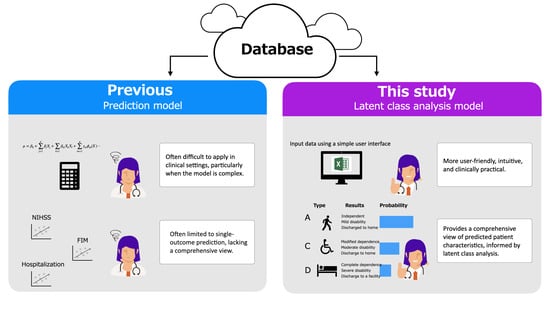
Classifying Patient Characteristics and Determining a Predictor in Acute Stroke Patients: Application of Latent Class Analysis in Rehabilitation Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Database
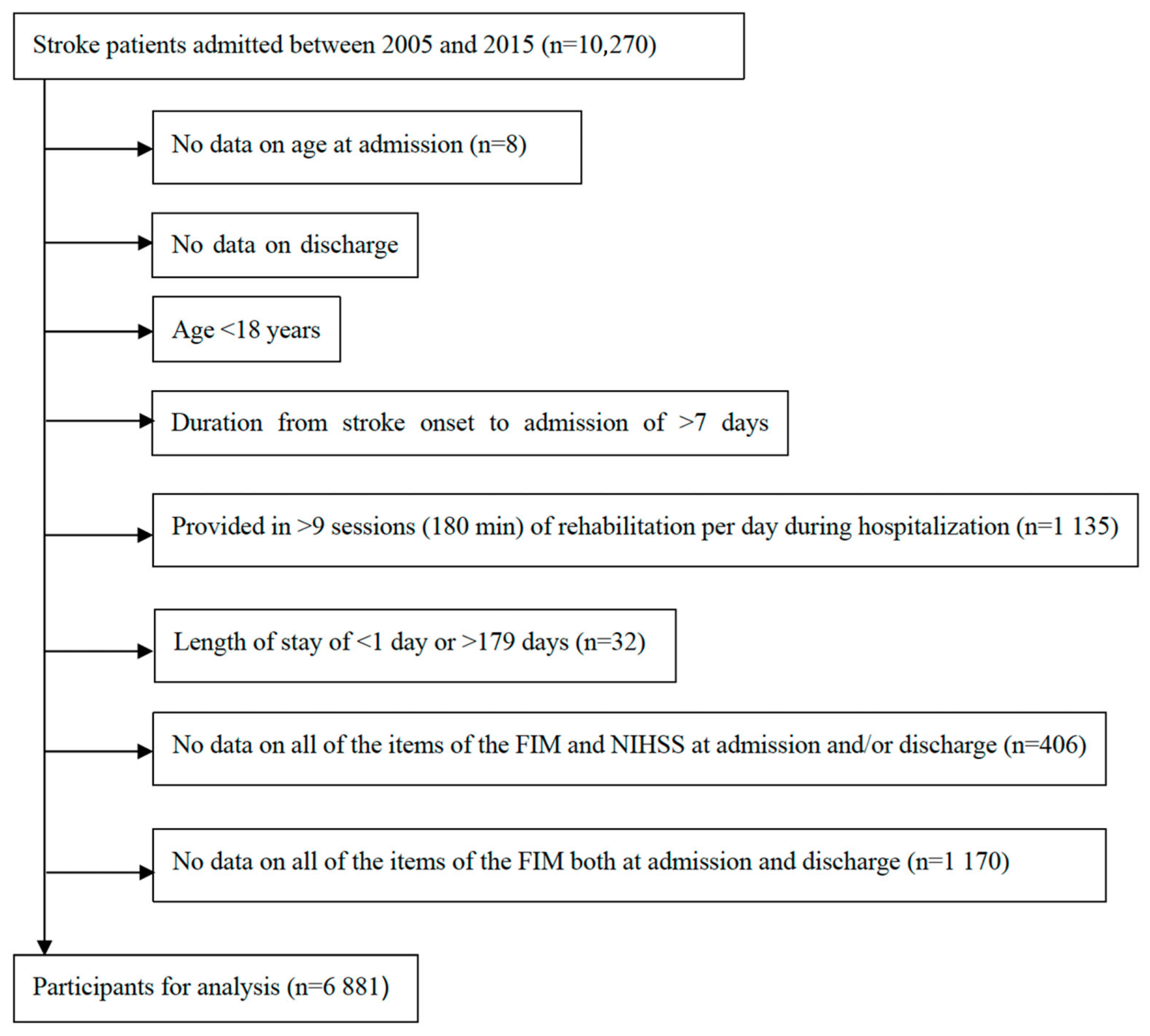
2.3. Participants
2.4. Statistical Analyses
2.5. Outcome Variables
2.6. Predictor Variables
3. Results
3.1. Patient Characteristics
3.2. Latent Classes of Patient Characteristics at Discharge
3.3. Predictors of Class Membership
3.4. Model Application
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | activities of daily living |
| LCA | latent class analysis |
| FIM | functional independent measure |
| NIHSS | National Institutes of Health Stroke Scale |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| JARD | Japan Association of Rehabilitation Database |
| AIC | Akaike information criterion |
| BIC | Bayesian information criterion |
References
- Wold Health Organization. Stroke, Cerebrovascular Accident. Available online: http://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html (accessed on 2 September 2023).
