Surgical Management of Pulmonary Typical Carcinoids: A Single-Centre Experience Comparing Anatomical and Non-Anatomical Resections
Abstract
1. Introduction
2. Materials and Methods
- -
- Non-anatomical resection, including segmentectomies performed for tumors greater than 2 cm in diameter, wedge resections, and bronchotomy with bronchoplasty (with exclusive resection of the bronchial tissue);
- -
- Anatomical resection, including segmentectomy for tumors less than or equal to 2 cm in diameter, lobectomy, pneumonectomy, and sleeve lobectomy.
- -
- Peripheral (in the outer third of the lung);
- -
- Central;
- -
- Endo-peribronchial, defined as any tumour visualized directly during bronchoscopy or in association with lung atelectasis and/or obstructive pneumonia [22] or close to the nearest bronchus at a CT scan.
Statistics
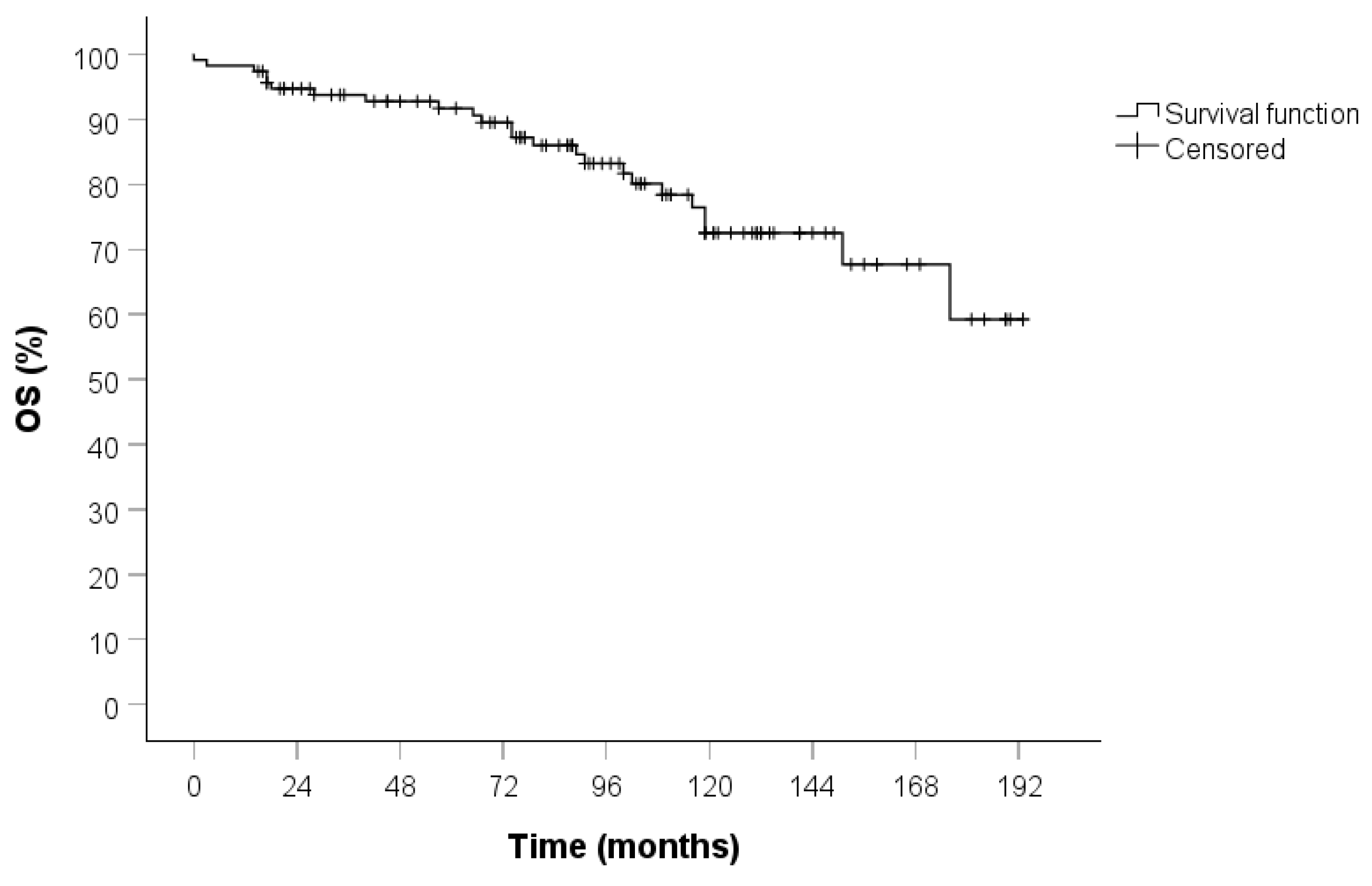
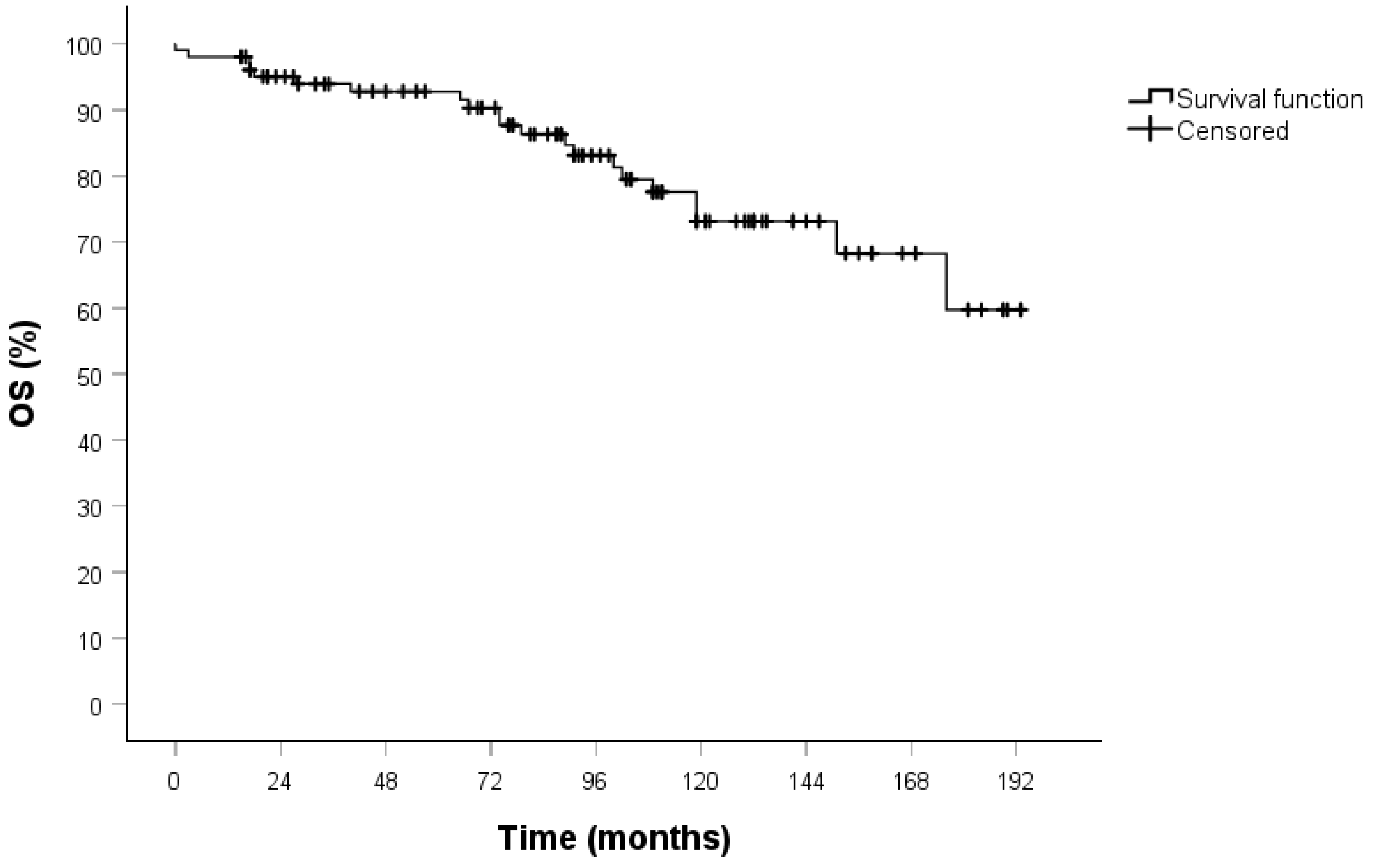
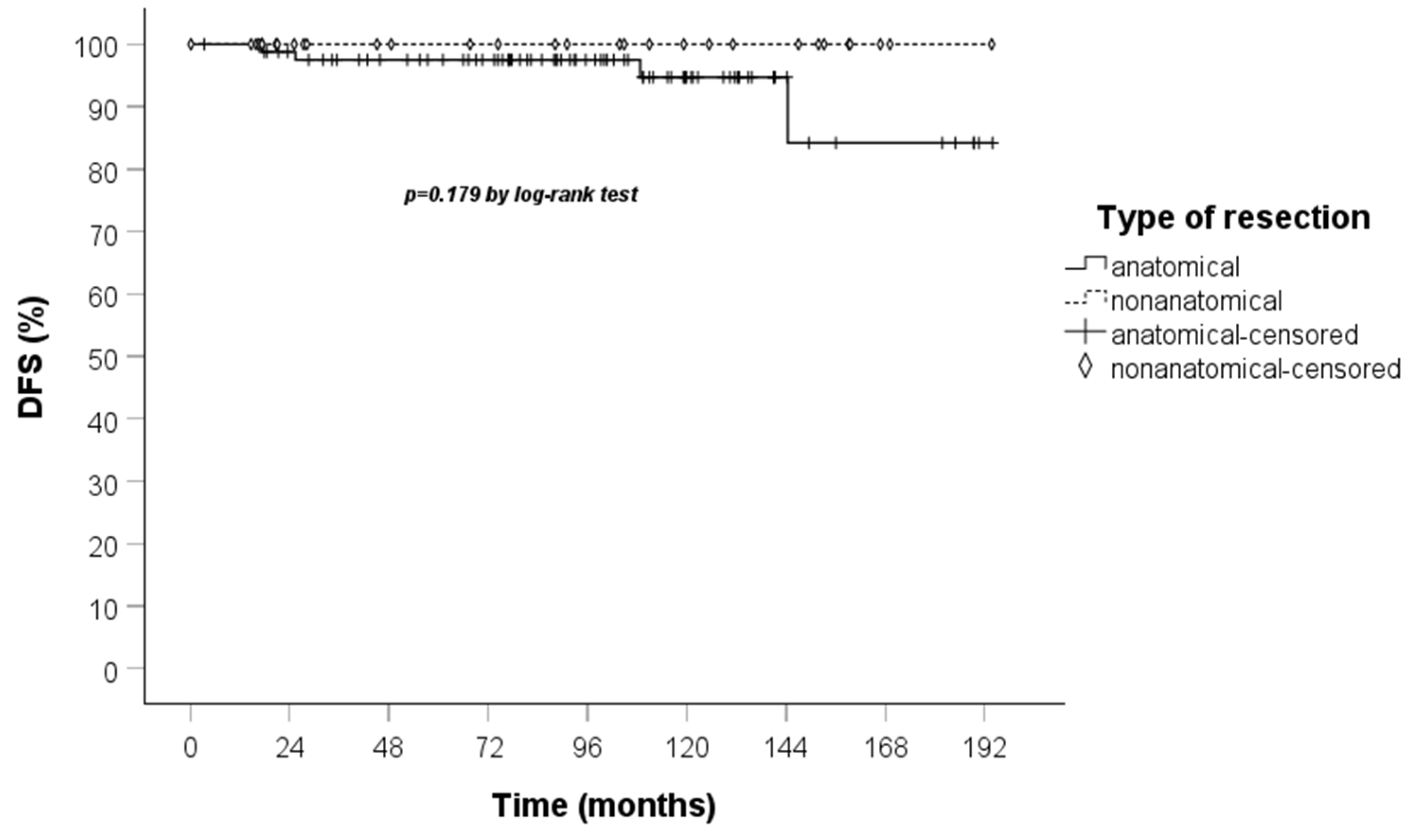
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TC | Typical carcinoid |
| OS | Overall survival |
