Influence of Remimazolam and Propofol on Intraoperative Motor Evoked Potentials During Spinal Surgery: A Randomized Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. General Anesthesia and Intervention Protocol
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TIVA | Total intravenous anesthesia |
| MEP | Motor evoked potential |
| BIS | Bispectral index |
| TCI | Target-controlled infusion |
| TOF | Train-of-four |
| EEG | Electroencephalogram |
References
- Riley, M.R.; Doan, A.T.; Vogel, R.W.; Aguirre, A.O.; Pieri, K.S.; Scheid, E.H. Use of motor evoked potentials during lateral lumbar interbody fusion reduces postoperative deficits. Spine J. 2018, 18, 1763–1778. [Google Scholar] [CrossRef] [PubMed]
- de Haan, P.; Kalkman, C.J.; Jacobs, M.J. Spinal cord monitoring with myogenic motor evoked potentials: Early detection of spinal cord ischemia as an integral part of spinal cord protective strategies during thoracoabdominal aneurysm surgery. Semin. Thorac. Cardiovasc. Surg. 1998, 10, 19–24. [Google Scholar] [CrossRef]
- Lotto, M.L.; Banoub, M.; Schubert, A. Effects of anesthetic agents and physiologic changes on intraoperative motor evoked potentials. J. Neurosurg. Anesthesiol. 2004, 16, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, L.; Stevenson, M.; Hobbs, G.J.; Jardine, A.; Webb, J.K. Intraoperative motor evoked potentials to transcranial electrical stimulation during two anaesthetic regimens. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2001, 112, 1076–1087. [Google Scholar] [CrossRef]
- Thees, C.; Scheufler, K.M.; Nadstawek, J.; Pechstein, U.; Hanisch, M.; Juntke, R.; Zentner, J.; Hoeft, A. Influence of fentanyl, alfentanil, and sufentanil on motor evoked potentials. J. Neurosurg. Anesthesiol. 1999, 11, 112–118. [Google Scholar] [CrossRef]
- Kakinohana, M.; Fuchigami, T.; Nakamura, S.; Kawabata, T.; Sugahara, K. Propofol reduces spinal motor neuron excitability in humans. Anesth. Analg. 2002, 94, 1586–1588. [Google Scholar] [CrossRef]
- Kannabiran, N.; Bidkar, P.U. Total intravenous anesthesia in neurosurgery. J. Neuroanaesth. Crit. Care 2018, 5, 141–149. [Google Scholar] [CrossRef]
- van Dongen, E.P.; ter Beek, H.T.; Aarts, L.P.; Schepens, M.A.; Morshuis, W.J.; Benning, F.J.; de Boer, A.; Boezeman, E.H. The effect of two low-dose propofol infusions on the relationship between six-pulse transcranial electrical stimulation and the evoked lower extremity muscle response. Acta Anaesthesiol. Scand. 2000, 44, 799–803. [Google Scholar] [CrossRef]
