The Role of Vestibular Physical Therapy in Managing Persistent Postural-Perceptual Dizziness: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
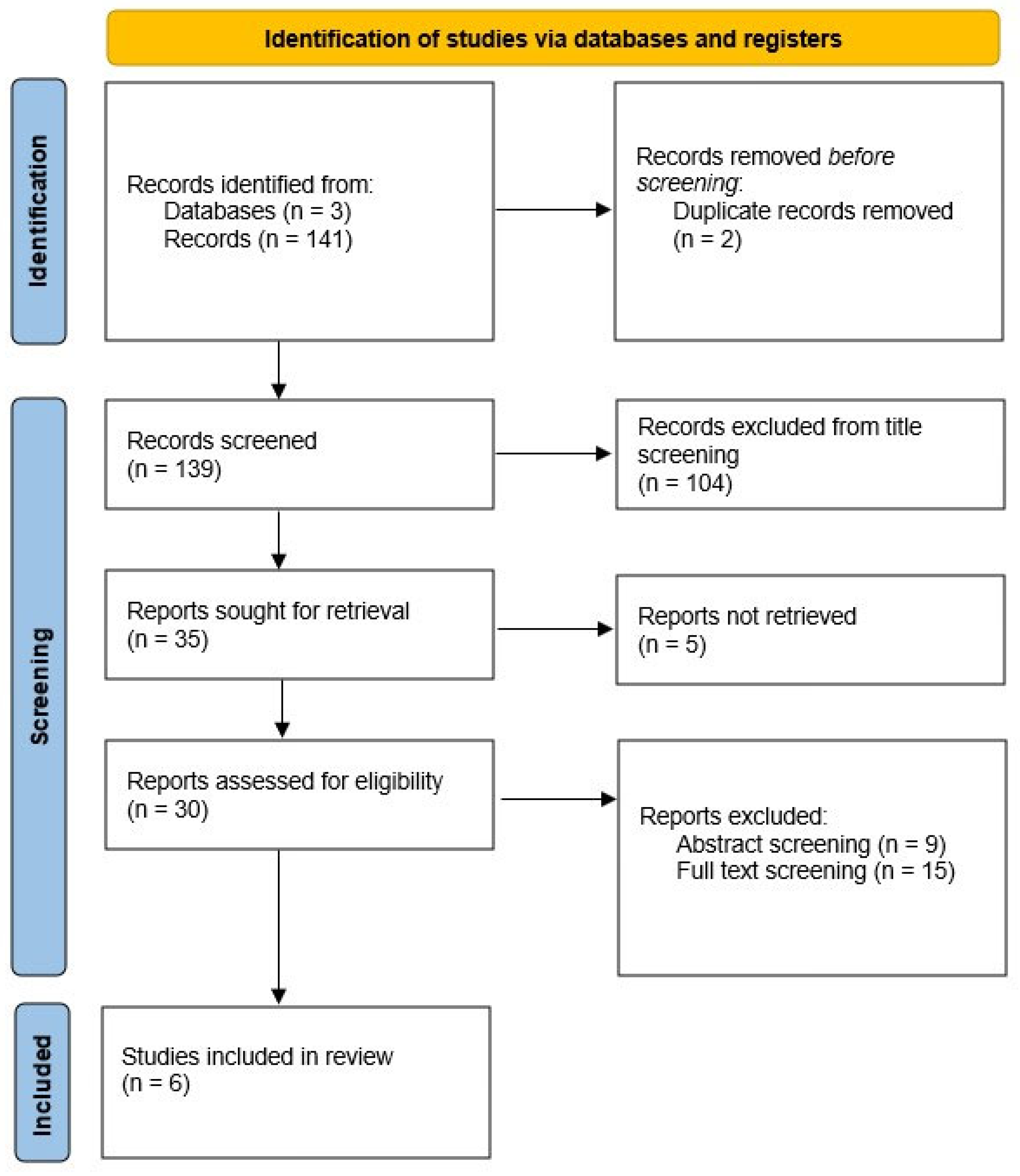
2.2. Study Selection and Data Collection Process
2.3. Risk of Bias
2.4. Meta-Analysis
2.5. PICO Question
3. Results
3.1. Risk of Bias
3.2. Results of Meta-Analysis of DHI
3.3. Adverse Events and Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPPD | Persistent Postural-Perceptual Dizziness |
| VPT | Vestibular Physical Therapy |
| DHI | Dizziness Handicap Inventory |
| VEMPs | Vestibular Evoked Myogenic Potentials |
| vHIT | Video Head Impulse Test |
Appendix A
References
- Staab, J.P.; Eckhardt-Henn, A.; Horii, A.; Jacob, R.; Strupp, M.; Brandt, T.; Bronstein, A. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J. Vestib. Res. 2017, 27, 191–208. [Google Scholar] [CrossRef]
- Staab, J.P. Persistent postural-perceptual dizziness: Review and update on key mechanisms of the most common functional neuro-otologic disorder. Neurol. Clin. 2023, 41, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Harrington-Benton, N.A.; Judd, O.; Kaski, D.; Maarsingh, O.R.; MacKeith, S.; Ray, J.; Van Vugt, V.A.; Burton, M.J. Pharmacological interventions for persistent postural-perceptual dizziness (PPPD). Cochrane Database Syst. Rev. 2023, 3, CD015188. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, K.; Shikino, K.; Yamauchi, Y.; Yanagita, Y.; Yokokawa, D.; Ikegami, A.; Tsukamoto, T.; Noda, K.; Uehara, T.; Ikusaka, M. The clinical key features of persistent postural perceptual dizziness in the general medicine outpatient setting: A case series study of 33 patients. Intern. Med. 2020, 59, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Dlugaiczyk, J.; Ertl-Wagner, B.B.; Rujescu, D.; Westhofen, M.; Dieterich, M. Vestibular disorders—Diagnosis, new classification and treatment. Dtsch. Arztebl. Int. 2020, 117, 300–310. [Google Scholar]
- Staibano, P.; Lelli, D.; Tse, D. A retrospective analysis of two tertiary care dizziness clinics: A multidisciplinary chronic dizziness clinic and an acute dizziness clinic. J. Otolaryngol. Head. Neck Surg. 2019, 48, 11. [Google Scholar] [CrossRef]
- Dieterich, M.; Staab, J.P.; Brandt, T. Functional (psychogenic) dizziness. Handb. Clin. Neurol. 2016, 139, 447–468. [Google Scholar]
- Indovina, I.; Passamonti, L.; Mucci, V.; Chiarella, G.; Lacquaniti, F.; Staab, J.P. Brain correlates of persistent postural-perceptual dizziness: A review of neuroimaging studies. J. Clin. Med. 2021, 10, 4274. [Google Scholar] [CrossRef]
- Riccelli, R.; Passamonti, L.; Toschi, N.; Nigro, S.; Chiarella, G.; Petrolo, C.; Lacquaniti, F.; Staab, J.P.; Indovina, I. Altered insular and occipital responses to simulated vertical self-motion in patients with persistent postural-perceptual dizziness. Front. Neurol. 2017, 8, 529. [Google Scholar] [CrossRef]
