.; Hammers, H.J.; Barthélémy, P.; Plimack, E.; Porta, C.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 8-year follow-up results of efficacy and safety from the phase III CheckMate 214 trial. Ann. Oncol. 2024, 35, 1026–1038. [Google Scholar] [CrossRef]
- Miao, D.; Margolis, C.A.; Gao, W.; Voss, M.H.; Li, W.; Martini, D.J.; Norton, C.; Bossé, D.; Wankowicz, S.M.; Cullen, D.; et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018, 359, 801–806. [Google Scholar] [CrossRef]
- Au, L.; Hatipoglu, E.; Robert de Massy, M.; Litchfield, K.; Beattie, G.; Rowan, A.; Schnidrig, D.; Thompson, R.; Byrne, F.; Horswell, S.; et al. Determinants of anti-PD-1 response and resistance in clear cell renal cell carcinoma. Cancer Cell 2021, 39, 1497–1518.e11. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]

| Before Matching | |||
|---|---|---|---|
| Parameter | No CRN 5072 cases | CRN 888 cases | p-value |
| Age at index, years Mean, SD | 63.8, 11.1 | 61.5, 10.3 | <0.0001 |
| Gender Male Female | 3529, 69.5% 1543, 30.4% | 608, 68.4% 280, 31.5% | 0.50 0.44 |