- Schüttler, J.; Eisenried, A.; Lerch, M.; Fechner, J.; Jeleazcov, C.; Ihmsen, H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology 2020, 132, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Shamseer, L.; Altman, D.G.; Weeks, L.; Peters, J.; Kober, T.; Dias, S.; Schulz, K.F.; Plint, A.C.; Moher, D. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 2012, 11, MR000030. [Google Scholar] [CrossRef]
- Groudine, S.B.; Soto, R.; Lien, C.; Drover, D.; Roberts, K. A randomized, dose-finding, phase II study of the selective relaxant binding drug, Sugammadex, capable of safely reversing profound rocuronium-induced neuromuscular block. Anesth. Analg. 2007, 104, 555–562. [Google Scholar] [CrossRef]
- Lee, W. The potential risks of sugammadex. Anesth. Pain Med. 2019, 14, 117–122. [Google Scholar] [CrossRef]
- Jamaludin, M.R.; Lai, K.W.; Chuah, J.H.; Zaki, M.A.; Hasikin, K.; Abd Razak, N.A.; Dhanalakshmi, S.; Saw, L.B.; Wu, X. Machine Learning Application of Transcranial Motor-Evoked Potential to Predict Positive Functional Outcomes of Patients. Comput. Intell. Neurosci. 2022, 2022, 2801663. [Google Scholar] [CrossRef] [PubMed]
- Ubags, L.H.; Kalkman, C.J.; Been, H.D.; Drummond, J.C. Differential effects of nitrous oxide and propofol on myogenic transcranial motor evoked responses during sufentanil anaesthesia. Br. J. Anaesth. 1997, 79, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Inghilleri, M.; Berardelli, A.; Marchetti, P.; Manfredi, M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp. Brain Res. 1996, 109, 467–472. [Google Scholar] [CrossRef]
- Sihle-Wissel, M.; Scholz, M.; Cunitz, G. Transcranial magnetic-evoked potentials under total intravenous anaesthesia and nitrous oxide. Br. J. Anaesth. 2000, 85, 465–467. [Google Scholar] [CrossRef]
- Sneyd, J.R.; Gambus, P.L.; Rigby-Jones, A.E. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: A narrative review. Br. J. Anaesth. 2021, 127, 41–55. [Google Scholar] [CrossRef]
- Koo, C.-H.; Lee, S.U.; Kim, H.-G.; Lee, S.; Bae, Y.K.; Oh, A.-Y.; Jeon, Y.-T.; Ryu, J.-H. Effect of remimazolam on intraoperative hemodynamic stability in patients undergoing cerebrovascular bypass surgery: A prospective randomized controlled trial. Korean J. Anesth. 2025, 78, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiao, J.; Nie, Y.; Shao, W.; Zhang, H.; Huang, S. Comparison of the bispectral indices of patients receiving remimazolam and propofol for general anesthesia: A randomized crossover trial. Anaesth. Crit. Care Pain Med. 2024, 43, 101377. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.S.; Kim, J.Y.; Han, D.W.; Yang, J.Y.; Kim, M.J.; Song, Y. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: A randomized non-inferiority trial. J. Clin. Anesth. 2022, 82, 110955. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.; Kim, H.; Park, Y.; Lee, U.; Kwon, J.; Koo, B.-N.; Moon, J.-Y. Electroencephalogram Correlates of Delayed Emergence After Remimazolam-Induced Anesthesia Compared to Propofol. Anesth. Analg. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Shirozu, K.; Nobukuni, K.; Tsumura, S.; Imura, K.; Nakashima, K.; Takamori, S.; Higashi, M.; Yamaura, K. Neurological sedative indicators during general anesthesia with remimazolam. J. Anesth. 2022, 36, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.W.; Beutler, A.S.; Chesnut, R.M.; Patel, P.M.; Kennelly, N.A.; Kalkman, C.J.; Drummond, J.C.; Garfin, S.R. Myogenic motor-evoked potential monitoring using partial neuromuscular blockade in surgery of the spine. Spine (Phila Pa 1976) 1996, 21, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, C.J.; Drummond, J.C.; Kennelly, N.A.; Patel, P.M.; Partridge, B.L. Intraoperative monitoring of tibialis anterior muscle motor evoked responses to transcranial electrical stimulation during partial neuromuscular blockade. Anesth. Analg. 1992, 75, 584–589. [Google Scholar] [CrossRef]
- Liu, H.Y.; Xia, T.J.; Zhu, Z.Z.; Zhao, X.; Qian, Y.; Ma, Z.L.; Gu, X.P. Effect of neuromuscular blockade on transcranial electric motor evoked potentials during surgical correction for idiopathic scoliosis under total intravenous anesthesia. J. Clin. Monit. Comput. 2019, 33, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Toyota, Y.; Narasaki, S.; Watanabe, T.; Miyoshi, H.; Saeki, N.; Tsutsumi, Y.M. Intraoperative responses of motor evoked potentials to the novel intravenous anesthetic remimazolam during spine surgery: A report of two cases. JA Clin. Rep. 2020, 6, 97. [Google Scholar] [CrossRef]
- Aratani, Y.; Tokinaga, Y.; Tanioku, T.; Maruyama, T.; Kawamata, T. A Case of Decreased Amplitude in Motor Evoked Potentials Under Remimazolam Anesthesia. Cureus 2022, 14, e27593. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, J.; Park, Y.G.; Cho, Y.E.; Kim, D.; Lee, D.J.; Kwak, K.W.; Lee, J.; Han, D.W. Comparison of intraoperative neurophysiological monitoring between propofol and remimazolam during total intravenous anesthesia in the cervical spine surgery: A prospective, double-blind, randomized controlled trial. Korean J. Anesthesiol. 2025, 78, 16–29. [Google Scholar] [CrossRef]
