Oral and Dental Sequelae After Oncological Treatment in Children: A Systematic Review
Abstract
1. Introduction
- P (patient, problem, population): patients who had cancer during their childhood from 0 to 14 years of age and have received treatment.
- I (intervention): use of different therapies for the treatment of childhood cancer (chemotherapy, radiotherapy, immunotherapy).
- C (comparison, control): childhood cancer-free patients aged 0–14 years.
- O (outcome): oral and dental sequelae after treatment.
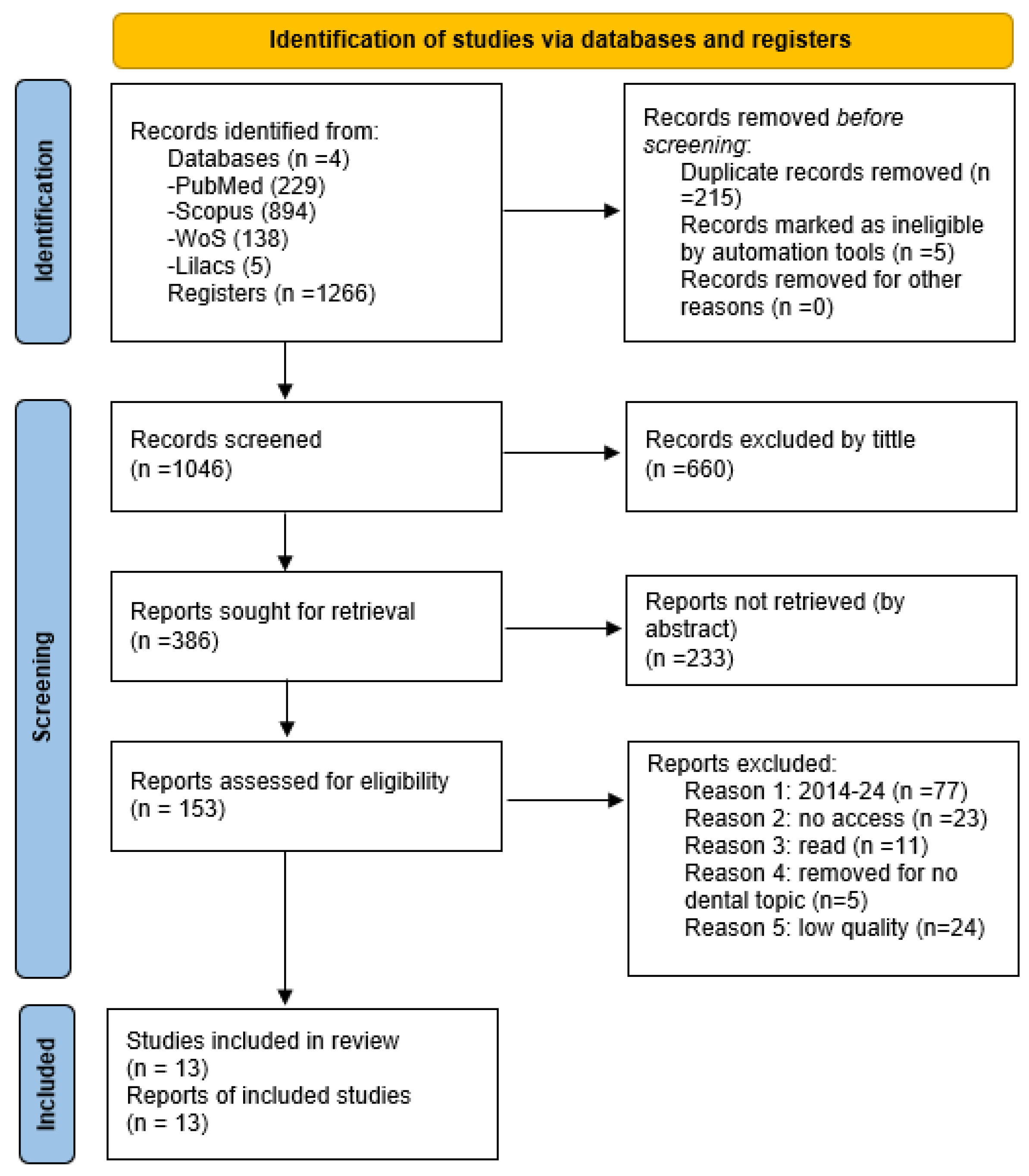
2. Methods
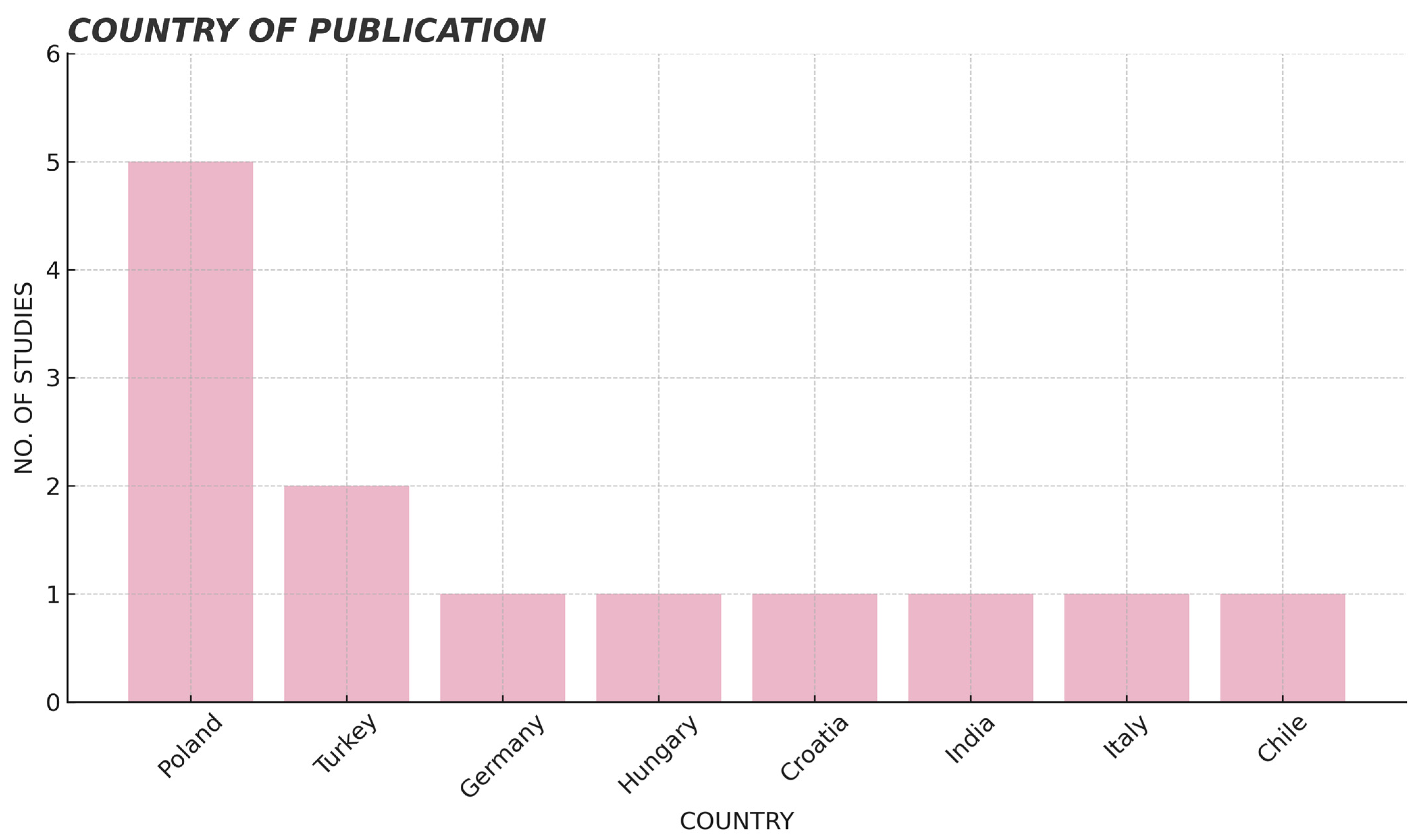
3. Results
| Study | Type of Condition Studied | Country | Study Design | Criteria | Total Score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Exposure | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||||
| Krasuska-Sławińska et al., 2016 [22]. | Congenital defects in permanent teeth | Poland | Cases and controls | * | * | - | * | * | * | * | * | 7 |
| Kilin et al., 2018 [26]. | Dental anomalies | Turkey | Cases and controls | * | * | - | * | * | * | * | * | 7 |
| Nemeth et al., 2014 [27]. | Late oral sequelae | Hungary | Cases and controls | * | * | * | * | * | * | * | * | 8 |
| Proc et al., 2016 [23] | Dental complications | Poland | Cases and controls | * | * | - | * | * | * | * | * | 7 |
| Katarzyna Olszewska et al., 2016 [24] | Oral microflora | Poland | Cases and controls | * | * | - | * | * | * | * | * | 7 |
| Cansu Kış et al., 2021 [16] | Mandibular bone | Turkey | Cases and controls | * | * | - | * | * | * | * | * | 7 |
| Study | Type of Condition Studied | Country | Study Design | Criteria | Total Score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Exhibition | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||||
| Longo et al., 2023 [28] | Periodontal tissues | Germany | Retrospective cohort | * | * | * | * | * | - | * | * | 7 |
| Shayani 2021 [17] | Caries and gingivitis | Chile | Retrospective cohort | * | * | * | - | * | * | * | * | 7 |