| Race White Black Asian American Indian or Alaska Native Others | 4177, 82.3% 546, 10.7% 273, 5.3% 52, 1.0% 24, 0.4% | 714, 80.4% 81, 9.1% 55, 6.2% 20, 2.3% 18, 2.0% | 0.18 0.01 0.42 0.24 0.17 |
| Ethnicity Non-Hispanic/Latino Hispanic/Latino Unknown | 3719, 73.3% 398, 7.8% 955, 18.8% | 674, 75.9% 84, 9.5% 130, 14.6% | 0.10 0.10 0.002 |
| BMI [kg/m2], Mean, SD | 30, 6.9 | 29.1, 6.2 | 0.001 |
| Creatinine (Mass/volume), Mean, SD | 1.35, 0.9 | 1.08, 0.5 | <0.0001 |
| Hemoglobin (Mass/volume), Mean, SD | 12.7, 2.3 | 12.2, 2.3 | <0.0001 |
| Leukocyte count cells/mm3, Mean, SD | 9820, 1910 | 7890, 3110 | 0.35 |
| Platelet count in lakhs/mm3, Mean, SD | 268, 111 | 295, 113 | <0.0001 |
| Calcium (Mass/volume), Mean, SD | 9.39, 0.7 | 9.43, 0.7 | 0.15 |
| HTN disease I10-I15 | 2738, 53.9% | 552, 62.2% | <0.0001 |
| Ischemic heart disease I20-25 | 941, 18.5% | 200, 22.5% | 0.005 |
| Liver diseases, K70-77 | 837, 16.5% | 180, 20.3% | 0.005 |
| Chronic lower respiratory tract diseases, J40-J4A | 858, 16.9% | 178, 20% | 0.02 |