| CCI | Charlson–Deyo comorbidity index |
| DFS | Disease-free survival |
| L-NETs | Lung neuroendocrine tumors |
| WHO | World Health Organization |
| AC | Atypical carcinoid |
| LCNEC | Large cell neuroendocrine carcinoma |
| SCLC | Small cell lung cancer |
| HPF | High-power fields |
| NCCN | National Comprehensive Cancer Network |
| ESMO | European Society for Medical Oncology |
| IASCL | International Association for the Study of Lung Cancer |
| CommNET | Commonwealth Neuroendocrine Tumour Research Collaboration |
| NANETS | North American Neuroendocrine Tumour Society |
| CT | Computed tomography |
| CDCC | Clavien–Dindo Complications Classification |
| CSS | Cancer specific survival |
| NSCLC | Non-small cell lung cancer |
| SEER | Surveillance, Epidemiology, and End Results |
| NET-WG | European Society of Thoracic Surgeon Neuroendocrine Tumours Working Group |
References
- Rekhtman, N. Lung neuroendocrine neoplasms: Recent progress and persistent challenges. Mod. Pathol. 2022, 35 (Suppl. 1), 36–50. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicolson, A. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2015. [Google Scholar]
- Travis, W.D. Pathology and diagnosis of neuroendocrine tumors: Lung neuroendocrine. Thorac. Surg. Clin. 2014, 24, 257–266. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroen-docrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Korse, C.M.; Taal, B.G.; van Velthuysen, M.-L.F.; Visser, O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: Experience of two decades of cancer registry. Eur. J. Cancer 2013, 49, 1975–1983. [Google Scholar] [CrossRef]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. Off. J. Eur. SocMed. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef]
- Wolin, E.M. Challenges in the diagnosis and management of well-differentiated neuroendocrine tumors of the lung (typical and atypical carcinoid): Current status and future considerations. Oncologist 2015, 20, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Neuroendocrine and Adrenal Tumors. Version 2.2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf (accessed on 20 December 2024).
