Comparative Effectiveness of Cognitive Behavioral Therapies in Schizophrenia and Schizoaffective Disorder: A Systematic Review and Meta-Regression Analysis
Abstract
1. Introduction
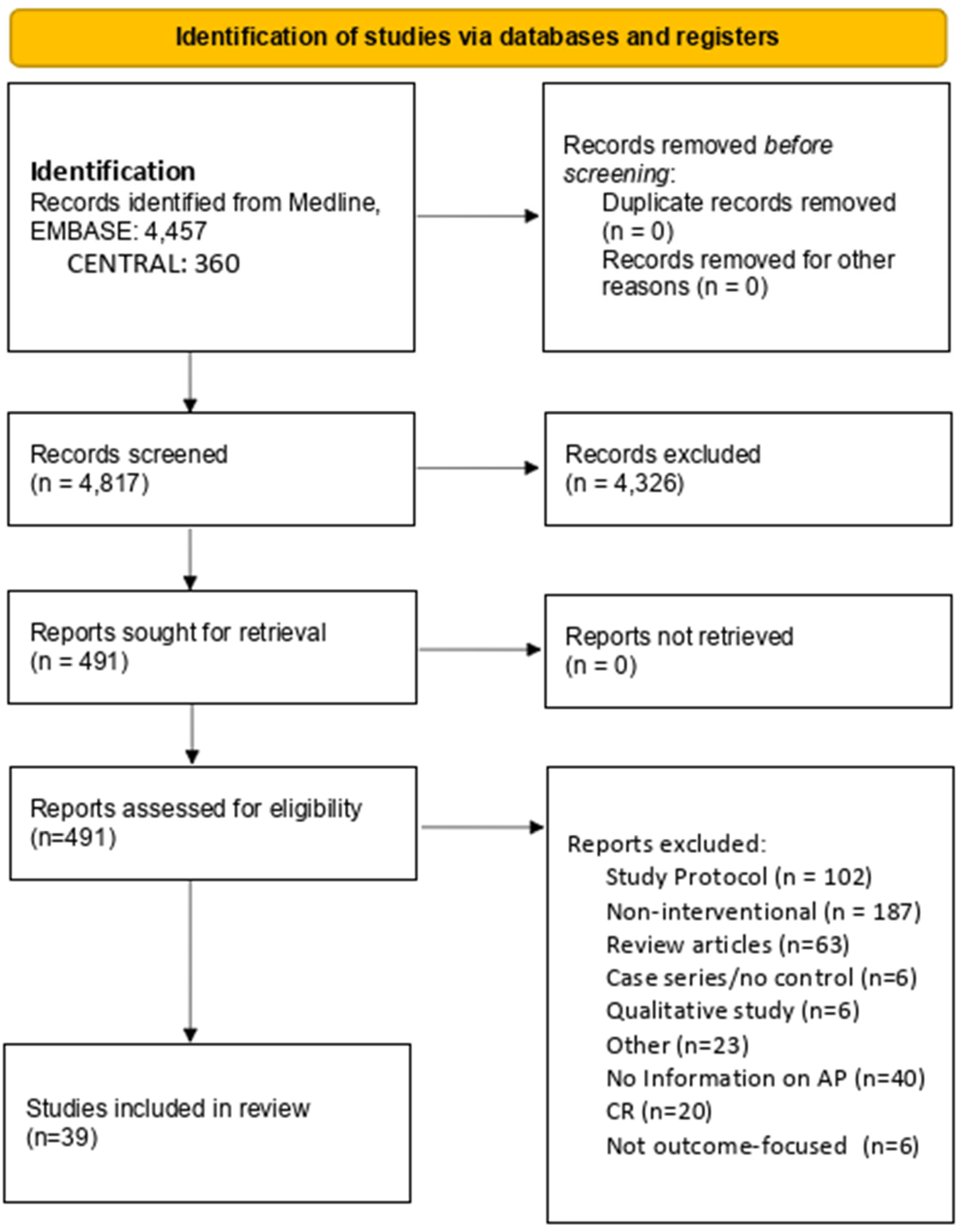
2. Methods
3. Results
| PMID | Author | Year | Country | Sample Size (AP) | Treatment and Control | Design Notes | Mean (SD) Age | Sex (% F) | Education |
|---|---|---|---|---|---|---|---|---|---|
| 16648530 | Baker, A. [53] | 2006 | Australia | 49/55 (15) | CBT, TAU | Substance use | 28.12 | 0.22 | |
| 30376124 | Balzan, R.P. [67] | 2019 | Australia | 27/27 (6/5) | MCT, TAU | PANSS General proxy for depression | 37.21 (8.74) | 0.41 | 11.41 years (2) |
| 21106618 | Barrowclough, C. [47] | 2010 | UK | 129/118 (13/14) | CBT, TAU | 24-month follow-up used | 37.9 (9.7) | 0.14 | 16.1 (1.8) left education |
| 38908265 | Chien, W.T. [62] | 2024 | Hong Kong | 42/42 (8/7) | AIM-AT, TAU | Early-stage psychosis | 27.1 (6.4) | 0.31 | 0.24 university |
| 33580033 | Dellazizzo, L. [68] | 2021 | Canada | 37/37 (9/8) | VRT, CBT | Both arms active treatment | 42.5 (12.7) | 0.24 | 12.2 (3.6) |
| 18851771 | Farhall, J. [69] | 2009 | Australia | 45/49 (1/6) | CBT, TAU | Second follow-up used | 32.9 (10.2) | 0.41 | 0.12 |
| 37716893 | Farrelly, S. [70] | 2024 | UK | 11/10 (3/2) | CBT, TAU | Psychotic depression and BD in AP | 40.5 (13.1) | 0.29 | 0.24 university |
| 24176646 | Favrod, J. [71] | 2014 | Switzerland | 26/26 (5/4) | MCT, TAU | 36.7 (10.1) | 0.35 | 0.12 post-secondary | |
| 34246324 | Freeman, D. [72] | 2021 | UK | 66/64 (13/11) | CBT, Befriending | 41.6 (12.1) | 0.40 | ||
| 25468186 | Freeman, D. [36] | 2014 | UK | 15/15 (2/4) | CBT, TAU | 12-week follow-up used | 41.7 (12.3) | 0.33 | |
| 26360083 | Freeman, D. [42] | 2015 | UK | 73/77 (5/6) | CBT, TAU | Six sessions | 41.5 (11.5) | 0.43 | |
| 33825827 | Garety, P. [50] | 2021 | UK | 161/171 (30/34) | CBT, TAU | 24-week follow-up used | 42.6 (11.6) | 0.30 | 0.24 post-secondary |
| 26352221 | Gaudiano, B.A. [58] | 2015 | USA | 6/7 (1/1) | ACT, TAU | 50 (17.0) | 0.54 | 14 (2.5) | |
| 15893293 | Gaudiano, B.A. [51] | 2006 | USA | 19/21 (5) | ACT, TAU | 40 (10) | 0.39 | 0.17 post-secondary | |
| 37716204 | Gaudiano, B.A. [61] | 2023 | USA | 23/23 (13/11) | ACT, TAU | Inpatients | 40.03 (11.63) | 0.48 | 11.5 (5.2) |
| 22130905 | Gleeson, J.F. [48] | 2013 | Australia | 41/40 (6/2) | rpCBT, TAU | BPRS used for proxy | 20.1 (3.1) | 0.37 | 12.0 (2) |
| 18005494 | Jackson, H.J. [63] | 2008 | Australia | 31/31 (10/10) | CBT, Befriending | BD and MDD in AP | 22.3 (3.6) | 0.27 | 0.13 in occupation |
| 39610049 | Katsushima, M. [49] | 2025 | Japan | 12/12 (2/2) | CBT, TAU | Seven sessions | 33.5 (10.8) | 0.58 | |
| 23635846 | Kråkvik, B. [66] | 2013 | Norway | 23/22 (1/1) | CBT, TAU | 36.4 (10) | 0.36 | ||
| 35485835 | Lepage, M. [54] | 2023 | Canada | 30/21 (11) | CBT, CR | SOFAS as proxy for depression | 24.6 (4.4) | 0.34 | 12 years (2.2) |
| 22663901 | Lincoln, T.M. [28] | 2012 | Germany | 40/40 (6/7) | CBT, WL | ||||
| 32994792 | López-Navarro, E. [73] | 2020 | Spain | 26/26 (6/5) | MBT, IRT | 39.70 (9) | 0.21 | 12 years (2) | |
| 30318868 | MacDougall, A.G. [55] | 2019 | Canada | 9/8 (1) | MAP, TAU | 23.7 (NA) | 0.24 | 0.06 post-secondary | |
| 30001930 | Morrison, A.P. [74] | 2018 | UK | 242/245 (28/20) | CBT, TAU | 42.5 (10.6) | 0.28 | 30/11/2000 | |
| 27092862 | Morrison, A.P. [52] | 2016 | UK | 15/14 (1) | CT, TAU | 34.3 (13.3) | 0.21 | ||
| 24508320 | Morrison, A.P. [23] | 2014 | UK | 37/37 (2) | CT, TAU | 31.3 (12.5) | 0.47 | ||
| 28828697 | Pos, K. [75] | 2018 | Netherlands | 19/14 (2/1) | MCT, OT | 23.3 (3.6) | 0.20 | higher secondary school most prevalent | |
| 27979820 | Shawyer, F. [60] | 2017 | Australia | 49/47 (14/9) | ACT, TAU | PANSS general proxy for depression | 36.1 (9.1) | 0.39 | 0.20 university |
| 31129983 | Sheaves, B. [64] | 2019 | UK | 11/9 (5/4) | CBT, TAU | 41 (12.5) | 0.42 | ||
| 31935529 | Sönmez, N. [76] | 2020 | Norway | 32/31 (7/3) | CBT, TAU | Six-month follow-up | 27.9 (18–51) | 0.41 | 0.35 in occupation |
| 15259826 | Startup, M. [77] | 2004 | Australia | 47/43 (6/1) | CBT, TAU | ||||