- Popkirov, S.; Staab, J.P.; Stone, J. Persistent postural-perceptual dizziness (PPPD): A common, characteristic and treatable cause of chronic dizziness. Pract. Neurol. 2018, 18, 5–13. [Google Scholar] [CrossRef]
- Axer, H.; Finn, S.; Wassermann, A.; Guntinas-Lichius, O.; Klingner, C.M.; Witte, O.W. Multimodal treatment of persistent postural-perceptual dizziness. Brain Behav. 2020, 10, e01864. [Google Scholar] [CrossRef]
- Sulway, S.; Whitney, S.L. Advances in vestibular rehabilitation. Adv. Otorhinolaryngol. 2019, 82, 164–169. [Google Scholar]
- Whitney, S.L.; Sparto, P.J. Principles of vestibular physical therapy rehabilitation. NeuroRehabilitation 2011, 29, 157–166. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, D.; Tian, E.; Wang, J.; Guo, Z.; Kong, W. Central vestibular dysfunction: Don’t forget vestibular rehabilitation. Expert Rev. Neurother. 2022, 22, 669–680. [Google Scholar] [CrossRef]
- Webster, K.E.; Kamo, T.; Smith, L.; Harrington-Benton, N.A.; Judd, O.; Kaski, D.; Maarsingh, O.R.; MacKeith, S.; Ray, J.; Van Vugt, V.A.; et al. Non-pharmacological interventions for persistent postural-perceptual dizziness (PPPD). Cochrane Database Syst. Rev. 2023, 3, CD015333. [Google Scholar]
- Tramontano, M.; Princi, A.A.; De Angelis, S.; Indovina, I.; Manzari, L. Vestibular rehabilitation in patients with persistent postural-perceptual dizziness: A scoping review. Hear. Balance Commun. 2021, 19, 282–290. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.5 (updated August 2024); Cochrane: London, UK, 2024. [Google Scholar]
- Sherrington, C.; Herbert, R.D.; Maher, C.G.; Moseley, A.M. PEDro: A database of randomized trials and systematic reviews in physiotherapy. Man. Ther. 2000, 5, 223–226. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Choi, S.Y.; Choi, J.H.; Oh, E.H.; Oh, S.-J.; Choi, K.-D. Effect of vestibular exercise and optokinetic stimulation using virtual reality in persistent postural-perceptual dizziness. Sci. Rep. 2021, 11, 14437. [Google Scholar] [CrossRef]
- Eldøen, G.; Kvalheim, S.E.; Thesen, T.; Mygland, Å.; Ljøstad, U.; Bakke, S.; Holo, M.H.; Løge, I.; Jonsbu, E. Web-based vestibular rehabilitation in persistent postural-perceptual dizziness. Brain Behav. 2021, 11, e2346. [Google Scholar] [CrossRef]
- Herdman, D.; Norton, S.; Murdin, L.; Frost, K.; Pavlou, M.; Moss-Morris, R. The INVEST trial: A randomised feasibility trial of psychologically informed vestibular rehabilitation versus current gold standard physiotherapy for people with persistent postural perceptual dizziness. J. Neurol. 2022, 269, 4753–4763. [Google Scholar] [CrossRef]
- Ibrahim, N.M.K.; Hazza, N.M.A.; Yaseen, D.M.; Galal, E.M. Effect of vestibular rehabilitation games in patients with persistent postural perceptual dizziness and its relation to anxiety and depression: Prospective study. Eur. Arch. Otorhinolaryngol. 2024, 281, 2861–2869. [Google Scholar] [CrossRef]