| Chronic kidney disease, N18 | 1138, 22.4% | 149, 16.7% | 0.0002 |
| Cerebrovascular disorders, I60-I69 | 430, 8.5% | 87, 9.8% | 0.19 |
| Diabetes mellitus, E08-E13 | 1210, 23.9% | 230, 26% | 0.18 |
| Overweight and obesity, E65-E68 | 1135, 22.4% | 221, 24.8% | 0.09 |
| Tobacco use, Z72.0 | 251, 4.9% | 44, 5% | 0.99 |
| After Matching | |||
|---|---|---|---|
| Parameter | No CRN 888 cases | CRN 888 cases | p-value |
| Age at index, years Mean, SD | 62.2, 11.7 | 61.5, 10.3 | 0.17 |
| Gender Male Female | 648, 72.9% 240, 27% | 646, 72.7% 242, 27.3% | 0.91 0.91 |
| Race White Black Asian American Indian or Alaska Native Others | 726, 81.8% 96, 10.8% 32, 3.6% 10, 1.1% 24, 2.7% | 714, 80.4% 81, 9.1% 55, 6.2% 20, 2.3% 18, 2% | 0.46 0.28 0.7 0.48 0.85 |
| Ethnicity Non-Hispanic/Latino Hispanic/Latino Unknown | 697, 78.5% 73, 8.2% 118, 13.3% | 674, 76% 84, 9.4% 130, 14.6% | 0.19 0.35 0.41 |
| BMI [kg/m2], Mean, SD | 29.9, 7.0 | 29.1,6.2 | 0.01 |
| Creatinine (Mass/volume), Mean, SD | 1.27, 0.7 | 1.08, 0.5 | 0.0001 |