- Goetz, S.M.; Alavi, S.M.M.; Deng, Z.D.; Peterchev, A.V. Statistical Model of Motor-Evoked Potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1539–1545. [Google Scholar] [CrossRef]
- Wiltshire, H.R.; Kilpatrick, G.J.; Tilbrook, G.S.; Borkett, K.M. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth. Analg. 2012, 115, 284–296. [Google Scholar] [CrossRef]
- Melton, M.S.; Nielsen, K.C.; Tucker, M.; Klein, S.M.; Gan, T.J. New Medications and Techniques in Ambulatory Anesthesia. Anesthesiol. Clin. 2014, 32, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Fechner, J. Remimazolam—Current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J. Anesthesiol. 2022, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, X.; Wen, C.; Li, D.; Lei, X. Remimazolam: An Updated Review of a New Sedative and Anaesthetic. Drug Des. Dev. Ther. 2022, 16, 3957–3974. [Google Scholar] [CrossRef] [PubMed]
- Bienert, A.; Zaba, Z.; Grzeskowiak, E.; Drobnik, L.; Dyderski, S.; Zaba, C. Measured context-sensitive half-time of propofol in describing its pharmacodynamic effect offset: A-524. Eur. J. Anaesthesiol.|EJA 2006, 23, 136. [Google Scholar] [CrossRef]
- Hill, S. Pharmacokinetics of drug infusions. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 76–80. [Google Scholar] [CrossRef]
- Masui, K.; Stöhr, T.; Pesic, M.; Tonai, T. A population pharmacokinetic model of remimazolam for general anesthesia and consideration of remimazolam dose in clinical practice. J. Anesth. 2022, 36, 493–505. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, H.-B.; Kim, M.S.; Lim, B.G.; Oh, S.K. Effects of Propofol and Remimazolam on Intraoperative Motor Evoked Potentials in Spine Surgery. In Proceedings of the Congress of Asian Society for Neuroanesthesia and Critical Care (ASNACC), Bangkok, Thailand, 1 March 2025. [Google Scholar]
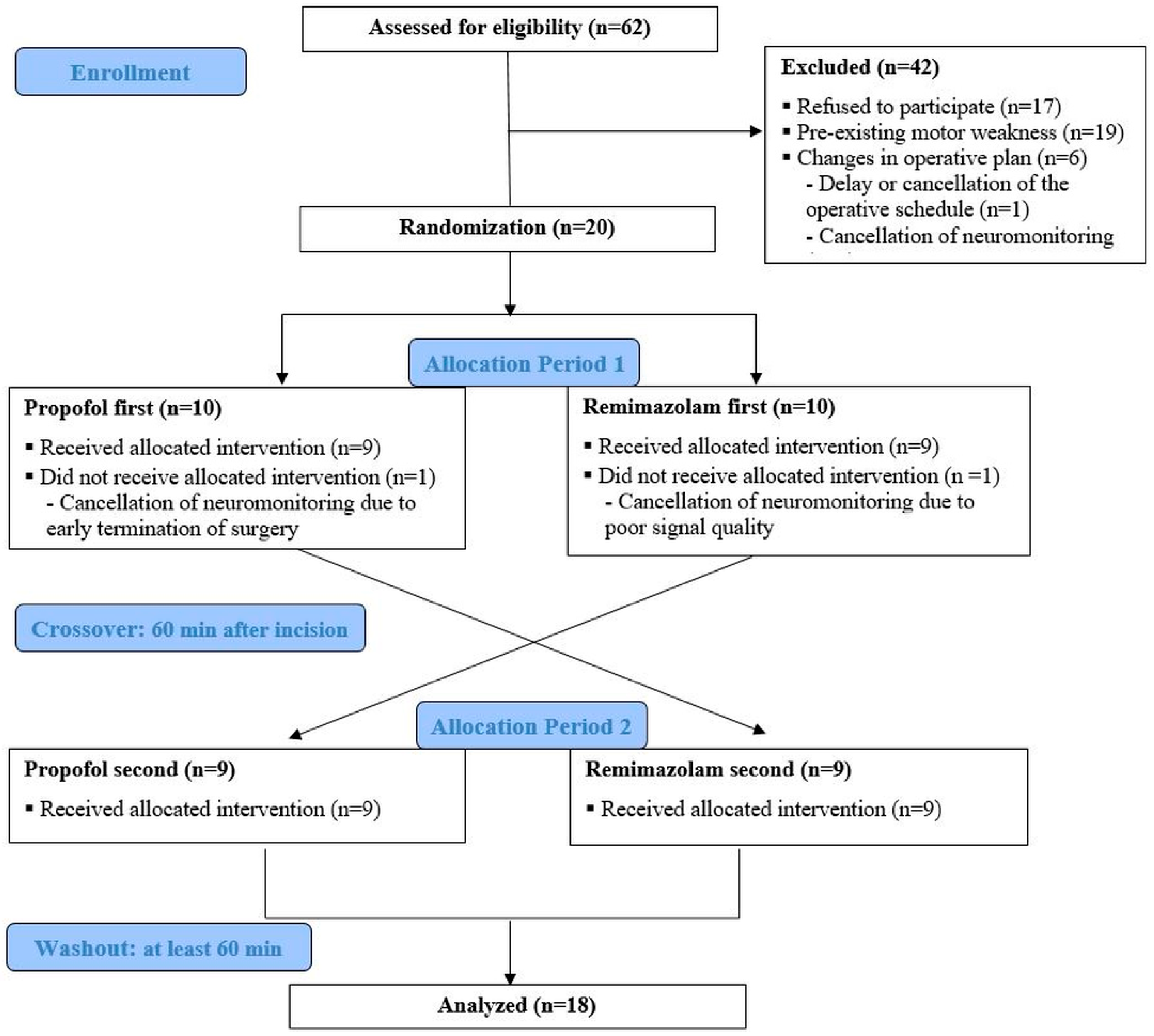
| Total Enrolled (n = 18) | Propofol First Assignment (n = 9) | Remimazolam First Assignment (n = 9) | p-Value | |
|---|---|---|---|---|
| Age (yr) | 40.50 (15.4) | 38.8 (18.2) | 42.2 (12.8) | 0.066 |
| ASA (I/II/III) | 10/7/1 | 6/2/1 | 4/5/0 | 0.261 |
| Height (m) | 168.5 (5.4) | 168.7 (5.3) | 168.3 (5.7) | 0.796 |
| Weight (kg) | 65.0 (14.0) | 61.2 (12.7) | 68.7 (15.0) | 0.556 |