| 24853059 | Tarrier, N. [78] | 2014 | UK | 25/24 (8) | CBSP, TAU | 34.9 (13.1) | 0.37 | 0.17 post-secondary | |
| 22941746 | van der Gaag, M. [65] | 2012 | Netherlands | 98/103 (1) | CBT, TAU | Ultra-high-risk focus of patient cohort | 22.8 (5.5) | 0.51 | 13.9 years (2.7) |
| 25066223 | van Oosterhout, B. [79] | 2014 | Netherlands | 75/79 (3/5) | MCT, TAU | 24-month follow-up DACOBS SC proxy for depression | 37.5 (9.9) | 0.29 | ‘medium’ on average |
| 21975193 | White, R. [59] | 2011 | UK | 14/13 (3/3) | ACT, TAU | 34 (9.7) | 0.22 | 0.15 | |
| 29494866 | Wood, L. [80] | 2018 | UK | 15/15 (4/2) | CBT, Psychoeducation | ISMI proxy for negative | 33.6 (12.9) | 0.23 | 0.37 |
| Contributed to systematic review only | |||||||||
| 34470506 | Wojtalik, J.A. [81] | 2022 | USA, Canada | 58/44 (NA) | CET, enriched TAU | 24.8 (5.5) | 0.26 | 0.68 | |
| 28166848 | Ochoa, S. [82] | 2017 | Spain | 65/57 (5/4) | MCT, TAU | 0.30 | 0.23 post-secondary | ||
| 26298541 | López-Navarro, E. [83] | 2015 | Spain | 22/22 (5/4) | MBI, CBT | 26 weeks | 38.8 (8.1) | 0.17 | 0.25 left education after 18 |
| 29207980 | Husain, M.O [84] | 2017 | Pakistan | 18/18 (NA) | CBT, TAU | ||||
Primary Outcomes
4. Discussion
Limitations
5. Conclusions
Funding
Conflicts of Interest
References
- Boland, R.; Verduin, M.L.; Ruiz, P. Kaplan & Sadock’s Synopsis of Psychiatry, 12th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2021. [Google Scholar]
- Biedermann, F.; Fleischhacker, W.W. Psychotic disorders in DSM-5 and ICD-11. CNS Spectr. 2016, 21, 349–354. [Google Scholar] [CrossRef]
- Heckers, S.; Barch, D.M.; Bustillo, J.; Gaebel, W.; Gur, R.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tandon, R.; Tsuang, M.; et al. Structure of the psychotic disorders classification in DSM-5. Schizophr. Res. 2013, 150, 11–14. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III), 3rd ed.; American Psychiatric Association: Washington, DC, USA, 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R), 3rd ed.; American Psychiatric Association: Washington, DC, USA, 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Harrow, M.; Grossman, L.S.; Herbener, E.S.; Davies, E.W. Ten-year outcome: Patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br. J. Psychiatry 2000, 177, 421–426. [Google Scholar] [CrossRef]
- Grossman, L.S.; Harrow, M.; Goldberg, J.F.; Fichtner, C.G. Outcome of schizoaffective disorder at two long-term follow-ups: Comparisons with outcome of schizophrenia and affective disorders. Am. J. Psychiatry 1991, 148, 1359–1365. [Google Scholar] [CrossRef]
- Miller, J.N.; Black, D.W. Schizoaffective disorder: A review. Ann. Clin. Psychiatry 2019, 31, 47–53. [Google Scholar]
- Kantrowitz, J.T.; Citrome, L. Schizoaffective disorder: A review of current research themes and pharmacological management. CNS Drugs 2011, 25, 317–331. [Google Scholar] [CrossRef]
- Kuller, A.M.; Ott, B.D.; Goisman, R.M.; Wainwright, L.D.; Rabin, R.J. Cognitive behavioral therapy and schizophrenia: A survey of clinical practices and views on efficacy in the United States and United kingdom. Community Ment. Health J. 2010, 46, 2–9. [Google Scholar] [CrossRef]
- Beck, A.T. Cognitive Therapy and the Emotional Disorders; International Universities Press: Oxford, UK, 1976; p. 356. [Google Scholar]
- Hofmann, S.G.; Asnaani, A.; Vonk, I.J.; Sawyer, A.T.; Fang, A. The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cognit. Ther. Res. 2012, 36, 427–440. [Google Scholar] [CrossRef]
- Harris, R. ACT Made Simple: An Easy-to-Read Primer on Acceptance and Commitment Therapy, 2nd ed.; New Harbinger Publications: Oakland, CA, USA, 2019; p. 373. [Google Scholar]
- Hayes, S.C.; Strosahl, K.D.; Wilson, K.G. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change; Guilford Press: New York, NY, USA, 1999; p. 304. [Google Scholar]
- Wells, A. Metacognitive Therapy for Anxiety and Depression; Guilford Press: New York, NY, USA, 2009. [Google Scholar]
- Segal, Z.V.; Williams, J.M.G.; Teasdade, J.D. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse; Guilford Press: New York, NY, USA, 2002. [Google Scholar]