- Kuwabara, J.; Kondo, M.; Kabaya, K.; Watanabe, W.; Shiraishi, N.; Sakai, M.; Toshishige, Y.; Ino, K.; Nakayama, M.; Iwasaki, S.; et al. Acceptance and commitment therapy combined with vestibular rehabilitation for persistent postural-perceptual dizziness: A pilot study. Am. J. Otolaryngol. 2020, 41, 102609. [Google Scholar] [CrossRef] [PubMed]
- Nada, E.H.; Ibraheem, O.A.; Hassaan, M.R. Vestibular rehabilitation therapy outcomes in patients with persistent postural-perceptual dizziness. Ann. Otol. Rhinol. Laryngol. 2019, 128, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liang, Q.; Yuan, J.; Li, S.; Ge, Y.; Yang, J.; Tsang, R.C.C.; Wei, Q. Vestibular rehabilitation therapy on balance and gait in patients after stroke: A systematic review and meta-analysis. BMC Med. 2023, 21, 322. [Google Scholar] [CrossRef] [PubMed]
- Alashram, A.R. Effects of Cawthorne-Cooksey exercises on vestibular symptoms: A systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2024, 39, 132–141. [Google Scholar] [CrossRef]
- Tramontano, M.; Paolocci, G.; Piatti, D.; Attanasio, G.; Conti, L.C.; Bergamini, E.; Manzari, L.; Lacquaniti, F.; Staab, J.P.; Bosco, G.; et al. Dynamic postural stability, symmetry, and smoothness of gait in patients with persistent postural-perceptual dizziness. J. Vestib. Res. 2025, 35, 82–90. [Google Scholar] [CrossRef]
- Fife, T.D.; Colebatch, J.G.; Kerber, K.A.; Brantberg, K.; Strupp, M.; Lee, H.; Walker, M.F.; Ashman, E.; Fletcher, J.; Callaghan, B.; et al. Practice guideline: Cervical and ocular vestibular evoked myogenic potential testing: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2017, 89, 2288–2296. [Google Scholar] [CrossRef]

| Authors, Year | Study Design | Participants | Intervention | Comparator | Outcomes | Conclusion |
|---|---|---|---|---|---|---|
| Choi, 2021 [21] | RCT | VPT+OS: 15 (8F) VPT: 13 (8F) | VPT with virtual reality, with or without optokinetic stimulation (OS), 20 min/week for 4 weeks | VPT using a virtual reality system | DHI, ADL, VVAS, Beck Anxiety Inventory, TUG test, Computerized Dynamic Posturography, Simulator Sickness Questionnaire | VPT group improved in all outcomes measures, where VPT+OS only improved in ADL and TUG. |
| Eldøen, 2021 [22] | Descriptive feasibility study | 10 patients (1 dropout) | 6-week web-based VPT program with daily exercises and educational content | NA | VSS, Niigata PPPD Questionnaire, PHQ-9, EQ VAS, semi-structured interview | Web-based VPT rehabilitation is a feasible and possibly effective treatment in PPPD patients. |