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.; Frilling, A.; de Herder, W.; Hörsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Cooke, D.T.; Jett, J.R.; David, E.A. Extent of Resection and Lymph Node Assessment for Clinical Stage T1aN0M0 Typical Carcinoid Tumors. Ann. Thorac. Surg. 2018, 105, 207–213. [Google Scholar] [CrossRef]
- Fox, M.; Van Berkel, V.; Bousamra, M., II; Sloan, S.; Martin, R.C., II. Surgical management of pulmonary carcinoid tumors: Sublobar resection versus lobectomy. Am. J. Surg. 2013, 205, 200–208. [Google Scholar] [CrossRef]
- Raz, D.J.; Nelson, R.A.; Grannis, F.W.; Kim, J.Y. Natural history of typical pulmonary carcinoid tumors: A comparison of nonsurgical and surgical treatment. Chest 2015, 147, 1111–1117. [Google Scholar] [CrossRef]
- Yendamuri, S.; Gold, D.; Jayaprakash, V.; Dexter, E.; Nwogu, C.; Demmy, T. Is sublobar resection sufficient for carcinoid tumors? Ann. Thorac. Surg. 2011, 92, 1774–1778, discussion 1778–1779. [Google Scholar] [CrossRef] [PubMed]
- Bachman, K.C.; Worrell, S.G.; Linden, P.A.; Gray, K.E.; Argote-Greene, L.M.; Towe, C.W. Wedge Resection Offers Similar Survival to Segmentectomy for Typical Carcinoid Tumors. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mei, T. Sublobar resection versus lobectomy for patients with stage T1-2N0M0 pulmonary typical carcinoid tumours: A population-based propensity score matching analysis. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac125. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, K.; Liu, J.; Zeng, Y.; Bie, F.; Wang, G.; Du, J. Wedge resection is equal to segmental resection for pulmonary typical carcinoid patients at localized stage: A population-based analysis. PeerJ 2019, 7, e7519. [Google Scholar] [CrossRef]
- Filosso, P.L.; Guerrera, F.; Falco, N.R.; Thomas, P.; Yuste, M.G.; Rocco, G.; Welter, S.; Casado, P.M.; Rendina, E.A.; Venuta, F.; et al. Anatomical resections are superior to wedge resections for overall survival in patients with Stage 1 typical carcinoids†. Eur. J. Cardio Thorac. Surg. 2019, 55, 273–279. [Google Scholar] [CrossRef]
- Del Calvo, H.; Nguyen, D.T.; Chan, E.Y.; Chihara, R.; Graviss, E.A.; Kim, M.P. Anatomic Pulmonary Resection is Associated with Improved Survival in Typical Carcinoid Lung Tumor Patients. J. Surg. Res. 2022, 275, 352–360. [Google Scholar] [CrossRef]
- Ducrocq, X.; Thomas, P.; Massard, G.; Barsotti, P.; Giudicelli, R.; Fuentes, P.; Wihlm, J.-M. Operative risk and prognostic factors of typical bronchial carcinoid tumors. Ann. Thorac. Surg. 1998, 65, 1410–1414. [Google Scholar] [CrossRef]
- Daddi, N.; Ferolla, P.; Urbani, M.; Semeraro, A.; Avenia, N.; Ribacchi, R.; Puma, F.; Daddi, G. Surgical treatment of neuroendocrine tumors of the lung. Eur. J. Cardio Thorac. Surg. 2004, 26, 813–817. [Google Scholar] [CrossRef][Green Version]