- Gilbert, P. Developing a compassion-focused approach in cognitive behavioural therapy. In Cognitive Behaviour Therapy: A Guide for the Practising Clinician, Vol 2; Routledge/Taylor & Francis Group: New York, NY, USA, 2009; pp. 205–220. [Google Scholar]
- Hayes, S.C.; Hofmann, S.G. The third wave of cognitive behavioral therapy and the rise of process-based care. World Psychiatry 2017, 16, 245–246. [Google Scholar] [CrossRef]
- Tai, S.; Turkington, D. The evolution of cognitive behavior therapy for schizophrenia: Current practice and recent developments. Schizophr. Bull. 2009, 35, 865–873. [Google Scholar] [CrossRef]
- Thase, M.E.; Kingdon, D.; Turkington, D. The promise of cognitive behavior therapy for treatment of severe mental disorders: A review of recent developments. World Psychiatry 2014, 13, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.P.; Turkington, D.; Pyle, M.; Spencer, H.; Brabban, A.; Dunn, G.; Christodoulides, T.; Dudley, R.; Chapman, N.; Callcott, P.; et al. Cognitive therapy for people with schizophrenia spectrum disorders not taking antipsychotic drugs: A single-blind randomised controlled trial. Lancet 2014, 383, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Tarrier, N.; Yusupoff, L.; Kinney, C.; McCarthy, E.; Gledhill, A.; Haddock, G.; Morris, J. Randomised controlled trial of intensive cognitive behaviour therapy for patients with chronic schizophrenia. BMJ 1998, 317, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Tarrier, N. CBT for psychosis: Effectiveness, diversity, dissemination, politics, the future and technology. World Psychiatry 2014, 13, 256–257. [Google Scholar] [CrossRef][Green Version]
- Turkington, D.; Kingdon, D.; Weiden, P.J. Cognitive behavior therapy for schizophrenia. Am. J. Psychiatry 2006, 163, 365–373. [Google Scholar] [CrossRef]
- Tarrier, N.; Wykes, T. Is there evidence that cognitive behaviour therapy is an effective treatment for schizophrenia? A cautious or cautionary tale? Behav. Res. Ther. 2004, 42, 1377–1401. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Ziegler, M.; Mehl, S.; Kesting, M.L.; Lüllmann, E.; Westermann, S.; Rief, W. Moving from efficacy to effectiveness in cognitive behavioral therapy for psychosis: A randomized clinical practice trial. J. Consult. Clin. Psychol. 2012, 80, 674–686. [Google Scholar] [CrossRef]
- Heriot-Maitland, C.; Gumley, A.; Wykes, T.; Longden, E.; Irons, C.; Gilbert, P.; Peters, E. A case series study of compassion-focused therapy for distressing experiences in psychosis. Br. J. Clin. Psychol. 2023, 62, 762–781. [Google Scholar] [CrossRef]
- Lazzari, C.; Kotera, Y.; Rabottini, M. Should mindfulness-based cognitive therapy be used for psychosis? A systematic review of the literature and meta-analysis. Riv. Psichiatr. 2022, 57, 203–211. [Google Scholar] [CrossRef]
- Marshall, M.; Rathbone, J. Early intervention for psychosis. Cochrane Database Syst. Rev. 2011, 6, Cd004718. [Google Scholar] [CrossRef]
- Morris, E.M.J.; Johns, L.C.; Gaudiano, B.A. Acceptance and commitment therapy for psychosis: Current status, lingering questions and future directions. Psychol. Psychother. 2024, 97, 41–58. [Google Scholar] [CrossRef]
- Moritz, S.; Klein, J.P.; Lysaker, P.H.; Mehl, S. Metacognitive and cognitive-behavioral interventions for psychosis: New developments . Dialogues Clin. Neurosci. 2019, 21, 309–317. [Google Scholar] [CrossRef]
- Pfammatter, M.; Junghan, U.M.; Brenner, H.D. Efficacy of psychological therapy in schizophrenia: Conclusions from meta-analyses. Schizophr. Bull. 2006, 32 (Suppl. S1), S64–S80. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Garety, P.A.; Kuipers, E.; Fowler, D.; Bebbington, P.E. A cognitive model of persecutory delusions. Br. J. Clin. Psychol. 2002, 41, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Pugh, K.; Dunn, G.; Evans, N.; Sheaves, B.; Waite, F.; Cernis, E.; Lister, R.; Fowler, D. An early Phase II randomised controlled trial testing the effect on persecutory delusions of using CBT to reduce negative cognitions about the self: The potential benefits of enhancing self confidence. Schizophr. Res. 2014, 160, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Garety, P.A.; Freeman, D. The past and future of delusions research: From the inexplicable to the treatable. Br. J. Psychiatry 2013, 203, 327–333. [Google Scholar] [CrossRef]