| Hemoglobin (Mass/volume), Mean, SD | 12.7, 2.3 | 12.2, 2.3 | 0.0002 |
| Leukocyte count cells/mm3, Mean, SD | 8820, 2410 | 7890, 3110 | 0.38 |
| Platelet count in lakhs/mm3, Mean, SD | 266,107 | 295, 113 | <0.0001 |
| Calcium (Mass/volume), Mean, SD | 9.39, 0.9 | 9.43, 0.7 | 0.38 |
| HTN disease I10-I15 | 554, 62.4% | 552, 62.2% | 0.92 |
| Ischemic heart disease I20-25 | 184, 20.7% | 200, 22.5% | 0.35 |
| Liver diseases, K70-77 | 161, 18.1% | 180, 20.3% | 0.25 |
| Chronic lower respiratory tract diseases, J40-J4A | 181, 20.4% | 178, 20.1% | 0.86 |
| Chronic kidney disease, N18 | 153, 17.2% | 149, 16.8% | 0.80 |
| Cerebrovascular disorders, I60-I69 | 88, 9.9% | 87, 9.8% | 0.93 |
| Diabetes Mellitus, E08-E13 | 221, 25.1% | 230, 25.9% | 0.62 |
| Overweight and obesity, E65-E68 | 212, 23.8% | 221, 24.8% | 0.62 |
| Tobacco use, Z72.0 | 42, 4.7% | 44, 5.0% | 0.82 |
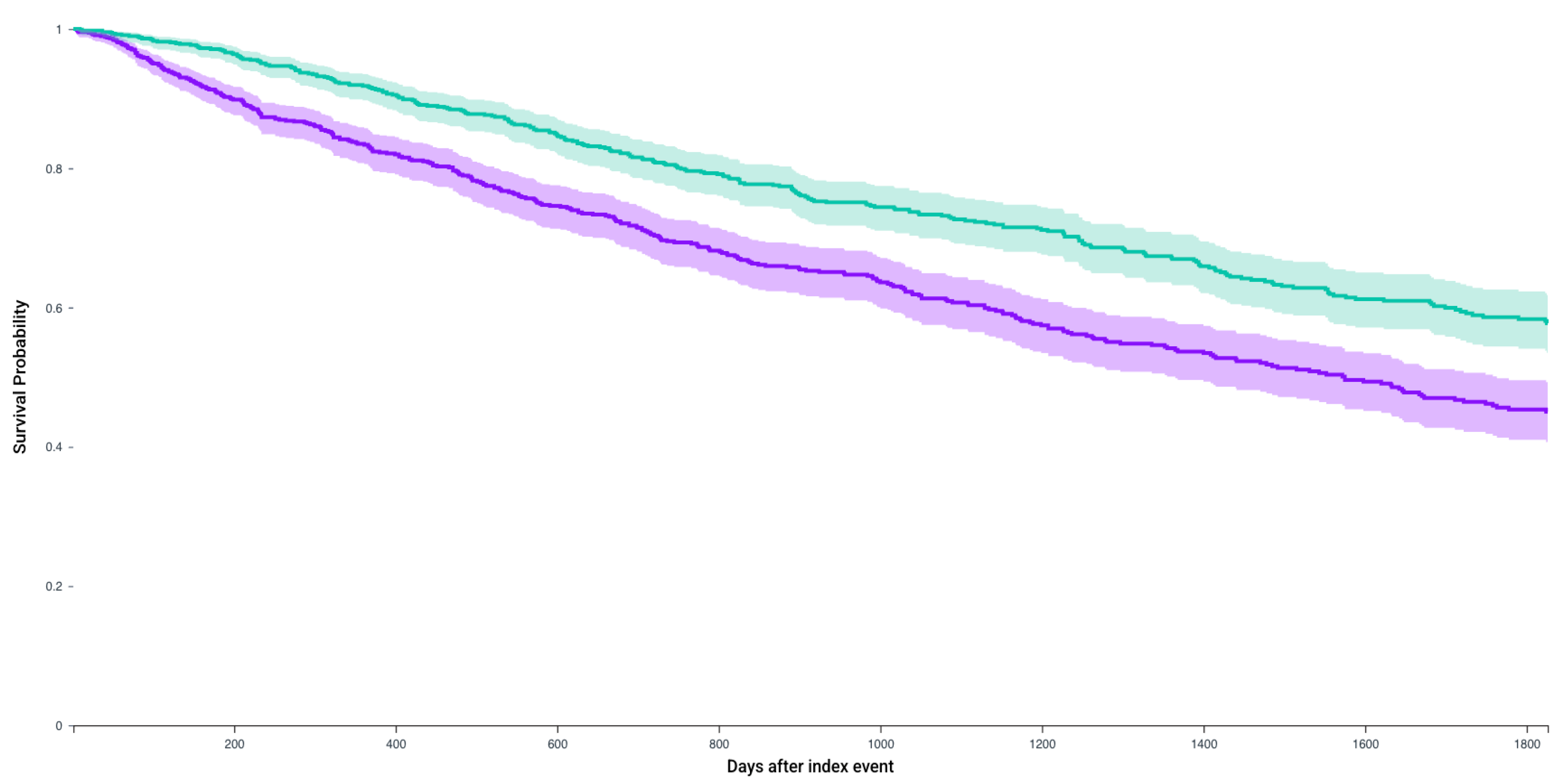
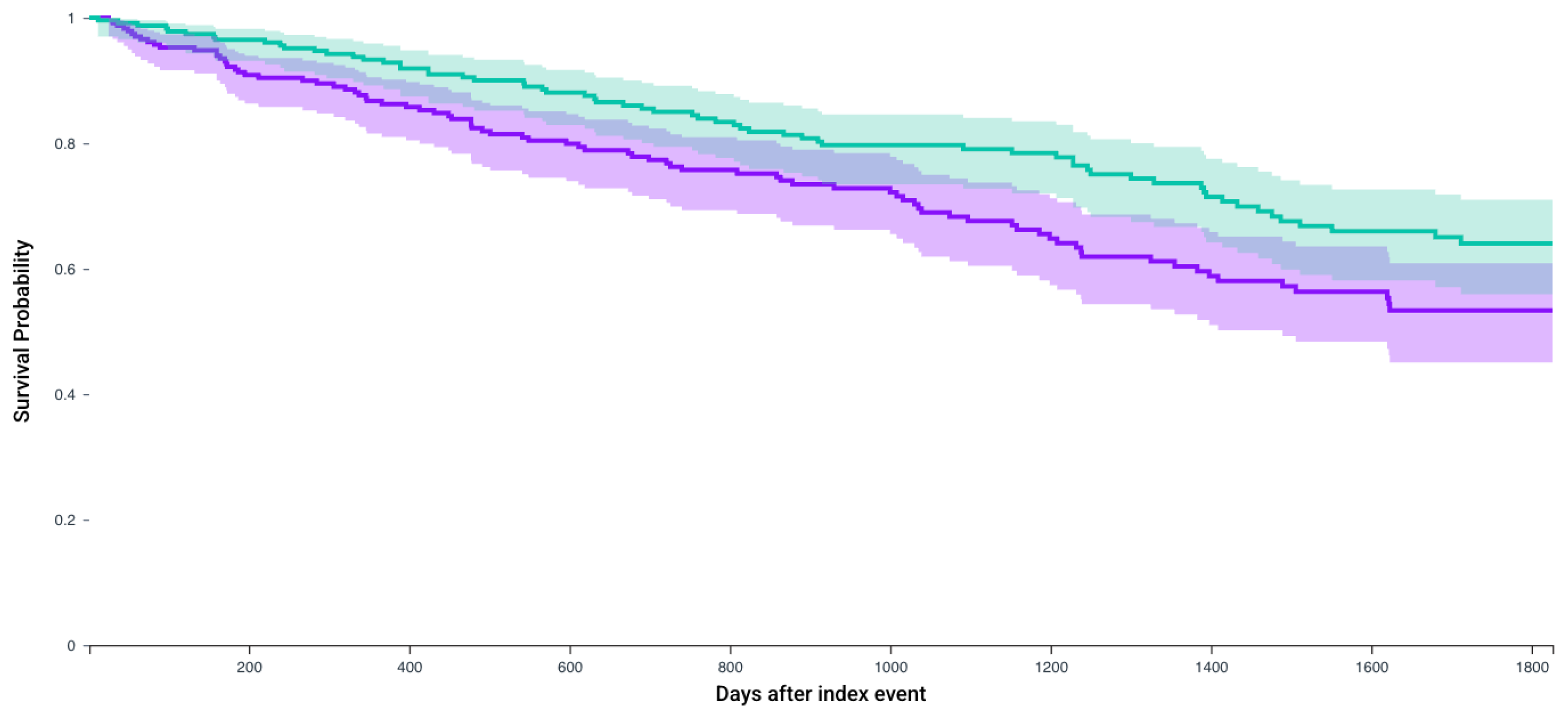
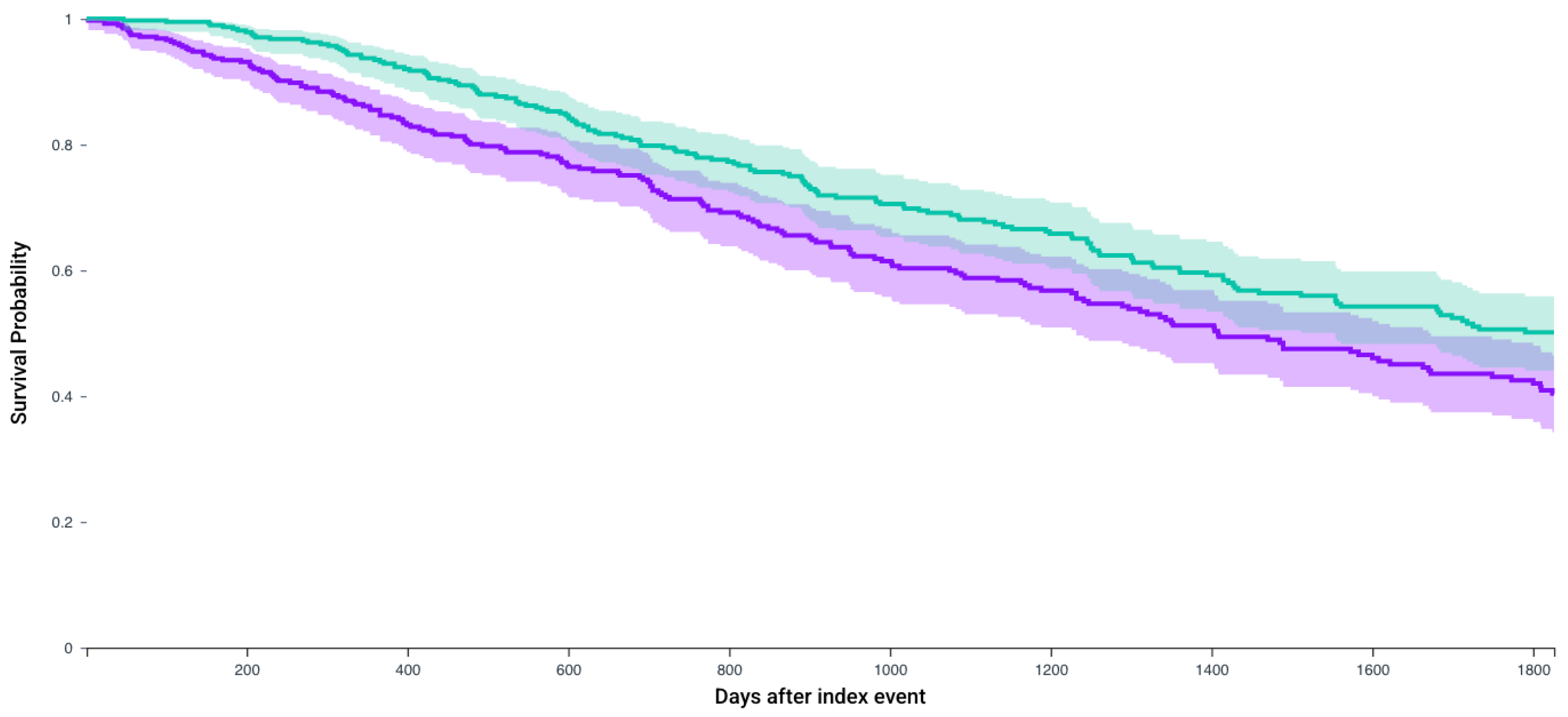
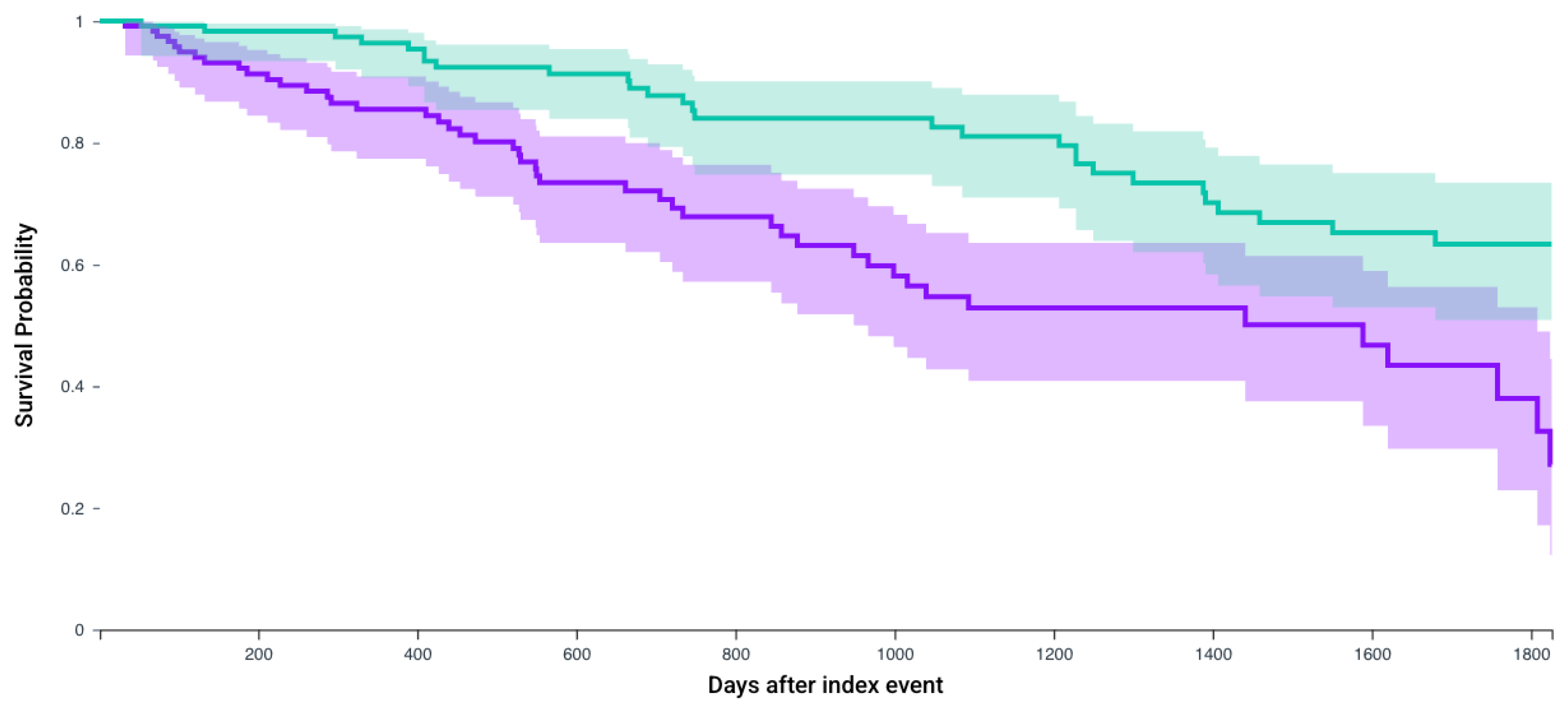