- Singh, S.; Bergsland, E.K.; Card, C.M.; Hope, T.A.; Kunz, P.L.; Laidley, D.T.; Lawrence, B.; Leyden, S.; Metz, D.C.; Michael, M.; et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients with Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J. Thorac. Oncol. 2020, 15, 1577–1598. [Google Scholar] [CrossRef]
- Detterbeck, F.C. Management of carcinoid tumors. Ann. Thorac. Surg. 2010, 89, 998–1005. [Google Scholar] [CrossRef]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits. 2019, 12, 188–197. [Google Scholar]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Travis, W.D. Advances in neuroendocrine lung tumors. Ann. Oncol. 2010, 21, vii65–vii71. [Google Scholar] [CrossRef]
- Lababede, O.; Meziane, M.A. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist 2018, 23, 844–848. [Google Scholar] [CrossRef]
- Travis, W.D.; Rush, W.; Flieder, D.B.; Falk, R.; Fleming, M.V.; Gal, A.A.; Koss, M.N. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am. J. Surg. Pathol. 1998, 22, 934–944. [Google Scholar] [CrossRef]
- Travis, W.D.; Colby, T.V.; Corrin, B.; Shimosato, Y.; Brambilla, E. Histological Typing of Lung and Pleural Tumors; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1999. [Google Scholar]
- Arrigoni, M.G.; Woolner, L.B.; Bernatz, P.E. Atypical carcinoid tumors of the lung. J. Thorac. Cardiovasc. Surg. 1972, 64, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Yazici, U.; Gulgosteren, M.; Agackiran, Y.; Kaya, S.; Gulhan, E.; Tastepe, I.; Karaoglanoglu, N. Long-term outcomes and prognostic factors of patients with surgically treated pulmonary carcinoid: Our institutional experience with 104 patients. Eur. J. Cardio-Thorac. Surg. 2011, 39, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.G.; Shibani, A.; Panjwani, N.; Arab, A.; Shepherd, J.; Stitt, L.W.; Inculet, R. Usefulness of Ki-67, Mitoses, and Tumor Size for Predicting Metastasis in Carcinoid Tumors of the Lung: A Study of 48 Cases at a Tertiary Care Centre in Canada. Lung Cancer Int. 2015, 2015, 545601. [Google Scholar] [CrossRef] [PubMed]
- Herde, R.F.; Kokeny, K.E.; Reddy, C.B.; Akerley, W.L.; Hu, N.; Boltax, J.P.; Hitchcock, Y.J. Primary Pulmonary Carcinoid Tumor: A Long-term Single Institution Experience. Am. J. Clin. Oncol. 2018, 41, 24–29. [Google Scholar] [CrossRef]
- Furqan, M.; Tien, Y.-Y.; Schroeder, M.C.; Parekh, K.R.; Keech, J.; Allen, B.G.; Thomas, A.; Zhang, J.; Clamon, G.; Abu Hejleh, T. Lobar versus sub-lobar surgery for pulmonary typical carcinoid, a population-based analysis. J. Thorac. Dis. 2018, 10, 5850–5859. [Google Scholar] [CrossRef]
- Soldath, P.; Petersen, R.H. The Surgical Management of Lung Neuroendocrine Neoplasms. Cancers 2023, 15, 1695. [Google Scholar] [CrossRef]