- Kennedy, L.; Xyrichis, A. Cognitive Behavioral Therapy Compared with Non-specialized Therapy for Alleviating the Effect of Auditory Hallucinations in People with Reoccurring Schizophrenia: A Systematic Review and Meta-analysis. Community Ment. Health J. 2017, 53, 127–133. [Google Scholar] [CrossRef]
- Pontillo, M.; De Crescenzo, F.; Vicari, S.; Pucciarini, M.L.; Averna, R.; Santonastaso, O.; Armando, M. Cognitive behavioural therapy for auditory hallucinations in schizophrenia: A review. World J. Psychiatry 2016, 6, 372–380. [Google Scholar] [CrossRef]
- Peralta, V.; Cuesta, M.J. The nosology of psychotic disorders: A comparison among competing classification systems. Schizophr. Bull. 2003, 29, 413–425. [Google Scholar] [CrossRef][Green Version]
- Malaspina, D.; Owen, M.J.; Heckers, S.; Tandon, R.; Bustillo, J.; Schultz, S.; Barch, D.M.; Gaebel, W.; Gur, R.E.; Tsuang, M.; et al. Schizoaffective Disorder in the DSM-5. Schizophr. Res. 2013, 150, 21–25. [Google Scholar] [CrossRef]
- Freeman, D.; Dunn, G.; Startup, H.; Pugh, K.; Cordwell, J.; Mander, H.; Černis, E.; Wingham, G.; Shirvell, K.; Kingdon, D. Effects of cognitive behaviour therapy for worry on persecutory delusions in patients with psychosis (WIT): A parallel, single-blind, randomised controlled trial with a mediation analysis. Lancet Psychiatry 2015, 2, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.P.; Law, H.; Carter, L.; Sellers, R.; Emsley, R.; Pyle, M.; French, P.; Shiers, D.; Yung, A.R.; Murphy, E.K.; et al. Antipsychotic drugs versus cognitive behavioural therapy versus a combination of both in people with psychosis: A randomised controlled pilot and feasibility study. Lancet Psychiatry 2018, 5, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.P.; Barratt, S. What are the components of CBT for psychosis? A Delphi study. Schizophr. Bull. 2010, 36, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Spencer, H.M.; Dudley, R.; Freeston, M.H.; Turkington, D. What are the essential ingredients of a CBT case conceptualization for voices and delusions in schizophrenia spectrum disorders? A study of expert consensus. Schizophr. Res. 2020, 224, 74–81. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Barrowclough, C.; Haddock, G.; Wykes, T.; Beardmore, R.; Conrod, P.; Craig, T.; Davies, L.; Dunn, G.; Eisner, E.; Lewis, S.; et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: Randomised controlled trial. BMJ 2010, 341, c6325. [Google Scholar] [CrossRef]
- Gleeson, J.F.; Cotton, S.M.; Alvarez-Jimenez, M.; Wade, D.; Gee, D.; Crisp, K.; Pearce, T.; Spiliotacopoulos, D.; Newman, B.; McGorry, P.D. A randomized controlled trial of relapse prevention therapy for first-episode psychosis patients: Outcome at 30-month follow-up. Schizophr. Bull. 2013, 39, 436–448. [Google Scholar] [CrossRef]
- Katsushima, M.; Nakamura, H.; Shiko, Y.; Hanaoka, H.; Shimizu, E. Effectiveness of a Videoconference-Based Cognitive Behavioral Therapy Program for Patients with Schizophrenia: Pilot Randomized Controlled Trial. JMIR Form. Res. 2025, 9, e59540. [Google Scholar] [CrossRef]
- Garety, P.; Ward, T.; Emsley, R.; Greenwood, K.; Freeman, D.; Fowler, D.; Kuipers, E.; Bebbington, P.; Rus-Calafell, M.; McGourty, A.; et al. Effects of SlowMo, a Blended Digital Therapy Targeting Reasoning, on Paranoia Among People With Psychosis: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 714–725. [Google Scholar] [CrossRef]
- Gaudiano, B.A.; Herbert, J.D. Acute treatment of inpatients with psychotic symptoms using Acceptance and Commitment Therapy: Pilot results. Behav. Res. Ther. 2006, 44, 415–437. [Google Scholar] [CrossRef]
- Morrison, A.P.; Burke, E.; Murphy, E.; Pyle, M.; Bowe, S.; Varese, F.; Dunn, G.; Chapman, N.; Hutton, P.; Welford, M.; et al. Cognitive therapy for internalised stigma in people experiencing psychosis: A pilot randomised controlled trial. Psychiatry Res. 2016, 240, 96–102. [Google Scholar] [CrossRef]