| Atif et al., 2022 [18] | Dentition | India | Cross-sectional cohort | * | * | * | * | * | - | * | * | 7 |
| Guagnano et al., 2022 [19] | Dentition | Italy | Cross-sectional cohort | * | * | * | - | * | * | * | * | 7 |
| Proc et al., 2019 [29] | Enamel demineralization | Poland | Cross-sectional cohort | * | * | * | - | * | * | * | * | 7 |
| Proc et al., 2022 [20] | Malocclusion | Poland | Cross-sectional cohort | * | * | * | * | * | * | * | * | 8 |
| Articles | Zulijani et al., 2022 [21] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of condition studied | Late dental defect | |||||||||||
| Country | Croatia | |||||||||||
| Type of study | Case report. | |||||||||||
| Criteria | ||||||||||||
| Subject | 1 | * | ||||||||||
| Key words | 2 | * | ||||||||||
| Summary | 3a | 3b | 3c | 3d | * | * | * | * | ||||
| Introduction | 4 | * | ||||||||||
| Patient information | 5a | 5b | 5c | 5d | * | * | * | * | ||||
| Clinical findings | 6 | * | ||||||||||
| Timeline | 7 | * | ||||||||||
| Evaluation diagnostic | 8a | 8b | 8c | 8d | * | - | * | * | ||||
| Therapeutic intervention | 9a | 9b | 9c | * | * | - | ||||||
| Follow-up and results | 10a | 10b | 10c | 10d | * | - | - | - | ||||
| Discussion | 11a | 11b | 11c | 11d | * | * | * | * | ||||
| Patient perspective | 12 | * | ||||||||||
| Informed consent | 13 | * | ||||||||||
| Total | 25 | |||||||||||
3.1. Study Design
3.2. Groups or Sample
3.3. Age of Participants
3.4. Type of Treatment Performed (Chemotherapy, Radiotherapy, Immunotherapy)
3.5. Oral/Dental Sequela
4. Discussion
5. Conclusions
- The lesions are different according to the therapy used. In children treated with chemotherapy, the appearance of sequelae related to the alteration of the odontogenesis process is common. On the other hand, children treated with radiotherapy have greater involvement in local areas such as the salivary glands. In addition, there is a correlation between radiotherapy and the presence of dental malocclusion.