| Herdman, 2022 [23] | RCT | INVEST: 20 (16F) VPT: 20 (16F) | Six sessions of individual CBT-informed VPT (INVEST). Exercises were customized and focused on normalizing any maladaptive postural strategies early on and habituation | Standard VPT customized exercise program, performed in the clinic and at home, which included a range of general exercises (e.g., walking programs) and more specific adaptation, habituation, visual desensitization, static and dynamic balance exercises | DHI, VVAS, %TSI, EQ5D, B-IPQ, CBRQ, PHQ-9, GAD-7, PHQ-ADS, mini-BESTest | The CBT-informed VPT (INVEST) produced slightly better effects than traditional VPT, both in terms of average reduction in DHI and adherence to the treatment. |
| Ibrahim, 2024 [24] | Prospective study | 30 patients | Clinic-based VPT (3×/week for 6 weeks) Home-based VPT: (15 min twice daily for 6 weeks: VOR X1 and walk with head movement exercises) | NA | HADS; DHI; SOT | VPT can reduce symptoms in patients with PPPD. |
| Kuwabara, 2020 [25] | Pilot study | 27 patients | Combined ACT + VPT program. Patients were encouraged to conduct sets of nodding and head-shaking exercises (eyes open, eyes closed, fixating finger) | NA | DHI VSS HADS AAQ-II FFMQ | ACT combined with VPT for PPPD is a promising treatment with good feasibility. |
| Nada, 2019 [26] | RCT | VPT:30 (19 F) Control group: 30 (17 F) | VPT (gaze stability and walking exercises) | VPT+placebo integration | DHI | Customized home-based VPT suited to the patient’s aggravating factors is effective in PPPD management. |
| First Author, Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nada, 2019 [26] | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 4 |
| Choi, 2021 [21] | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Herdman, 2022 [23] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piatti, D.; De Angelis, S.; Paolocci, G.; Minnetti, A.; Manzari, L.; Verdecchia, D.H.; Indovina, I.; Tramontano, M. The Role of Vestibular Physical Therapy in Managing Persistent Postural-Perceptual Dizziness: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 5524. https://doi.org/10.3390/jcm14155524
Piatti D, De Angelis S, Paolocci G, Minnetti A, Manzari L, Verdecchia DH, Indovina I, Tramontano M. The Role of Vestibular Physical Therapy in Managing Persistent Postural-Perceptual Dizziness: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(15):5524. https://doi.org/10.3390/jcm14155524
Chicago/Turabian StylePiatti, Diego, Sara De Angelis, Gianluca Paolocci, Andrea Minnetti, Leonardo Manzari, Daniel Hector Verdecchia, Iole Indovina, and Marco Tramontano. 2025. "The Role of Vestibular Physical Therapy in Managing Persistent Postural-Perceptual Dizziness: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 15: 5524. https://doi.org/10.3390/jcm14155524
APA StylePiatti, D., De Angelis, S., Paolocci, G., Minnetti, A., Manzari, L., Verdecchia, D. H., Indovina, I., & Tramontano, M. (2025). The Role of Vestibular Physical Therapy in Managing Persistent Postural-Perceptual Dizziness: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(15), 5524. https://doi.org/10.3390/jcm14155524