- Baker, A.; Bucci, S.; Lewin, T.J.; Kay-Lambkin, F.; Constable, P.M.; Carr, V.J. Cognitive-behavioural therapy for substance use disorders in people with psychotic disorders: Randomised controlled trial. Br. J. Psychiatry 2006, 188, 439–448. [Google Scholar] [CrossRef]
- Lepage, M.; Bowie, C.R.; Montreuil, T.; Baer, L.; Percie du Sert, O.; Lecomte, T.; Joober, R.; Abdel-Baki, A.; Jarvis, G.E.; Margolese, H.C.; et al. Manualized group cognitive behavioral therapy for social anxiety in first-episode psychosis: A randomized controlled trial. Psychol. Med. 2023, 53, 3335–3344. [Google Scholar] [CrossRef]
- MacDougall, A.G.; Price, E.; Vandermeer, M.R.J.; Lloyd, C.; Bird, R.; Sethi, R.; Shanmugalingam, A.; Carr, J.; Anderson, K.K.; Norman, R.M.G. Youth-focused group mindfulness-based intervention in individuals with early psychosis: A randomized pilot feasibility study. Early Interv. Psychiatry 2019, 13, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Garety, P.; Waller, H.; Emsley, R.; Jolley, S.; Kuipers, E.; Bebbington, P.; Dunn, G.; Fowler, D.; Hardy, A.; Freeman, D. Cognitive mechanisms of change in delusions: An experimental investigation targeting reasoning to effect change in paranoia. Schizophr. Bull. 2015, 41, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, R.; Muñoz-Sanjose, A.; Rodriguez-Vega, B.; Bayon, C.; Lahera, G.; Palao, A.; Bravo-Ortiz, M.F. Mindfulness-Based Social Cognition Training (SocialMind) for People With Psychosis: A Feasibility Trial. Front. Psychiatry 2019, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Gaudiano, B.A.; Busch, A.M.; Wenze, S.J.; Nowlan, K.; Epstein-Lubow, G.; Miller, I.W. Acceptance-based Behavior Therapy for Depression With Psychosis: Results From a Pilot Feasibility Randomized Controlled Trial. J. Psychiatr. Pract. 2015, 21, 320–333. [Google Scholar] [CrossRef]
- White, R.; Gumley, A.; McTaggart, J.; Rattrie, L.; McConville, D.; Cleare, S.; Mitchell, G. A feasibility study of Acceptance and Commitment Therapy for emotional dysfunction following psychosis. Behav. Res. Ther. 2011, 49, 901–907. [Google Scholar] [CrossRef]
- Shawyer, F.; Farhall, J.; Thomas, N.; Hayes, S.C.; Gallop, R.; Copolov, D.; Castle, D.J. Acceptance and commitment therapy for psychosis: Randomised controlled trial. Br. J. Psychiatry 2017, 210, 140–148. [Google Scholar] [CrossRef]
- Gaudiano, B.A.; Ellenberg, S.; Johnson, J.E.; Mueser, K.T.; Miller, I.W. Effectiveness of acceptance and commitment therapy for inpatients with psychosis: Implementation feasibility and acceptability from a pilot randomized controlled trial. Schizophr. Res. 2023, 261, 72–79. [Google Scholar] [CrossRef]
- Chien, W.T.; Chong, Y.Y.; Bressington, D.; McMaster, C.W. A randomized controlled trial of an acceptance-based, insight-inducing medication adherence therapy (AIM-AT) for adults with early-stage psychosis. Psychiatry Res. 2024, 339, 116046. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.J.; McGorry, P.D.; Killackey, E.; Bendall, S.; Allott, K.; Dudgeon, P.; Gleeson, J.; Johnson, T.; Harrigan, S. Acute-phase and 1-year follow-up results of a randomized controlled trial of CBT versus Befriending for first-episode psychosis: The ACE project. Psychol. Med. 2008, 38, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Sheaves, B.; Holmes, E.A.; Rek, S.; Taylor, K.M.; Nickless, A.; Waite, F.; Germain, A.; Espie, C.A.; Harrison, P.J.; Foster, R.; et al. Cognitive Behavioural Therapy for Nightmares for Patients with Persecutory Delusions (Nites): An Assessor-Blind, Pilot Randomized Controlled Trial. Can. J. Psychiatry 2019, 64, 686–696. [Google Scholar] [CrossRef] [PubMed]
- van der Gaag, M.; Nieman, D.H.; Rietdijk, J.; Dragt, S.; Ising, H.K.; Klaassen, R.M.; Koeter, M.; Cuijpers, P.; Wunderink, L.; Linszen, D.H. Cognitive behavioral therapy for subjects at ultrahigh risk for developing psychosis: A randomized controlled clinical trial. Schizophr. Bull. 2012, 38, 1180–1188. [Google Scholar] [CrossRef]