- It is not possible to determine which therapy has a greater deleterious effect. It could be said that the most aggressive therapy is chemotherapy because of its systemic effect, while radiotherapy has a field of action more localized. Thus, the combination of both therapies in the studies makes comparison difficult, which could mean a line of research for future studies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Cohort Results
| Author and Year | Type of Study | Participants (No., Age, Type) | Type of Medication | Type of Cancer, Diagnostic Criteria, Type of Tooth Examined and Result Recorded | Results | p-Value | Conclusion |
|---|---|---|---|---|---|---|---|
| Longo et al., 2023 [28] | Retrospective cohort | No. of participants: 72 GS: 36 (cancer patients) GC: 36 (healthy patients) Age: 12.0 ± 3.9/4.0 | Chemotherapy (QT): n = 36 Chemotherapy (QT) and radiotherapy (RT): n = 9 | Type of cancer: lymphoid leukemia, myelodysplastic syndrome, Hodgkin’s lymphoma, Non-Hodgkin’s lymphoma, Burkitt’s lymphoma, intracranial and intraspinal cancer, renal carcinoma, gonadal carcinoma, rhabdomyosarcoma, other sarcomas Diagnostic criteria:
Type of tooth examined: permanent dentition Recorded result: periodontal status and oral microbiome | Saliva samples Porphyromonas gingivalis
Aggregatibacter actinomycetemcomitans
Tannerella forsythia
Fusobacterium nucleatum
Clinical parameters IP CC: 30.5% (5–73)/GC: 22.6% (2–61) IG CC 28.8% (5–60)/GC 17.3% (2–44) PS CC 1.77 (1.43–2.43)/GC 1.61 (1.30–2.09) CAL CC 1.77 (1.42–2.44)/GC 1.57 (1.21–2.09) CAL ≥ 4 mm CC 28 GC 16 Periodontal status Periodontal health: CC 3 GC 7 Gingivitis CC 16 GC 24 Periodontitis CC 17 GC 5 | p = 0.33 p = 0.37 p = 0.29 p = 0.02 p = 0.02 p ≤ 0.001 p ≤ 0.008 p ≤ 0.001 p ≤ 0.001 | Antineoplastic therapy in cancer patients has a negative impact on periodontal and microbiological clinical parameters. |
| Shayani et al., 2022 [17] | Retrospective cohort | No. of participants: 69 GS (Study group): 23 GC (control group): 46 Age: 3.25–12.9 years) | QT | Type of cancer: acute lymphoblastic leukemia Diagnostic criteria:
Type of tooth examined: permanent dentition Recorded outcome: dental caries, presence of new lesions during treatment and gingivitis | Clinical parameters: Caries index: Exposed: [4.48 (SD 3.51)] Not exposed: [3.01 (SD 2.92)] DMFT index: Exposed:1.58 (SD 2.87) Unexposed: 0.31 (SD 0.64) No. of teeth with presence of new lesions during treatment Exposed: 60.87% Not exposed: 28.26% Gingivitis Exposed: 47.83%; −69.57% Not exposed: 84.78% | p = 0.54 p < 0.05 p = 0.008 p = 0.001 | The ALL IC-BFM 2009 chemotherapy protocol in patients with acute lymphoblastic leukemia is a risk factor in the development of new caries and gingivitis lesions. |
Appendix A.2. Results of Cases and Controls
| Author and Year | Type of Study | Participants (No., Age) | Type of Medication | Type of Cancer, Diagnostic Criteria, Type of Tooth Examined and Result Recorded | Results | p-Value | Conclusion |
|---|---|---|---|---|---|---|---|
| Krasuska-Sławińska et al., 2016 [22] | Cases and controls | No. of participants: 120 GS: 60 (cancer patients) GC:60 (healthy patients) Age: <18 | Chemotherapy Drugs VCR, CTX, ADM, VP-16, CDDP, IF, ACTD | Diagnosis: Burkitt’s lymphoma (15.0%), nephroblastoma (13.0%), neuroblastoma (10.0%), histiocytosis (8.3%), rhabdomyosarcoma (6.7%), Ewing’s disease sarcoma (6.7%), medulloblastoma (5.0%), neurofibromatosis type I (5.0%) and others (19.7%) Criteria: Clinical
Data analysis: Mann Whitney U and Spearman’s RHO metric test Type of tooth examined: permanent dentition Recorded result: dental alterations | GS CG Enamel defects: 53/88.3 24/40.0 Opacities: 34/56.6 19/32.3 Hypoplasia: 7/11.6 1/1.6 Combination of injuries: 12/20 4/6.6 Tooth resorption: 36/60.0 25/41.6 V-shaped: 18/30.00 0/0.0 Microdontia: 12/21.67 0/0.0 Without dental yolk: 16/26.67 4/6.66 From >0: 45/75.00 2643.33 Others: Taurodontism: 12/20.00 3/5.00 Mesiodens: 1/1.67 0/0.0 Dentinoma: 18/30.0 9/15.0 Impacted teeth: 6/10.00 2/3.33 | p = 0.000 p = 0.006 p = 0.001 p = 0.0068 p = 0.0450 p = 0.000 p = 0.0003 p = 0.0035 p = 0.0004 p = 0.0135 p = 0.3254 p = 0.0505 p = 0.1466 | Chemotherapy in the pediatric setting affects the appearance of new congenital dental alterations; hypodontia, microdontia, root resorption, taurodontism and congenital enamel defects. The drugs used in chemotherapy have a negative influence on the dental level, and their effect is more detrimental with the increase in the dose, the duration of therapy and the severity of the effects derived from chemotherapy such as mucositis and vomiting. |