| N, In Each Cohort | No CRN Survival Probability, % (Mean Follow-Up in Days) | CRN Survival Probability, % (Mean Follow-Up in Days) | Hazard Ratio, 95% CI | p-Value | |
|---|---|---|---|---|---|
| Patients who receive any of the first-line regimens | 888 | 45.0% (729.5 days) | 57.7% (887 days) | 1.56 (1.33–1.83) | <0.0001 |
| Patients who receive Axitinib and Pembrolizumab | 235 | 53.3% (743.4 days) | 64% (857.3 days) | 1.51 (1.08–2.09) | 0.01 |
| Patients who receive Cabozantanib and Nivolumab | 387 | 40.4% (642.1 days) | 50.1% (672 days) | 1.37 (1.10–1.71) | 0.004 |
| Patients who receive Lenvatinib and Pembrolizumab | 123 | 22.8% (470.9 days) | 37.4% (561.5 days) | 2.50 (1.55–4.03) | 0.012 |
| Patients who receive Nivolumab and Ipilimumab | 479 | 46.1% (748.4 days) | 56.4% (844.2 days) | 1.51 (1.22–1.87) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manivasagam, S.S.; Aminsharifi, A.; Raman, J.D. Revisiting Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma: Real-World Evidence of Survival Benefit with First-Line Immunotherapy and Targeted Therapy Regimens. J. Clin. Med. 2025, 14, 5543. https://doi.org/10.3390/jcm14155543
Manivasagam SS, Aminsharifi A, Raman JD. Revisiting Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma: Real-World Evidence of Survival Benefit with First-Line Immunotherapy and Targeted Therapy Regimens. Journal of Clinical Medicine. 2025; 14(15):5543. https://doi.org/10.3390/jcm14155543
Chicago/Turabian StyleManivasagam, Sri Saran, Alireza Aminsharifi, and Jay D. Raman. 2025. "Revisiting Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma: Real-World Evidence of Survival Benefit with First-Line Immunotherapy and Targeted Therapy Regimens" Journal of Clinical Medicine 14, no. 15: 5543. https://doi.org/10.3390/jcm14155543
APA StyleManivasagam, S. S., Aminsharifi, A., & Raman, J. D. (2025). Revisiting Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma: Real-World Evidence of Survival Benefit with First-Line Immunotherapy and Targeted Therapy Regimens. Journal of Clinical Medicine, 14(15), 5543. https://doi.org/10.3390/jcm14155543