- Kråkvik, B.; Gråwe, R.W.; Hagen, R.; Stiles, T.C. Cognitive behaviour therapy for psychotic symptoms: A randomized controlled effectiveness trial. Behav. Cogn. Psychother. 2013, 41, 511–524. [Google Scholar] [CrossRef]
- Balzan, R.P.; Mattiske, J.K.; Delfabbro, P.; Liu, D.; Galletly, C. Individualized Metacognitive Training (MCT+) Reduces Delusional Symptoms in Psychosis: A Randomized Clinical Trial. Schizophr. Bull. 2019, 45, 27–36. [Google Scholar] [CrossRef]
- Dellazizzo, L.; Potvin, S.; Phraxayavong, K.; Dumais, A. One-year randomized trial comparing virtual reality-assisted therapy to cognitive-behavioral therapy for patients with treatment-resistant schizophrenia. NPJ Schizophr. 2021, 7, 9. [Google Scholar] [CrossRef]
- Farhall, J.; Freeman, N.C.; Shawyer, F.; Trauer, T. An effectiveness trial of cognitive behaviour therapy in a representative sample of outpatients with psychosis. Br. J. Clin. Psychol. 2009, 48, 47–62. [Google Scholar] [CrossRef]
- Farrelly, S.; Peters, E.; Azis, M.; David, A.S.; Hunter, E.C.M. A brief CBT intervention for depersonalisation-derealisation disorder in psychosis: Results from a feasibility randomised controlled trial. J. Behav. Ther. Exp. Psychiatry 2024, 82, 101911. [Google Scholar] [CrossRef]
- Favrod, J.; Rexhaj, S.; Bardy, S.; Ferrari, P.; Hayoz, C.; Moritz, S.; Conus, P.; Bonsack, C. Sustained antipsychotic effect of metacognitive training in psychosis: A randomized-controlled study. Eur. Psychiatry 2014, 29, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Emsley, R.; Diamond, R.; Collett, N.; Bold, E.; Chadwick, E.; Isham, L.; Bird, J.C.; Edwards, D.; Kingdon, D.; et al. Comparison of a theoretically driven cognitive therapy (the Feeling Safe Programme) with befriending for the treatment of persistent persecutory delusions: A parallel, single-blind, randomised controlled trial. Lancet Psychiatry 2021, 8, 696–707. [Google Scholar] [CrossRef] [PubMed]
- López-Navarro, E.; Del Canto, C.; Mayol, A.; Fernández-Alonso, O.; Reig, J.; Munar, E. Does mindfulness improve inhibitory control in psychotic disorders? A randomized controlled clinical trial. Int. J. Clin. Health Psychol. 2020, 20, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.P.; Pyle, M.; Gumley, A.; Schwannauer, M.; Turkington, D.; MacLennan, G.; Norrie, J.; Hudson, J.; Bowe, S.E.; French, P.; et al. Cognitive behavioural therapy in clozapine-resistant schizophrenia (FOCUS): An assessor-blinded, randomised controlled trial. Lancet Psychiatry 2018, 5, 633–643. [Google Scholar] [CrossRef]
- Pos, K.; Meijer, C.J.; Verkerk, O.; Ackema, O.; Krabbendam, L.; de Haan, L. Metacognitive training in patients recovering from a first psychosis: An experience sampling study testing treatment effects. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 57–64. [Google Scholar] [CrossRef]
- Sönmez, N.; Romm, K.L.; Østefjells, T.; Grande, M.; Jensen, L.H.; Hummelen, B.; Tesli, M.; Melle, I.; Røssberg, J.I. Cognitive behavior therapy in early psychosis with a focus on depression and low self-esteem: A randomized controlled trial. Compr. Psychiatry 2020, 97, 152157. [Google Scholar] [CrossRef]
- Startup, M.; Jackson, M.C.; Bendix, S. North Wales randomized controlled trial of cognitive behaviour therapy for acute schizophrenia spectrum disorders: Outcomes at 6 and 12 months. Psychol. Med. 2004, 34, 413–422. [Google Scholar] [CrossRef]
- Tarrier, N.; Kelly, J.; Maqsood, S.; Snelson, N.; Maxwell, J.; Law, H.; Dunn, G.; Gooding, P. The cognitive behavioural prevention of suicide in psychosis: A clinical trial. Schizophr. Res. 2014, 156, 204–210. [Google Scholar] [CrossRef]
- van Oosterhout, B.; Krabbendam, L.; de Boer, K.; Ferwerda, J.; van der Helm, M.; Stant, A.D.; van der Gaag, M. Metacognitive group training for schizophrenia spectrum patients with delusions: A randomized controlled trial. Psychol. Med. 2014, 44, 3025–3035. [Google Scholar] [CrossRef]
- Wood, L.; Byrne, R.; Enache, G.; Morrison, A.P. A brief cognitive therapy intervention for internalised stigma in acute inpatients who experience psychosis: A feasibility randomised controlled trial. Psychiatry Res. 2018, 262, 303–310. [Google Scholar] [CrossRef]