- La Salvia, A.; Persano, I.; Siciliani, A.; Verrico, M.; Bassi, M.; Modica, R.; Audisio, A.; Zanata, I.; Marinucci, B.T.; Trevisi, E.; et al. Prognostic significance of laterality in lung neuroendocrine tumors. Endocrine 2022, 76, 733–746. [Google Scholar] [CrossRef]
- Filosso, P.L.; Guerrera, F.; Evangelista, A.; Welter, S.; Thomas, P.; Casado, P.M.; Rendina, E.A.; Venuta, F.; Ampollini, L.; Brunelli, A.; et al. Prognostic model of survival for typical bronchial carcinoid tumours: Analysis of 1109 patients on behalf of the European Association of Thoracic Surgeons (ESTS) Neuroendocrine Tumours Working Group. Eur. J. Cardio Thorac. Surg. 2015, 48, 441–447, discussion 447. [Google Scholar] [CrossRef]
- Riely, G.J.; Wood, D.E.; Ettinger, D.S.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. Non–Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 249–274. [Google Scholar] [CrossRef]
- Filosso, P.L.; Rena, O.; Donati, G.; Casadio, C.; Ruffini, E.; Papalia, E.; Oliaro, A.; Maggi, G. Bronchial carcinoid tumors: Surgical management and long-term outcome. J. Thorac. Cardiovasc. Surg. 2002, 123, 303–309. [Google Scholar] [CrossRef]
- Nussbaum, D.P.; Speicher, P.J.; Gulack, B.C.; Hartwig, M.G.; Onaitis, M.W.; D’amico, T.A.; Berry, M.F. Defining the role of adjuvant chemotherapy after lobectomy for typical bronchopulmonary carcinoid tumors. Ann. Thorac. Surg. 2015, 99, 428–434. [Google Scholar] [CrossRef]
- Westin, G.F.M.; Alsidawi, S.; Leventakos, K.; Halfdanarson, T.R.; Molina, J.R. Impact of adjuvant chemotherapy in non-metastatic node positive bronchial neuroendocrine tumors (BNET). J. Clin. Oncol. 2017, 35, 8533. [Google Scholar] [CrossRef]

| Entire Population | Stage I Population | |
|---|---|---|
| Characteristics | Statistics | Statistics |
| Age | 63 (13) | 64 (12) |
| Gender | ||
| M | 31 (27) | 29 (28.4) |
| F | 84 (73) | 73 (71.6) |
| CCI | 3.6 (1.6) | 3.6 (1.6) |
| BMI | 28 (5.1) | 28.3 (5) |
| History of smoking | ||
| No | 56 (49) | 49 (48) |
| Ex | 41 (36) | 38 (37.3) |
| Yes | 18 (16) | 15 (14.7) |
| Side | ||
| Right | 67 (58) | 62 (60.8) |
| Left | 48 (42) | 40 (39.2) |
| Location of the tumor | ||
| Central | 64 (56) | 56 (54.9) |
| Peripheral | 51 (44) | 46 (45.1) |
| Endo-peribronchial lesion | ||
| No | 35 (30) | 30 (29.4) |
| Yes | 80 (70) | 72 (70.6) |
| Entire Population | Stage I Population | |
|---|---|---|
| Characteristics | Statistics | Statistics |
| Type of resection | ||
| Anatomical | 83 (72) | 72 (71) |
| Non-anatomical | 32 (28) | 30 (29) |
| Type of surgery | ||
| Open | 64 (56) | 57 (56) |
| Robot | 45 (39) | 39 (38) |
| Vats | 6 (5) | 6 (6) |
| Post-operative complications | ||
| No | 102 (89) | 87 (85) |