| Duration of surgery (min) | 242.8 (79.4) | 246.1 (95.5) | 242.4 (65.4) | 0.435 |
| Duration of anesthesia (min) | 347.7 (107.2) | 363.8 (133.4) | 332.6 (78.1) | 0.238 |
| Monitoring site (APB/AH/others) | 10/7/1 | 6/2/1 | 4/5/0 | 0.261 |
| Propofol infused (mg) | 1178 [925–1798] | 1000 [720–1272] | 1660 [1090–1927] | 0.136 |
| Remimazolam infused (mg) | 201 [138–300] | 300 [146–360] | 178 [112–213] | 0.063 |
| Remifentanil infused (µg) | 2399.7 (199.6) | 2232.9 (837.9) | 2354.4 (622.2) | 0.299 |
| Emergence time (min) | 22.5 [11.5–37.5] | 25.0 [16.0–50.0] | 20.0 [10.0–32.5] | 0.340 |
| Flumazenil use (n) | 7 | 6 | 1 | 0.050 |
| Length of hospital stay (day) | 7.0 [7.0–9.0] | 7.0 [7.0–9.0] | 7.0 [7.0–8.0] | 1.000 |
| Propofol Infusion | Remimazolam Infusion | p-Value | |
|---|---|---|---|
| MEP data | |||
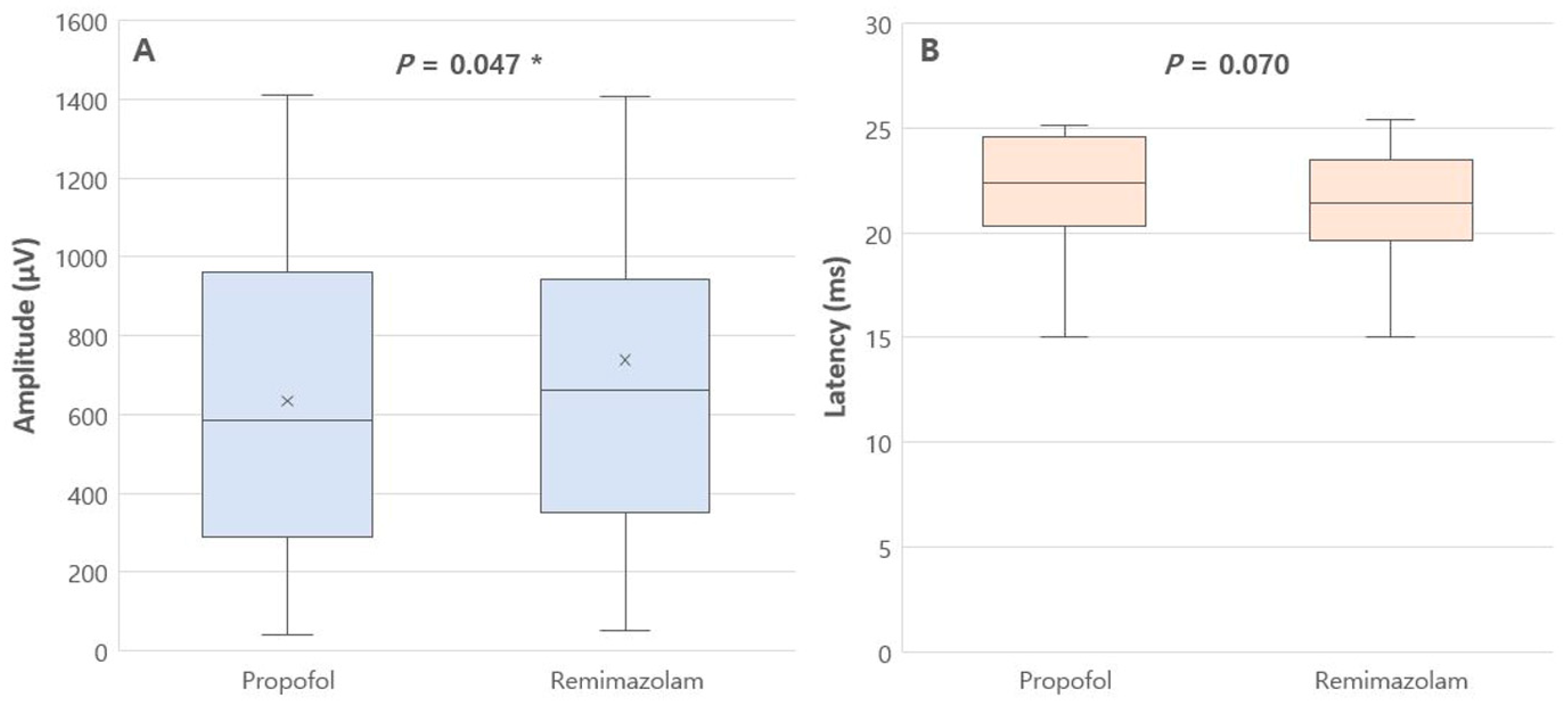
| Amplitude (μV) | 635.3 (399.1) | 738.4 (480.4) | 0.047 * |
| Latency (ms) | 22.4 [20.3–24.6] | 21.4 [19.6–23.5] | 0.070 |
| Hemodynamic data | |||
| Mean blood pressure (mmHg) | 79.36 [74.06–84.51] | 77.33 [73.80–82.30] | 0.378 |
| Heart rate (beats/min) | 68.39 [59.67–75.89] | 70.56 [66.94–72.19] | >0.999 |
| Other data | |||
| BIS value (0–100) | 41.28 [39.69–41.97] | 51.17 [42.86–56.83] | 0.004 * |
| TOF ratio (%) | 99.25 [97.97–100.44] | 99.76 [99.11–100.85] | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.R.; Kim, H.-B.; Kim, M.S.; Lim, B.G.; Oh, S.K. Influence of Remimazolam and Propofol on Intraoperative Motor Evoked Potentials During Spinal Surgery: A Randomized Crossover Trial. J. Clin. Med. 2025, 14, 5491. https://doi.org/10.3390/jcm14155491
Kim BR, Kim H-B, Kim MS, Lim BG, Oh SK. Influence of Remimazolam and Propofol on Intraoperative Motor Evoked Potentials During Spinal Surgery: A Randomized Crossover Trial. Journal of Clinical Medicine. 2025; 14(15):5491. https://doi.org/10.3390/jcm14155491
Chicago/Turabian StyleKim, Bo Rim, Hye-Bin Kim, Moo Soo Kim, Byung Gun Lim, and Seok Kyeong Oh. 2025. "Influence of Remimazolam and Propofol on Intraoperative Motor Evoked Potentials During Spinal Surgery: A Randomized Crossover Trial" Journal of Clinical Medicine 14, no. 15: 5491. https://doi.org/10.3390/jcm14155491
APA StyleKim, B. R., Kim, H.-B., Kim, M. S., Lim, B. G., & Oh, S. K. (2025). Influence of Remimazolam and Propofol on Intraoperative Motor Evoked Potentials During Spinal Surgery: A Randomized Crossover Trial. Journal of Clinical Medicine, 14(15), 5491. https://doi.org/10.3390/jcm14155491