- Wojtalik, J.A.; Mesholam-Gately, R.I.; Hogarty, S.S.; Greenwald, D.P.; Litschge, M.Y.; Sandoval, L.R.; Shashidhar, G.; Guimond, S.; Keshavan, M.S.; Eack, S.M. Confirmatory Efficacy of Cognitive Enhancement Therapy for Early Schizophrenia: Results From a Multisite Randomized Trial. Psychiatr. Serv. 2022, 73, 501–509. [Google Scholar] [CrossRef]
- Ochoa, S.; López-Carrilero, R.; Barrigón, M.L.; Pousa, E.; Barajas, A.; Lorente-Rovira, E.; González-Higueras, F.; Grasa, E.; Ruiz-Delgado, I.; Cid, J.; et al. Randomized control trial to assess the efficacy of metacognitive training compared with a psycho-educational group in people with a recent-onset psychosis. Psychol. Med. 2017, 47, 1573–1584. [Google Scholar] [CrossRef]
- López-Navarro, E.; Del Canto, C.; Belber, M.; Mayol, A.; Fernández-Alonso, O.; Lluis, J.; Munar, E.; Chadwick, P. Mindfulness improves psychological quality of life in community-based patients with severe mental health problems: A pilot randomized clinical trial. Schizophr. Res. 2015, 168, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.O.; Chaudhry, I.B.; Mehmood, N.; Rehman, R.U.; Kazmi, A.; Hamirani, M.; Kiran, T.; Bukhsh, A.; Bassett, P.; Husain, M.I.; et al. Pilot randomised controlled trial of culturally adapted cognitive behavior therapy for psychosis (CaCBTp) in Pakistan. BMC Health Serv. Res. 2017, 17, 808. [Google Scholar] [CrossRef] [PubMed]
- Zanello, A.; Mohr, S.; Merlo, M.C.; Huguelet, P.; Rey-Bellet, P. Effectiveness of a brief group cognitive behavioral therapy for auditory verbal hallucinations: A 6-month follow-up study. J. Nerv. Ment. Dis. 2014, 202, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Wykes, T.; Steel, C.; Everitt, B.; Tarrier, N. Cognitive behavior therapy for schizophrenia: Effect sizes, clinical models, and methodological rigor. Schizophr. Bull. 2008, 34, 523–537. [Google Scholar] [CrossRef]
- van der Gaag, M.; Valmaggia, L.R.; Smit, F. The effects of individually tailored formulation-based cognitive behavioural therapy in auditory hallucinations and delusions: A meta-analysis. Schizophr. Res. 2014, 156, 30–37. [Google Scholar] [CrossRef]
- Van Eck, R.M.; Burger, T.J.; Schenkelaars, M.; Vellinga, A.; de Koning, M.B.; Schirmbeck, F.; Kikkert, M.; Dekker, J.; de Haan, L. The impact of affective symptoms on personal recovery of patients with severe mental illness. Int. J. Soc. Psychiatry 2018, 64, 521–527. [Google Scholar] [CrossRef]
- Upthegrove, R.; Broome, M.R.; Caldwell, K.; Ives, J.; Oyebode, F.; Wood, S.J. Understanding auditory verbal hallucinations: A systematic review of current evidence. Acta Psychiatr. Scand. 2016, 133, 352–367. [Google Scholar] [CrossRef]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef]
- Strauss, G.P.; Bartolomeo, L.A.; Harvey, P.D. Avolition as the core negative symptom in schizophrenia: Relevance to pharmacological treatment development. NPJ Schizophr. 2021, 7, 16. [Google Scholar] [CrossRef]
- Daniel, D.G.; Cohen, A.S.; Velligan, D.; Harvey, P.D.; Alphs, L.; Davidson, M.; Potter, W.; Kott, A.; Schooler, N.; Brodie, C.R.; et al. Remote Assessment of Negative Symptoms of Schizophrenia. Schizophr. Bull. Open 2023, 4, sgad001. [Google Scholar] [CrossRef]
- Geissbühler, M.; Hincapié, C.A.; Aghlmandi, S.; Zwahlen, M.; Jüni, P.; da Costa, B.R. Most published meta-regression analyses based on aggregate data suffer from methodological pitfalls: A meta-epidemiological study. BMC Med. Res. Methodol. 2021, 21, 123. [Google Scholar] [CrossRef]
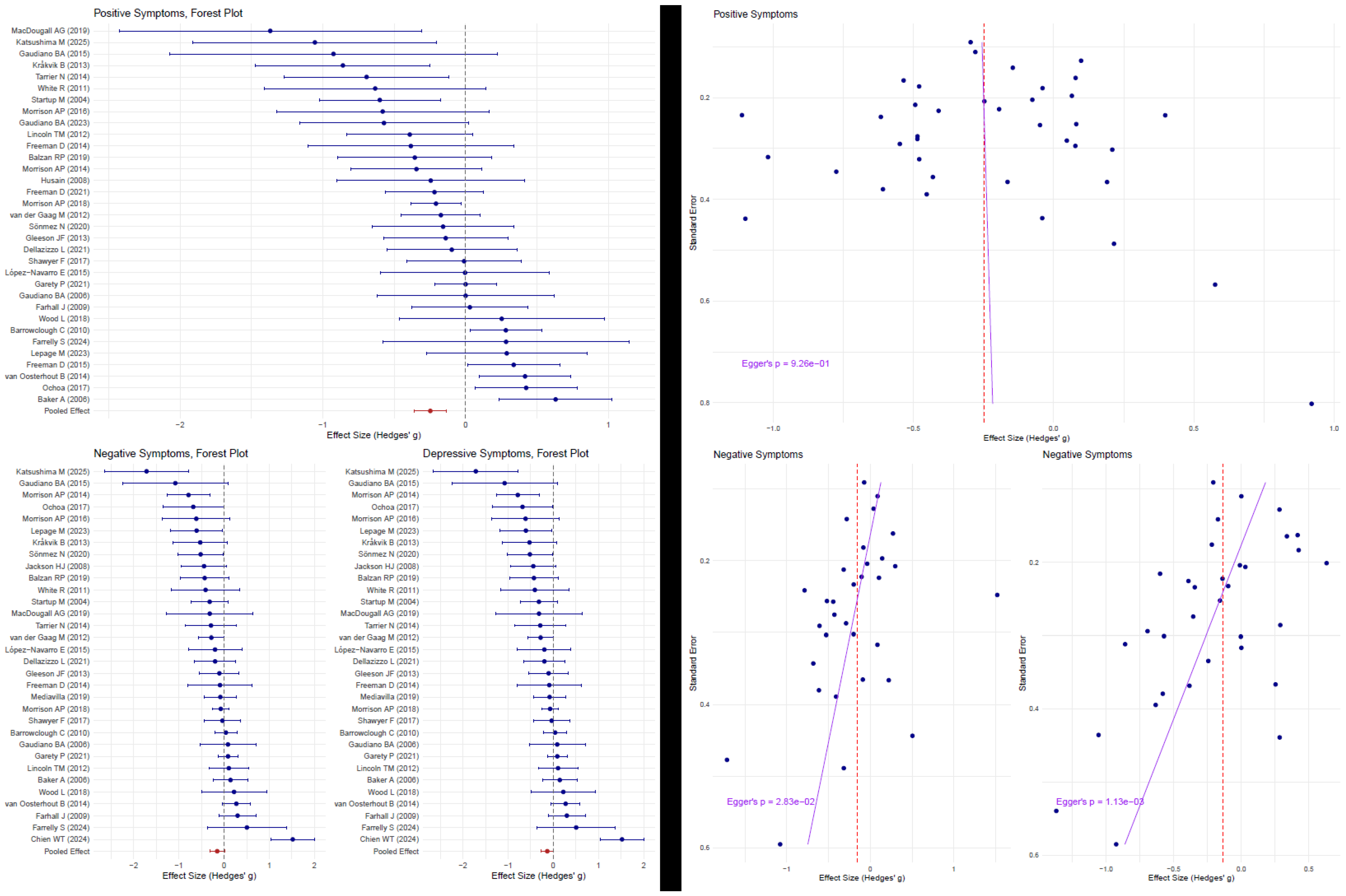
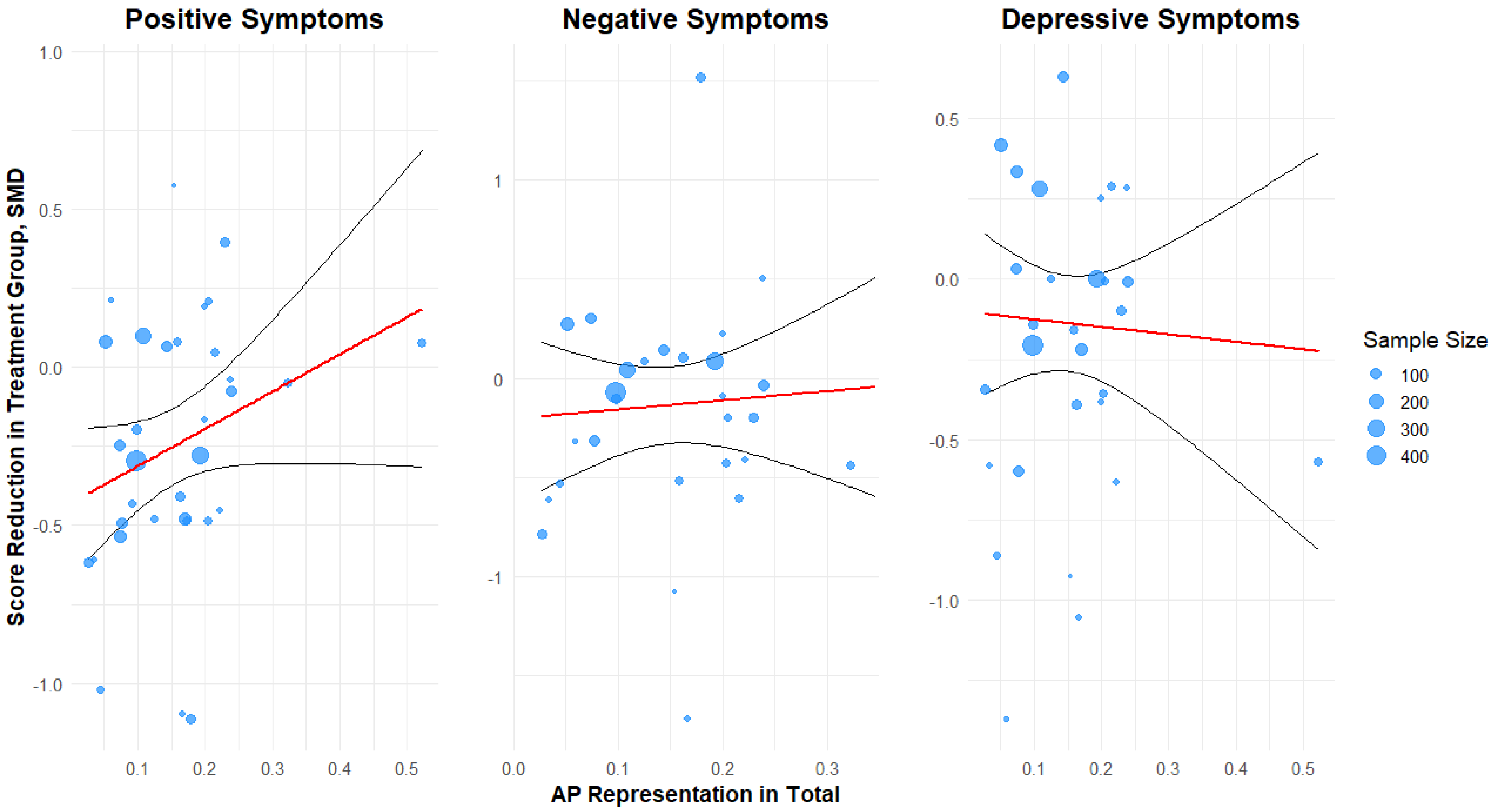
| Symptom Domain | Overall Effect Size | Association with AP (% per 10%) | Explained Variance (R2) | Residual Heterogeneity (I2) | Publication Bias |
|---|---|---|---|---|---|
| Positive Symptoms | −0.25 (−0.36 to −0.14, p < 0.001) | +0.10 (−0.03 to +0.22, p = 0.13) | 1% | 55.03% | z = −0.39, p = 0.69 |
| Negative Symptoms | −0.15 (−0.32 to +0.01, p = 0.07) | +0.05 (−0.18 to +0.28, p = 0.73) | 0% | 76.86% | z = −2.15, p = 0.03 |