| Yes | 13 (11) | 15 (15) |
| Chest drain duration (days) | 3.7 (2.5) | 3.8 (2.6) |
| Hospital stay (days) | 5 (1.7) | 5 (1.7) |
| pT stage | ||
| 1a | 22 (19) | 22 (22) |
| 1b | 47 (41) | 45 (44) |
| 1c | 29 (25) | 28 (27) |
| 2a | 8 (7) | 7 (7) |
| 2b | 4 (3) | |
| 3 | 3 (3) | |
| 4 | 2 (2) | |
| pN stage | ||
| 0 | 111 (97) | 102 (100) |
| 1 | 4 (3) | |
| pStage | ||
| IA1 | 22 (19) | 22 (22) |
| IA2 | 45 (39) | 45 (44) |
| IA3 | 28 (24) | 28 (27) |
| IB | 7 (6) | 7 (7) |
| IIA | 4 (3) | |
| IIB | 7 (6) | |
| IIIA | 2 (2) | |
| Ki67% | 5.4 (3.5) | 5.1 (3) |
| Mitoses | 0.91 (0.4) | 0.9 (0.4) |
| Tumor dimension (mm) | 20.5 (12.3) | 18.1 (7.9) |
| Univariate Analysis | Multivariate Analysis (Step-Wise Method) | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI Lower | 95% CI Upper | p-Value | HR | 95% CI Lower | 95% CI Upper | p-Value | |
| Gender: (0) M, (1) F | 0.785 | 0.325 | 1896 | 0.591 | ||||
| Age | 1.044 | 1.004 | 1.086 | 0.029 | 0.555 | |||
| History of smoking: (0) no, (1) ex, (2) yes | 1.061 | 0.619 | 1.820 | 0.829 | ||||
| BMI | 1.028 | 0.954 | 1.108 | 0.465 | ||||
| Charlons-Deyo Comorbidity Index | 1.696 | 1.303 | 2.208 | <0.001 | 1.753 | 1.322 | 2.325 | <0.001 |
| Side: (0) dx, (1) sx | 1.040 | 0.459 | 2.357 | 0.925 | ||||
| Endo-peribronchial lesion: (0) no, (1) si | 0.611 | 0.271 | 1.376 | 0.234 | ||||
| Location of the tumor: (0) central, (1) peripheral | 1.740 | 0.778 | 3.891 | 0.177 | ||||
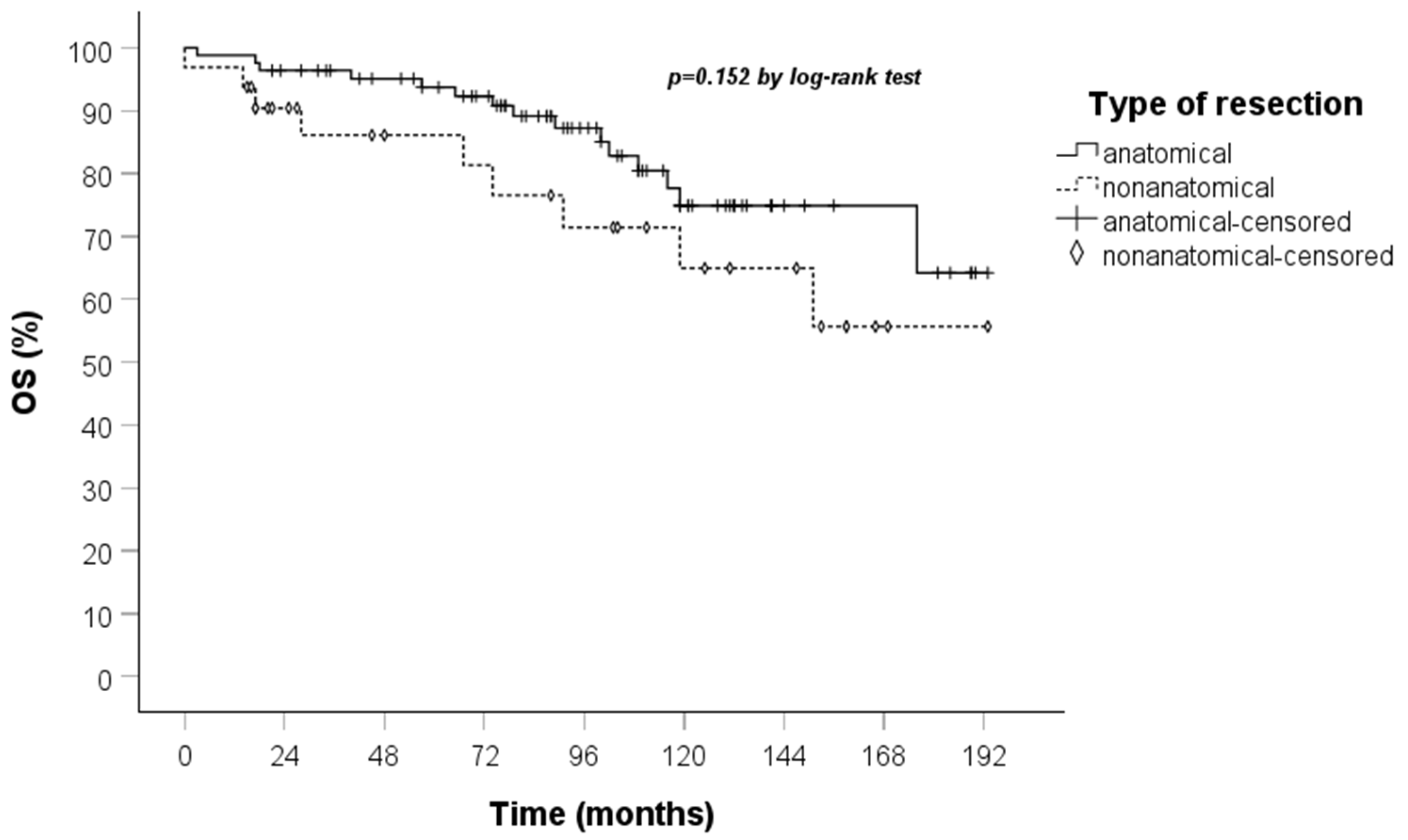
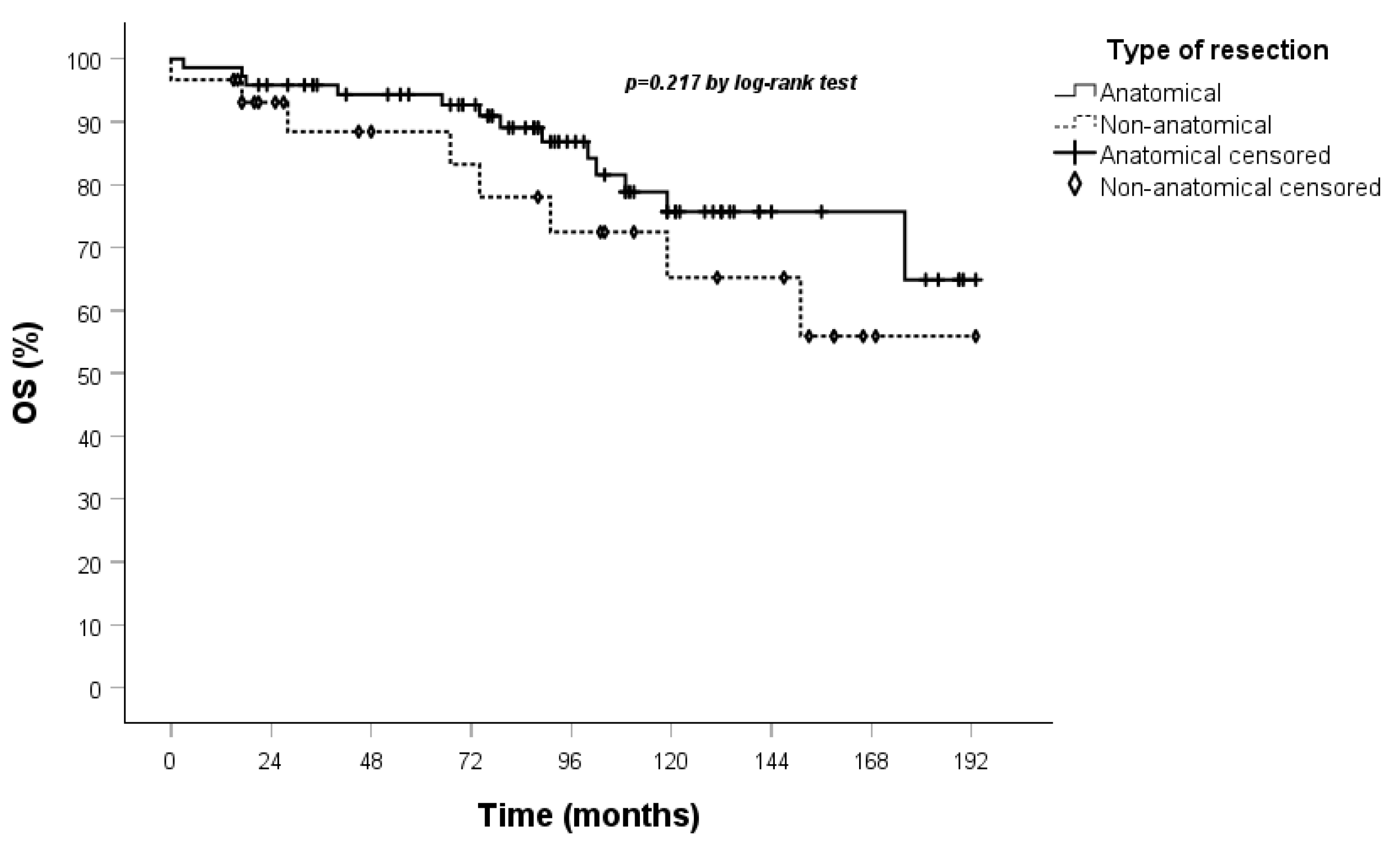
| Type of resection: (0) anatomical, (1) nonanatomical | 1.822 | 0.791 | 4.197 | 0.159 | 0.056 | |||
| Post-operative complications: (0) no, (1) yes | 1.742 | 0.689 | 4.400 | 0.241 | ||||
| Chest drain duration | 0.936 | 0.746 | 1.174 | 0.566 | ||||
| Hospital stay | 1.024 | 0.810 | 1.295 | 0.841 | ||||
| Mitoses | 0.944 | 0.310 | 2.869 | 0.919 | ||||
| Dimension of the tumor | 1.010 | 0.982 | 1.039 | 0.480 | ||||
| T | 1.174 | 0.645 | 2.138 | 0.599 | ||||
| pStage | 1.244 | 0.504 | 3.070 | 0.636 | ||||
| Left lower lobe: (0) no, (1) yes | 2.373 | 1.031 | 5.461 | 0.042 | 2.492 | 1.069 | 5.807 | 0.034 |
| Left upper lobe: (0) no, (1) yes | 0.676 | 0.200 | 2.287 | 0.529 | ||||
| Right lower lobe: (0) no, (1) yes | 0.737 | 0.251 | 2.164 | 0.578 | ||||
| Right upper lobe: (0) no, (1) yes | 1.045 | 0.311 | 3.509 | 0.943 | ||||
| Middle lobe: (0) no, (1) yes | 0.641 | 0.237 | 1.729 | 0.379 | ||||
| Univariate Analysis | Multivariate Analysis (Step-Wise Method) | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI Lower | 95% CI Upper | p-Value | HR | 95% CI Lower | 95% CI Upper | p-Value | |
| Gender: (0) M, (1) F | 1.115 | 0.408 | 3.046 | 0.832 | ||||
| Age | 1.036 | 0.994 | 1.081 | 0.096 | 0.994 | 0.943 | 1.048 | 0.830 |