| Kilin et al., 2018 [26] | Cases and controls | Total number of participants: 165 GS: Group A (9 months–4 years): 59 Group B (5–7 years): 34 Average age of treatment: 3.75 + −2.01 Mean age at examination: 9.54 + −1.25 (8–13 years) GC 72 Average age: 10.60 + −2.40 (8–16 years) | QT and RT | Type of cancer: leukemia, lymphoma, neuroblastoma, renal tumor, retinoblastoma, liver tumor, soft tissue sarcoma, germ cell tumor, Langerhans cell histiocytosis Diagnostic criteria:
Type of tooth examined: permanent dentition Recorded result: dental alterations | GS (A and B): Hypodontia 21 (22.6) Microdontia 60 (64.5) Root malformations 24 (25.8) Enamel defects 22 (23.7) Hyperodontia 1 (1.1) GC Enamel defects: 7 (9.7) Comparison of patient groups according to age of treatment. Group A Group B Hypodontia 17 (28.8) 4 (11.8) Microdontics 42 (71.2) 18 (52.9) Malformation radicular 13 (22.0) 11 (32.4) Enamel defects 14 (23.7) 8 (23.5) Hyperodontia 1 (1.7) 0 (0.0) | p = 0.058 p = 0.077 p = 0.273 p = 0.982 p = 1.000 p = 0.982 p = 1.000 p = 0.058 p = 0.077 p = 0.273 p = 0.982 p = 1.000 | Patients treated with chemotherapy before the age of 7 years constituted a high-risk group for dental anomalies. The frequency of microdontia and hypodontia increased even more when the patient received oncologic treatment before the age of 5 years. |
| Nemeth et al., 2014 [27] | Cases and controls | Total number of participants: 78 GS: 38 GC: 40 (healthy) Age: 12 years old | QT | Type of cancer: lymphoblastic lymphoma, neuroblastoma, sarcoma, osteosarcoma, Hodgkin’s lymphoma Diagnostic criteria:
Type of tooth examined: permanent dentition Recorded outcome: long-term oral effects of chemotherapy and correlation between salivary flow and karyological status | caries index: Patients with QT (GS)
Healthy patients (CG)
Stimulated and unstimulated total saliva flow rates Patients with QT (GS) SSF: 0.85 ± 0.49 USF: 0.28 ± 0.26 PS: 1.643 ± 2.42 Buffer capacity:
Healthy patients (CG) SSF: 1.13 ± 0.48 USF: 0.378 ± 0.24 PS: 0.456 ± 0.32 Buffer capacity:
Correlation between USF, SSF, PS and DMFT GS USF SSF PS DMFT −0.32 −0.35 −0.49 GC USF SSF PS (no data) | p = 0.01 p = ≤0.05 p ≤ 0.1 p ≤ 0.001 p ≤ 0.01 p ≤ 0.01 (p ≤ 0.01) GS USF: p = 0.02 SSF: p = 0.02 PS: p = 0.001 | Chemotherapy in children could cause a decrease in total stimulated salivary flow, hyposalivation and, consequently, an increased risk of caries. |
| Proc et al., 2016 [23] | Case and controls | Total number of participants: 582 Study group: 61 Control group: 521 (healthy) Age: 5–18 | RT in head and neck | Type of cancer: lymphoblastic leukemia, acute non-lymphoblastic leukemia, B-cell non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, germinal tumor, brain tumor, Wilms’ tumor, hepatoblastoma, neuroblastoma, primitive neuroectodermal tumor or rhabdomyosarcoma Diagnostic criterion: OPG Type of tooth examined: permanent dentition Recorded result: dental anomalies | Dental agenesis SG CC IC 4 (1.63) 5 (0.23) IL 4 (1.63) 28 (1.34) C 2 (0.81) 10 (0.47) 1PM 1 (0.4) 9 (0.43) 2PM 31 (12.7) 33 (1.58) 1M 4 (1.63) 4 (0.19) 2M 23 (9.42) 2 (0.095) 3M NA Healthy teeth 175 (71.72) 1993 (95.63) Microdontia SG CC IC 0 0 IL 0 14 (0.67) C 0 1 (0.047) 1PM 16 (6.55) 1 (0.047) 2PM 25 (10.24) 0 1M 0 0 2M 24 (9.83) 2 (0.095) 3M 12 (4.91) 2 (0.095) Healthy teeth 167 (68.44) 2064 (99.04) Root shortening CI 0 7 (0.33) IL 0 6 (0.28) C 0 4 (0.19) 1PM 4 (1.63) 5 (0.23) 2PM 8 (3.27) 4 (0.19) 1M 14 (5.73) 4 (0.19) 2M 12 (4.91) 6 (0.28) 3M 0 0 Healthy teeth 206 (84.42) 2048 (98.27) | p = 0.001 p = 0.712 p = 0.478 p = 0.945 p = 0.001 p = 0.001 p = 0.001 p = 0.001 - p = 0.199 p = 0.734 p = 0.001 p = 0.001 - p = 0.001 p = 0.001 p = 0.001 p = 0.368 p = 0.407 p = 0.495 p = 0.001 p = 0.001 p = 0.001 p = 0.001 p = 0.001 | Children under 5 years of age are in a high-risk group for dental complications following cancer treatment. Basic chemotherapy has a considerable impact on the occurrence of dental anomalies. |
| Katarzyna Olszewska et al., 2016 [24]. | Case and controls | Total number of participants: 104 GS: 52 (37 patients with hematologic cancer and 15 patients with solid tumors) GC: 52 healthy patients Age: 3–17.5a | QT Medication (not specified) | Type of cancer: leukemia, lymphoma and solid tumors Diagnostic criteria: Streptococcus mutans evaluation: Dentocult SM Strips Mutans tests (Orion Diagnostica) Evaluation No. of Lactobacillus spp: Dentocult LB tests (Orion Diagnostica, Espoo, Finland) Results analysis: STATISTICA 10 of Windows Software (StatSoft Inc., Tulsa, OK, USA) Type of teeth examined: permanent and deciduous dentition Recorded result: no. of Lactobacillus sp. and Streptococcus mutans | Evaluation no. of Streptococcus mutans: 0 1 2 3 GS T1 11 28 12 1 T2 7 11 19 15 T3 8 16 25 3 GC 12 20 16 4 Evaluation nº of Lactobacillus spp: 0 103104105106 CFU/mL SG T1 14 21 15 1 1 1 T2 9 8 12 15 8 T3 7 15 21 8 1 CG 11 22 11 5 3 | Streptococcus mutans p = T1:0.29 p = T2:0.0144 * p = T3: 0.3389 Lactobacillus spp. p = T1: 0.3234 p = T2:0.0071 * p = T3:0.1280 | The alteration of the bacterial flora presents changes and is statistically related to the degree of neutropenia induced by chemotherapy. |