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Cormier, D.J.; Frantz, M.A.; Rand, E.; Stein, J. Physiatrist referral preferences for postacute stroke rehabilitation. Medicine 2016, 95, e4356. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.M.; Brock, K.A.; Lunt, A.W.; Black, S.J. Factors influencing selection for rehabilitation after stroke: A questionnaire using case scenarios to investigate physician perspectives and level of agreement. Arch. Phys. Med. Rehabil. 2012, 93, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Smith, M.-C.; Byblow, W.D. Prediction Tools for Stroke Rehabilitation. Stroke 2019, 50, 3314–3322. [Google Scholar] [CrossRef]
- de Ridder, I.R.; Dijkland, S.A.; Scheele, M.; den Hertog, H.M.; Dirks, M.; Westendorp, W.F.; Nederkoorn, P.J.; van de Beek, D.; Ribbers, G.M.; Steyerberg, E.W.; et al. Development and validation of the Dutch Stroke Score for predicting disability and functional outcome after ischemic stroke: A tool to support efficient discharge planning. Eur. Stroke J. 2018, 3, 165–173. [Google Scholar] [CrossRef]
- Douiri, A.; Grace, J.; Sarker, S.J.; Tilling, K.; McKevitt, C.; Wolfe, C.D.; Rudd, A.G. Patient-specific prediction of functional recovery after stroke. Int. J. Stroke 2017, 12, 539–548. [Google Scholar] [CrossRef]
- Scrutinio, D.; Lanzillo, B.; Guida, P.; Mastropasqua, F.; Monitillo, V.; Pusineri, M.; Formica, R.; Russo, G.; Guarnaschelli, C.; Ferretti, C.; et al. Development and Validation of a Predictive Model for Functional Outcome after Stroke Rehabilitation: The Maugeri Model. Stroke 2017, 48, 3308–3315. [Google Scholar] [CrossRef]
- Nijland, R.H.; van Wegen, E.E.; Harmeling-van der Wel, B.C.; Kwakkel, G.; Investigators, E. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: Early prediction of functional outcome after stroke: The EPOS cohort study. Stroke 2010, 41, 745–750. [Google Scholar] [CrossRef]
- Stinear, C.M.; Byblow, W.D.; Ackerley, S.J.; Smith, M.C.; Borges, V.M.; Barber, P.A. PREP2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann. Clin. Transl. Neurol. 2017, 4, 811–820. [Google Scholar] [CrossRef]
- Bland, M.D.; Sturmoski, A.; Whitson, M.; Connor, L.T.; Fucetola, R.; Huskey, T.; Corbetta, M.; Lang, C.E. Prediction of Discharge Walking Ability from Initial Assessment in a Stroke Inpatient Rehabilitation Facility Population. Arch. Phys. Med. Rehabil. 2012, 93, 1441–1447. [Google Scholar] [CrossRef]
- Kinoshita, S.; Abo, M.; Okamoto, T.; Tanaka, N. Utility of the Revised Version of the Ability for Basic Movement Scale in Predicting Ambulation During Rehabilitation in Poststroke Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 1663–1669. [Google Scholar] [CrossRef]
- Kwah, L.K.; Harvey, L.A.; Diong, J.; Herbert, R.D. Models containing age and NIHSS predict recovery of ambulation and upper limb function six months after stroke: An observational study. J. Physiother. 2013, 59, 189–197. [Google Scholar] [CrossRef]