| Depressive Symptoms | −0.13 (−0.27 to +0.01, p = 0.06) | −0.02 (−0.18 to +0.14, p = 0.78) | 0% | 68.2% | z = −3.02, p = 0.003 |
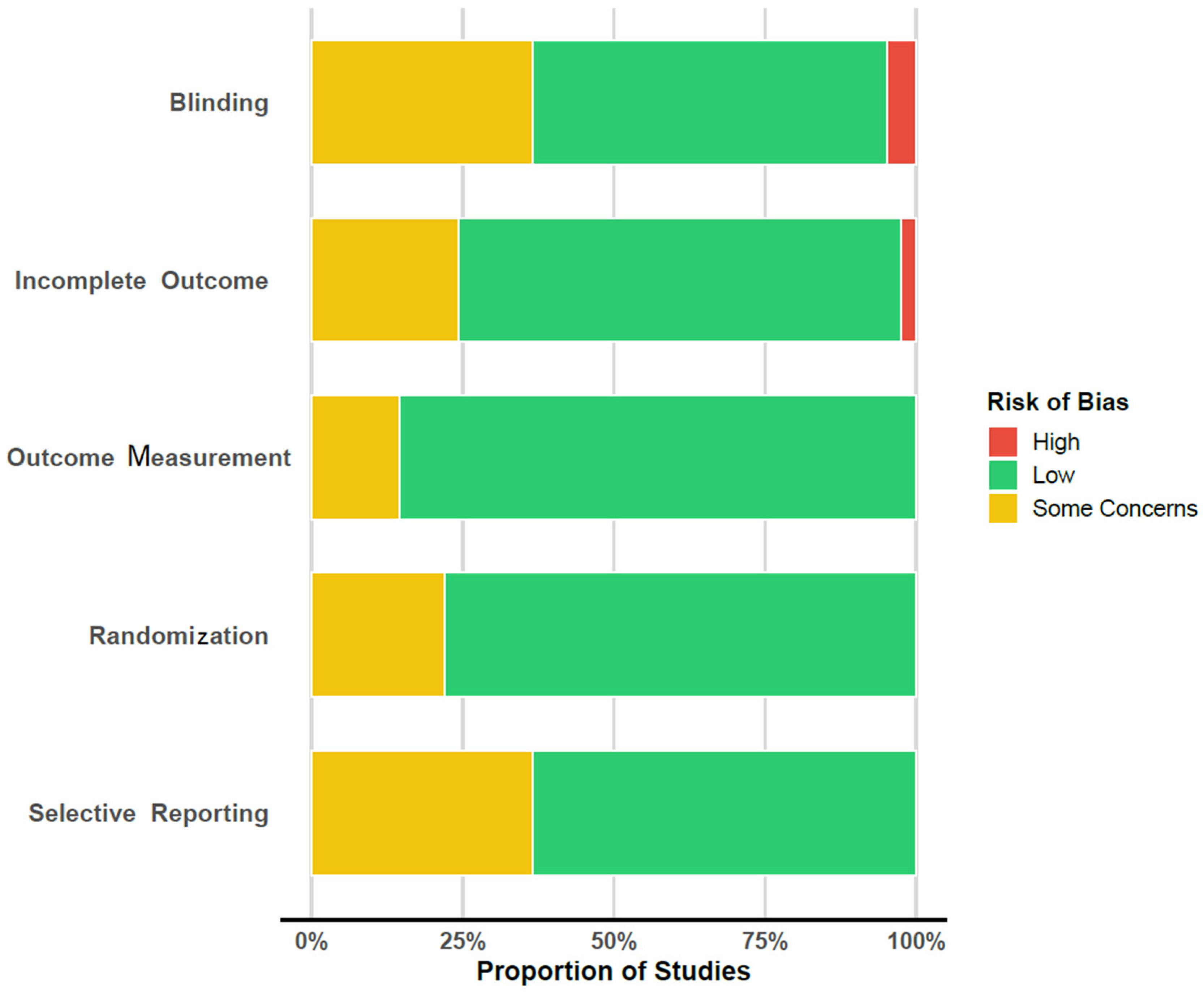
| Author | Year | Randomization | Blinding | Incomplete Outcome | Outcome Measure | Selective Reporting |
|---|---|---|---|---|---|---|
| Baker, A. [53] | 2006 | low | low | low | low | low |
| Balzan, R.P. [67] | 2019 | low | moderate | moderate | low | NP/PNI |
| Barrowclough, C. [47] | 2010 | low | low | moderate | low | NP/PNI |
| Chien, W.T. [62] | 2024 | low | moderate | low | low | low |
| Dellazizzo, L. [68] | 2021 | low | low | low | low | low |
| Farhall, J. [69] | 2009 | moderate | high | moderate | moderate | low |
| Farrelly, S. [70] | 2024 | low | low | low | low | low |
| Favrod, J. [71] | 2014 | low | low | low | low | low |
| Freeman, D. [72] | 2021 | low | low | low | low | low |
| Freeman, D. [36] | 2014 | low | low | low | low | low |
| Freeman, D. [42] | 2015 | low | low | low | low | low |
| Garety, P. [50] | 2021 | low | low | moderate | low | NP/PNI |
| Gaudiano, B.A. [58] | 2015 | low | low | low | low | NP/PNI |
| Gaudiano, B.A. [51] | 2006 | moderate | moderate | low | moderate | low |
| Gaudiano, B.A. [61] | 2023 | low | moderate | low | low | low |
| Gleeson, J.F. [48] | 2013 | moderate | low | low | low | low |
| Husain, M.O. [84] | 2017 | low | low | low | low | low |
| Jackson, H.J. [63] | 2008 | low | low | low | low | NP/PNI |
| Katsushima, M. [49] | 2025 | low | low | low | low | low |
| Krakvik, B. [66] | 2013 | moderate | high | moderate | moderate | low |
| Lepage, M. [54] | 2023 | low | moderate | low | low | NP/PNI |
| Lincoln, T.M. [28] | 2012 | low | moderate | low | low | NP/PNI |
| López-Navarro, E. [83] | 2015 | low | low | low | low | NP/PNI |
| López-Navarro, E. [73] | 2020 | low | low | low | low | low |
| MacDougall, A.G. [55] | 2019 | moderate | moderate | low | low | low |
| Morrison, A.P. [74] | 2018 | low | low | low | low | low |
| Morrison, A.P. [52] | 2016 | low | low | low | low | low |
| Morrison, A.P. [23] | 2014 | low | low | low | low | low |
| Ochoa, S. [82] | 2017 | moderate | moderate | low | low | low |
| Pos, K. [75] | 2018 | low | moderate | moderate | low | NP/PNI |
| Shawyer, F. [60] | 2017 | low | low | low | low | low |
| Sheaves, B. [64] | 2019 | low | low | low | low | low |
| Sonmez, N. [76] | 2020 | low | low | low | low | NP/PNI |
| Startup, M. [77] | 2004 | moderate | moderate | moderate | low | NP/PNI |
| Tarrier, N. [78] | 2014 | low | low | low | low | low |
| van der Gaag, M. [65] | 2012 | low | low | low | low | moderate (QoL Scale) |
| van Oosterhout, B. [79] | 2014 | low | low | moderate | low | NP/PNI |
| White, R. [59] | 2011 | low | low | low | low | NP/PNI |
| Wojtalik, J.A. [81] | 2022 | moderate | low | moderate | low | low |
| Wood, L. [80] | 2018 | low | low | high | moderate | low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karageorgiou, V.; Michopoulos, I.; Tsigkaropoulou, E. Comparative Effectiveness of Cognitive Behavioral Therapies in Schizophrenia and Schizoaffective Disorder: A Systematic Review and Meta-Regression Analysis. J. Clin. Med. 2025, 14, 5521. https://doi.org/10.3390/jcm14155521
Karageorgiou V, Michopoulos I, Tsigkaropoulou E. Comparative Effectiveness of Cognitive Behavioral Therapies in Schizophrenia and Schizoaffective Disorder: A Systematic Review and Meta-Regression Analysis. Journal of Clinical Medicine. 2025; 14(15):5521. https://doi.org/10.3390/jcm14155521
Chicago/Turabian StyleKarageorgiou, Vasilios, Ioannis Michopoulos, and Evdoxia Tsigkaropoulou. 2025. "Comparative Effectiveness of Cognitive Behavioral Therapies in Schizophrenia and Schizoaffective Disorder: A Systematic Review and Meta-Regression Analysis" Journal of Clinical Medicine 14, no. 15: 5521. https://doi.org/10.3390/jcm14155521
APA StyleKarageorgiou, V., Michopoulos, I., & Tsigkaropoulou, E. (2025). Comparative Effectiveness of Cognitive Behavioral Therapies in Schizophrenia and Schizoaffective Disorder: A Systematic Review and Meta-Regression Analysis. Journal of Clinical Medicine, 14(15), 5521. https://doi.org/10.3390/jcm14155521