| Cansu kis et al., 2022 [16] | Case and controls | Total number of participants: 98 GS: 49 GC: 49 (healthy) Age: 14.5 ±4.4 years and 14.6 ±4.8 years | QT (dexametha- sone; prednisolone; methotrexate) | Diagnosis: Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, Burkitt’s lymphoma, acute lymphoblastic leukemia, brain tumor, osteosarcoma, neuroblastoma Diagnostic criteria:
Type of teeth examined: permanent and deciduous dentition Recorded result: alterations of the mandibular bone | Categories Klemetti index (KI) C1
| Statistically significant difference in C3 study group p = 0.015 * | Chemotherapy is related to the affectation of the mandibular bone structures in the long term in the patient’s life. The Klemetti Index is considered a good tool for the clinical diagnosis of mandibular bone conditions. |
Appendix A.3. Results of Case Reports
| Author and Year | Type of Study | Participants (No., Age) | Type of Medication | Diagnosis, Criteria, Type of Tooth Examined and Result Recorded | Results | p-Value | Conclusion |
|---|---|---|---|---|---|---|---|
| Zulijani et al., 2022 [21] | Case Report | Total number of participants: 1 Age: 9 | QT Drugs: cyclophosphamide vincristine, methotrexate, etoposide and carboplatin | Diagnosis: Anaplastic Ependymoma grade III Criteria: intraoral and extraoral examination, OPG Teeth examined: primary and permanent dentition (16, 55, 54, 11, 21, 63, 64, 65, 26, 36, 75, 74, 33, 32, 31, 41, 42, 43, 84, 85 and 46) Recorded result: dental anomalies | Mucositis Microdoncia: 12, 14, 16, 22, 24, 26, 32, 34, 36, 42, 44, 46 | Not described | There is an alteration of dental development in children treated with antineoplastic therapy, so dentists should keep in mind that these patients should be followed up after the end of treatment to reduce the consequences of the treatment. |
Appendix A.4. Results of Cross-Sectional Studies
| Author and Year | Type of Study | Participants (No., Age) | Type of Medication | Diagnosis, Criteria, Type of Tooth Examined and Result Recorded | Results | p-Value | Conclusion |
|---|---|---|---|---|---|---|---|
| Atif et al., 2022 [18] | Cross-sectional | No. of participants: Study Group (GS): 120 Control group (CG): 121 Mean age at dental examination: 14.3 (GS)–14.4 (GC) years Mean age (cancer diagnosis): 5.67 years | Chemotherapy (not specified) QT Home (i) <4a (ii) 4–5a (iii) 6–7a | Type of cancer: acute lymphoblastic leukemia (45%), soft tissue sarcoma, Hodgkin’s lymphoma, medulloblastoma, Langerhans cell histiocytosis, Retinoblastoma, primitive neuroectodermal tumor, Non-Hodgkin’s lymphoma, Osteosarcoma Diagnostic criteria:
Type of tooth examined: permanent dentition Recorded result: microdontia, hypodontia, teeth with abnormal anomaly and enamel development defects | Study Group (GS): Microdontia: 21 (17.5%) Hypodontia 6 (5%) Abnormally shaped teeth: 10 (8.33%) Enamel developmental defects: 45 (37.5%) Control group (CG): Microdontia: 10 (8.2%) Hypodontia: 3 (2.5%) Abnormally shaped teeth: 2 (1.65%) Enamel developmental defects: 27 (22.3%) No presence of any anomaly: GS: 57 GC: 82 Correlation between age of onset and presence of dental anomalies Microdontia
| Microdontia: 0.032 Hypodontia: 0.302 Abnormally shaped teeth: 0.017 Defects of enamel development: 0.01 p = 0.005 * p = 0.106 p = 0.853 p = 0.349 | Statistically significant association between dental anomalies and antineoplastic therapy, with prevalence of microdontia, teeth with abnormal appearance and enamel defects among childhood cancer survivors. |
| Guagnano et al., 2022 [19] | Cross-sectional | No. of participants: 104 Study group (GS): 52 Control group (CG): 52 (GS) Age at time of dental examination: 10.6 ± 3.8 years (GC): 11.5 ± 4.5 years Age of diagnosis of cancer: 3.8 ± 2.6 years | Chemotherapy (n = 36) Chemotherapy and radiotherapy (n = 16) Hematopoietic stem cell/bone marrow transplantation (n = 19/5) | Type of cancer: acute lymphoblastic leukemia, acute myeloblastic leukemia, medulloblastoma, familial hematological lymphohistiocytosis, lymphoma, juvenile myelomonocytic leukemia, Wilms tumor, hepatoblastoma, rhabdomyosarcoma, Ewing sarcoma-PNET, severe aplastic anemia, Xanthoastrocytoma, histiocytosis, anaplastic broad cell lymphoma. Diagnostic criteria: Caries index:
Alterations of enamel mineralization: Aine classification scale Dental anomalies:
Type of tooth examined: permanent dentition Recorded result: dental alterations | Dental anomalies Defects in dental development according to the Defects Index in the study groups GS GC D1 3.57 ± 4.26 ** 0.85 ± 2.16 D2 0.74 ± 2.17 ** 0.00 D3 0.19 ± 0.50 ** 0.00 D4 1.91 ± 3.16 ** 0.00 D5 0.94 ± 1.75 *** 0.29 ± 0.71 <5 years >5 years D1 2.94 ± 4.16 5.07 ± 4.27 * D2 0.97 ± 2.56 0.21 ± 0.43 D3 0.24 ± 0.56 0.07 ± 0.27 D4 2.64 ± 3.53 ** 0.21 ± 0.58 D5 1.30 ± 1.98 * 0.07 ± 0.27 QT QT + RT Transplant D1 2.56 ± 3.07 5.73 ± 5.61 * 4.05 ± 4.81 D2 0.34 ± 1.12 1.60 ± 3.40 1.45 ± 3.04 * D3 0.13 ± 0.42 0.33 ± 0.62 0.36 ± 0.66 * D4 1.53 ± 2.58 2.73 ± 4.13 3.59 ± 3.94 *** D5 0.81 ± 1.49 1.20 ± 2.24 1.82 ± 2.24 *** Enamel defects NSAID classification CCS GC AINE 1 3.36 ± 5.31 *** 0.19 ± 0.71 AINE 2 0.64 ± 1.48 *** 0.04 ± 0.29 AINE 3 0.56 ± 1.47 *** 0.00 AINE 4 0.27 ± 0.86 *** 0.00 Caries rate dmft DMFT CCS: 4.15 ± 3.25 * 1.70 ± 2.21* * * * GC: 2.59 ± 3.04 1.09 ± 1.79 Males: 3.83 ± 3.56 3.46 ± 3.89 *. Females: 6.07 ± 5.27 1.75 ± 2.92 <5 years old: 4.87 ± 4.82 1.90 ± 3.25 > 5 years: 4.00 ± 1.85 4.40 ± 3.72 * 4.40 ± 3.72 * 4.40 ± 3.72 * 4.40 ± 3.72 * 4.00 ± 1.85 QT: 4.57 ± 4.23 2.58 ± 3.16 QT + RT: 5.11 ± 5.06 3.00 ± 4.41 Transplantation: 4.00 ± 3.73 1.52 ± 3.30 Non-transplant: 5.35 ± 4.91 3.72 ± 3.54 *** 3.72 ± 3.54 *** 3.72 ± 3.54 *** 3.72 ± 3.54 | * p < 0.05 ** p < 0.01 *** p < 0.001 Root malformations, agenesis and microdontia (p < 0.01) Enamel defects: p <0.001) p = 0.029 | These children are at high risk for dental developmental abnormalities and poor dental health and should be closely supervised by a specialist dentist. |
| Proc et al., 2019 [29] | Cross-sectional | No. of participants: 225 GS: 75 GC: 150 Age: 4–18 years old | QT RT | Type of cancer: acute lymphoblastic leukemia, Williams tumor, neuroblastoma, rhabdomyosarcoma, brain tumor, hepatoblastoma, acute non-lymphoblastic leukemia, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, primitive neuroectodermal tumor, germ cell tumor, ovarian tumor Diagnostic criteria Caries rate
Oral hygiene: IP of Silness-Löe Cancer risk: questionnaire Type of neoplasm on the incidence of caries Teeth examined: permanent and deciduous dentition Recorded result: caries and dental plaque index; Influence of radiotherapy and caries prevalence | caries index: dmft: GS 5.02 ± 4.1/GC 4.14 ± 2.94 DMFT: GS 2.60 ± 3.14/GC 1.59 ± 2.79 Correlation between oral hygiene and caries index: IP <1 dmft 25 DMFT 32 >1 dmft 25 DMFT 36 Influence between radiotherapy and the prevalence of caries. Variable No RTX with RTX d 3 (0.5–6.5) 2.5 (1–4) m 0 (0–0) 0 (0–0) f 0 (0–1) 2 (1–3) dmft 4 (1–8) 5 (4–6) D 0 (0–2) 2 (0–3) M 0 (0–0) 0 (0–0) F 0 (0–2) 1.5 (0–2) DMFT 2 (0–4) 4.5 (1–6) | p = 0.3722 p = 0.0053 dmft p = 0.0914 DMFT p = 0.0252 p = 0.762965 p = 0.747707 p = 0.012871 p = 0.499063 p = 0.043573 p = 0.574069 p = 0.230553 p = 0.048725 | Childhood cancer patients have an increased risk of advanced dental caries that could be prevented by oral health education. |
| Proc et al., 2022 [20] | Cross-sectional | No. of participants: 225 GS: 75 GC: 150 Age: 16 Dental examination: 6–155 months (mean 4.9 years) after cessation of antineoplastic treatment when patients were aged between 47 months (4 years) and 215 months (18 years) | Head and neck radiotherapy | Type of cancer: leukemia, B-cell non-Hodgkin’s lymphoma, Hodgkin’s lymphoma lymphoma, peripheral primitive neuroectodermal tumor, germinal tumor, and ovarian tumor Diagnostic criteria:
Type of tooth examined: permanent dentition Recorded outcome: dental malocclusions in relation to antineoplastic treatment | GS/GC
| p = 0.9727 p = 0.9361 p = 0.3670 p = 0.0628 p = 1.0000 p = 0.0136 p = 0.0012 p = 0.0387 p = 0.1091 p = 0.0651 p = 1.0000 p = 0.8195 p = 0.0310 p = 0.1091 p = 1.0000 p = 0.0662 | Oncological treatment may alter the development of occlusion in cancer patients. |
References
- Rodríguez, A.; Valdez, L.; Vega, J.; Gómez García, W. Cáncer infantil: Lo que debemos saber. Cienc. Y Salud 2023, 7, 69–76. [Google Scholar] [CrossRef]
- Cañete Nieto, A.; Pardo Romaguera, E.; Alfonso Comos, P.; Valero Poveda, S.; Porta Cebolla, S. Registro Español de Tumores Infantiles (RETI-SEHOP); Universidad de Valencia: Valencia, Spain, 2024; pp. 1–74. [Google Scholar]
- Effinger, K.E.; Migliorati, C.A.; Hudson, M.M.; McMullen, K.P.; Kaste, S.C.; Ruble, K.; Guilcher, G.M.; Shah, A.J.; Castellino, S.M. Oral and dental late effects in survivors of childhood cancer: A Children’s Oncology Group report. Support. Care Cance Off. J. Multinatl. Assoc. Support. Care Cancer 2014, 22, 2009–2019. [Google Scholar] [CrossRef]