| History of smoking: (0) no, (1) ex, (2) yes | 1.156 | 0.652 | 2.049 | 0.620 | ||||
| BMI | 1.008 | 0.929 | 1.093 | 0.853 | ||||
| Charlons-Deyo Comorbidity Index | 1.625 | 1.230 | 2.148 | <0.001 | 1.655 | 1.199 | 2.284 | 0.002 |
| Side: (0) dx, (1) sx | 1.259 | 0.527 | 3.008 | 0.605 | ||||
| Endo-peribronchial lesion: (0) no, (1) si | 0.605 | 0.255 | 1.440 | 0.256 | ||||
| Location of the tumor: (0) central, (1) peripheral | 1.817 | 0.764 | 4.319 | 0.177 | ||||
| Type of resection: (0) anatomical, (1) nonanatomical | 1.738 | 0.714 | 4.231 | 0.223 | ||||
| Post-operative complications: (0) no, (1) yes | 1.873 | 0.725 | 4.843 | 0.195 | ||||
| Chest drain duration | 0.888 | 0.681 | 1.159 | 0.383 | ||||
| Hospital stay | 1.013 | 0.787 | 1.306 | 0.917 | ||||
| Mitoses | 0.926 | 0.267 | 3.214 | 0.904 | ||||
| Dimension of the tumor | 0.994 | 0.942 | 1,.049 | 0.835 | ||||
| T | 0.042 | 0.000 | 21.761 | 0.320 | ||||
| pStage | ||||||||
| Left lower lobe: (0) no, (1) yes | 2.060 | 0.825 | 5.145 | 0.122 | ||||
| Left upper lobe: (0) no, (1) yes | 0.539 | 0.124 | 2.343 | 0.409 | ||||
| Right lower lobe: (0) no, (1) yes | 0.834 | 0.279 | 2.489 | 0.745 | ||||
| Right upper lobe: (0) no, (1) yes | 1.125 | 0.331 | 3.827 | 0.850 | ||||
| Middle lobe: (0) no, (1) yes | 0.751 | 0.272 | 2.070 | 0.580 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zirafa, C.C.; Manfredini, B.; Romano, G.; Ceccarelli, I.; Calabrò, F.; Morganti, R.; Alì, G.; Melfi, F.; Davini, F. Surgical Management of Pulmonary Typical Carcinoids: A Single-Centre Experience Comparing Anatomical and Non-Anatomical Resections. J. Clin. Med. 2025, 14, 5488. https://doi.org/10.3390/jcm14155488
Zirafa CC, Manfredini B, Romano G, Ceccarelli I, Calabrò F, Morganti R, Alì G, Melfi F, Davini F. Surgical Management of Pulmonary Typical Carcinoids: A Single-Centre Experience Comparing Anatomical and Non-Anatomical Resections. Journal of Clinical Medicine. 2025; 14(15):5488. https://doi.org/10.3390/jcm14155488
Chicago/Turabian StyleZirafa, Carmelina Cristina, Beatrice Manfredini, Gaetano Romano, Ilaria Ceccarelli, Fabrizia Calabrò, Riccardo Morganti, Greta Alì, Franca Melfi, and Federico Davini. 2025. "Surgical Management of Pulmonary Typical Carcinoids: A Single-Centre Experience Comparing Anatomical and Non-Anatomical Resections" Journal of Clinical Medicine 14, no. 15: 5488. https://doi.org/10.3390/jcm14155488
APA StyleZirafa, C. C., Manfredini, B., Romano, G., Ceccarelli, I., Calabrò, F., Morganti, R., Alì, G., Melfi, F., & Davini, F. (2025). Surgical Management of Pulmonary Typical Carcinoids: A Single-Centre Experience Comparing Anatomical and Non-Anatomical Resections. Journal of Clinical Medicine, 14(15), 5488. https://doi.org/10.3390/jcm14155488