- Carrillo, C.; Corrêa, F.; Lopes, N.; Fava, M.; Odone, V. Dental anomalies in children submitted to antineoplastic therapy. Clinics 2014, 69, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Busenhart, D.M.; Erb, J.; Rigakos, G.; Eliades, T.; Papageorgiou, S.N. Adverse effects of chemotherapy on the teeth and surrounding tissues of children with cancer: A systematic review with meta-analysis. Oral Oncol. 2018, 83, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Rabassa-Blanco, J.; Brunet-Llobet, L.; Marcote-Sinclair, P.; Balsells-Mejía, S.; Correa-Llano, M.G.; Miranda-Rius, J. Prevalence of, and risk factors for, dental sequelae in adolescents who underwent cancer therapy during childhood. Oral Dis. 2024, 30, 604–614. [Google Scholar] [CrossRef]
- Beatriz, A.R.; Frida, H.V.; Omar, V.S.; Alberto, L.F.J.; Iovanna, T.G.; Heraclio, R.R.; Elena, C.M.L. Efectos tardíos orales y dentales en sobrevivientes de cáncer infantil. Cancer 2022, 2022, 1–12. [Google Scholar]
- Olczak-Kowalczyk, D.; Krasuska-Sławińska, E.; Brożyna, A.; Turska-Szybka, A.; Dembowska-Bagińska, B. Dental Caries in Children and Adolescents During and After Antineoplastic Chemotherapy. J. Clin. Pediatr. Dent. 2018, 42, 225–230. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud. Manual de Cuidados Orales Para Pacientes Pediátricos con Cáncer; Organización Panamericana de la Salud: Washington, NC, USA, 2023. [Google Scholar]
- Alves, A.; Kizi, G.; Mascarenhas, P.; Ventura, I. Oral Complications of Chemotherapy on Paediatric Patients with Cancer: A Systematic Review and Meta-Analysis. Med. Sci. Forum 2021, 5, 25. [Google Scholar]
- Halton, J.M.; Ma, J.; Babyn, P.; Matzinger, M.A.; Kaste, S.C.; Scharke, M.; Fernandez, C.V.; Miettunen, P.; Ho, J.; Alos, N.; et al. Reductions in Bone Mineral Density Are Apparent Early in Children with Prevalent Osteonecrosis Lesions Following Leukemia Therapy. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2023, 38, 1104–1115. [Google Scholar] [CrossRef]
- Patni, T.; Lee, C.T.; Li, Y.; Kaste, S.; Zhu, L.; Sun, R.; Hudson, M.M.; Ness, K.K.; Neumann, A.; Robison, L.L. Factors for poor oral health in long-term childhood cancer survivors. BMC Oral Health 2023, 23, 73. [Google Scholar] [CrossRef]
- Ferrández-Pujante, A.; Pérez-Silva, A.; Serna-Muñoz, C.; Fuster-Soler, J.L.; Galera-Miñarro, A.M.; Cabello, I.; Ortiz-Ruiz, A.J. Prevention and Treatment of Oral Complications in Hematologic Childhood Cancer Patients: An Update. Children 2022, 9, 566. [Google Scholar] [CrossRef]
- Casariego, Z.J. La participación del odontólogo en el control del cáncer oral: Manejo en la prevención, tratamiento y rehabilitación. Revis. Av. Odontoestomatol. 2009, 25, 265–285. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kış, H.C.; Coşgunarslan, A.; Delikan, E.; Aksu, S. Does childhood chemotherapy affect mandibular bone structures in a lifetime? Dent. Med. Probl. 2022, 59, 495–501. [Google Scholar] [CrossRef]
- Shayani, A.; Aravena, P.C.; Rodríguez-Salinas, C.; Escobar-Silva, P.; Diocares-Monsálvez, Y.; Angulo-Gutiérrez, C.; Rivera, C. Chemotherapy as a risk factor for caries and gingivitis in children with acute lymphoblastic leukemia: A retrospective cohort study. Int. J. Paediatr. Dent. 2022, 32, 538–545. [Google Scholar] [CrossRef]
- Atif, M.; Mathur, V.P.; Tewari, N.; Bansal, K.; Rahul, M.; Bakhshi, S. Long-Term Effect of Anticancer Therapy on Dentition in Childhood Cancer Survivors: An Observational, Cross-Sectional Study. Indian J. Pediatr. 2022, 89, 327–332. [Google Scholar] [CrossRef]
- Guagnano, R.; Romano, F.; Berger, M.; Fagioli, F.; Vallone, V.; Bello, L.; Vitale, M.C.; Defabianis, P. Long-term effect of anticancer therapy on dentition of Italian children in remission from malignant disease: A cross-sectional study. Eur. J. Paediatr. Dent. 2022, 23, 131–136. [Google Scholar]
- Proc, P.; Szczepanska, J.; Herud, A.; Zubowska, M.; Fendler, W.; Lukomska-Szymanska, M.; Mlynarski, W. Comparative Study of Malocclusions between Cancer Patients and Healthy Peers. Int. J. Environ. Res. Public Health 2022, 19, 4045. [Google Scholar] [CrossRef]
- Zulijani, A.; Zigante, M.; Morelato, L.; Peric, B.; Milardovic, A. Oligomicrodontia in a Pediatric Cancer Survivor after Chemotherapy: A Case Report. Healthcare 2022, 10, 1521. [Google Scholar] [CrossRef]
- Krasuska-Sławińska, E.; Brożyna, A.; Dembowska-Bagińska, B.; Olczak-Kowalczyk, D. Factors influencing caries incidence in permanent teeth in children/adolescents under and after anti-neoplastic treatment. Contemp. Oncol. 2016, 20, 45–51. [Google Scholar] [CrossRef]
- Proc, P.; Szczepańska, J.; Skiba, A.; Zubowska, M.; Fendler, W.; Młynarski, W. Dental Anomalies as Late Adverse Effect among Young Children Treated for Cancer. Cancer Res. Treat. 2016, 48, 658–667. [Google Scholar] [CrossRef]
- Olszewska, K.; Mielnik-Błaszczak, M. An assessment of the number of cariogenic bacteria in the saliva of children with chemotherapy-induced neutropenia. Adv. Clin. Exp. Med. 2016, 25, 11–19. [Google Scholar] [CrossRef]
- Proc, P.; Szczepańska, J.; Zarzycka, B.; Szybka, M.; Borowiec, M.; Płoszaj, T.; Fendler, W.; Chrzanowski, J.; Zubowska, M.; Stolarska, M.; et al. Evaluation of Changes to the Oral Microbiome Based on 16S rRNA Sequencing among Children Treated for Cancer. Cancers 2021, 14, 7. [Google Scholar] [CrossRef]
- Kılınç, G.; Bulut, G.; Ertuğrul, F.; Ören, H.; Demirağ, B.; Demiral, A.; Aksoylar, S.; Kamer, E.S.; Ellidokuz, H.; Olgun, N. Long-term dental anomalies after pediatric cancer treatment in children. Turk. J. Hematol. 2019, 36, 155–161. [Google Scholar] [CrossRef]
- Nemeth, O.; Kivovics, M.; Pinke, I.; Marton, K.; Kivovics, P.; Garami, M. Late Effects of Multiagent Chemotherapy on Salivary Secretion in Children Cancer Survivors. J. Am. Coll. Nutr. 2014, 33, 186–191. [Google Scholar] [CrossRef]
- Longo, B.C.; Rohling, I.B.; Silva, P.L.; de Morais, M.E.; Paz, H.E.; Casarin, R.C.; Nishiyama, S.A.; de Souza, M.D.; Silva, C.O. Antineoplastic therapy in childhood cancer patients presents a negative impact in the periodontal tissues: A cohort study. Clin. Oral Investig. 2023, 27, 6637–6644. [Google Scholar] [CrossRef]
- Proc, P.; Szczepańska, J.; Herud, A.; Zubowska, M.; Fendler, W.; Młynarski, W. Dental caries among childhood cancer survivors. Medicine 2019, 98, e14279. [Google Scholar] [CrossRef]
- Longo, B.C.; Rohling, I.B.; Silva, P.L.; Paz, H.E.; Casarin, R.C.; Souza, M.D.B.; Silva, C.O. Antineoplastic therapy is an independent risk factor for dental caries in childhood cancer patients: A retrospective cohort study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2024, 32, 316. [Google Scholar] [CrossRef]
- Quezada, M. Tratamientos Antineoplásicos en Base a Quimio y Radioterapia y su Relación con Alteraciones Dentarias, Flujo Salival y Riesgo de Caries en Pacientes Pediátricos Oncológicos del Hospital de Niños. Chile, 2011. Available online: https://repositorio.uchile.cl/handle/2250/133598 (accessed on 23 July 2025).
- Pombo Lopes, J.; Rodrigues, I.; Machado, V.; Botelho, J. Chemotherapy and Radiotherapy Long-Term Adverse Effects on Oral Health of Childhood Cancer Survivors: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 110. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Tylavsky, F.A.; Smith, K.; Surprise, H.; Garland, S.; Yan, X.; McCammon, E.; Hudson, M.M.; Pui, C.H.; Kaste, S.C. Nutritional intake of long-term survivors of childhood acute lymphoblastic leukemia: Evidence for bone health interventional opportunities. Pediatr. Blood Cancer 2010, 55, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.J.; Merkx, M.A.; Hamann, M.N.; Brand, H.S.; de Haan, A.F.; Rosenberg, A.J.; Speksnijder, C.M. Tongue function and its influence on masticatory performance in patients treated for oral cancer: A five-year prospective study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2020, 28, 1491–1501. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrecillas-Quiles, L.; Gómez-Ríos, I.; Jiménez-García, I.; Serrano-Belmonte, I.; Ortiz-Ruiz, A.J.; Serna-Muñoz, C. Oral and Dental Sequelae After Oncological Treatment in Children: A Systematic Review. J. Clin. Med. 2025, 14, 5479. https://doi.org/10.3390/jcm14155479
Torrecillas-Quiles L, Gómez-Ríos I, Jiménez-García I, Serrano-Belmonte I, Ortiz-Ruiz AJ, Serna-Muñoz C. Oral and Dental Sequelae After Oncological Treatment in Children: A Systematic Review. Journal of Clinical Medicine. 2025; 14(15):5479. https://doi.org/10.3390/jcm14155479
Chicago/Turabian StyleTorrecillas-Quiles, Lidia, Inmaculada Gómez-Ríos, Irene Jiménez-García, Ildefonso Serrano-Belmonte, Antonio José Ortiz-Ruiz, and Clara Serna-Muñoz. 2025. "Oral and Dental Sequelae After Oncological Treatment in Children: A Systematic Review" Journal of Clinical Medicine 14, no. 15: 5479. https://doi.org/10.3390/jcm14155479
APA StyleTorrecillas-Quiles, L., Gómez-Ríos, I., Jiménez-García, I., Serrano-Belmonte, I., Ortiz-Ruiz, A. J., & Serna-Muñoz, C. (2025). Oral and Dental Sequelae After Oncological Treatment in Children: A Systematic Review. Journal of Clinical Medicine, 14(15), 5479. https://doi.org/10.3390/jcm14155479