- Sánchez-Blanco, I.; Ochoa-Sangrador, C.; López-Munaín, L.; Izquierdo-Sánchez, M.; Fermoso-Garcia, J. Predictive model of functional independence in stroke patients admitted to a rehabilitation programme. Clin. Rehabil. 1999, 13, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.-C.; Barber, P.A.; Stinear, C.M. The TWIST Algorithm Predicts Time to Walking Independently after Stroke. Neurorehabilit. Neural Repair 2017, 31, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; Van Wegen, E.E.; Harmeling-Van der Wel, B.C.; Kwakkel, G.; Investigators, E. Is accurate prediction of gait in nonambulatory stroke patients possible within 72 hours poststroke? The EPOS study. Neurorehabilit. Neural Repair 2011, 25, 268–274. [Google Scholar] [CrossRef]
- Faigle, R.; Marsh, E.B.; Llinas, R.H.; Urrutia, V.C.; Gottesman, R.F. Novel Score Predicting Gastrostomy Tube Placement in Intracerebral Hemorrhage. Stroke 2015, 46, 31–36. [Google Scholar] [CrossRef]
- Galovic, M.; Stauber, A.J.; Leisi, N.; Krammer, W.; Brugger, F.; Vehoff, J.; Balcerak, P.; Müller, A.; Müller, M.; Rosenfeld, J.; et al. Development and Validation of a Prognostic Model of Swallowing Recovery and Enteral Tube Feeding after Ischemic Stroke. JAMA Neurol. 2019, 76, 561. [Google Scholar] [CrossRef]
- Formann, A.K.; Kohlmann, T. Latent class analysis in medical research. Stat. Methods Med. Res. 1996, 5, 179–211. [Google Scholar] [CrossRef]
- Lanza, S.T.; Tan, X.; Bray, B.C. Latent Class Analysis with Distal Outcomes: A Flexible Model-Based Approach. Struct. Equ. Model. 2013, 20, 1–26. [Google Scholar] [CrossRef]
- Rienstra, M.; Geelhoed, B.; Yin, X.; Siland, J.E.; Vermond, R.A.; Mulder, B.A.; Van Der Harst, P.; Hillege, H.L.; Benjamin, E.J.; Van Gelder, I.C. Cluster Individuals Based on Phenotype and Determine the Risk for Atrial Fibrillation in the PREVEND and Framingham Heart Study Populations. PLoS ONE 2016, 11, e0165828. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Kinoshita, S.; Kakuda, W.; Momosaki, R.; Yamada, N.; Sugawara, H.; Watanabe, S.; Abo, M. Clinical management provided by board-certificated physiatrists in early rehabilitation is a significant determinant of functional improvement in acute stroke patients: A retrospective analysis of Japan rehabilitation database. J. Stroke Cerebrovasc. Dis. 2015, 24, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kondo, K.; Shiraishi, N.; Inoue, Y. An evaluation of the quality of post-stroke rehabilitation in Japan. Clin. Audit 2010, 2, 59–66. [Google Scholar] [CrossRef][Green Version]
- Kamo, T.; Momosaki, R.; Suzuki, K.; Asahi, R.; Azami, M.; Ogihara, H.; Nishida, Y. Effectiveness of Intensive Rehabilitation Therapy on Functional Outcomes after Stroke: A Propensity Score Analysis Based on Japan Rehabilitation Database. J. Stroke Cerebrovasc. Dis. 2019, 28, 2537–2542. [Google Scholar] [CrossRef]
- Bakk, Z.; Kuha, J. Two-Step Estimation of Models Between Latent Classes and External Variables. Psychometrika 2018, 83, 871–892. [Google Scholar] [CrossRef]
- Vermunt, J.K.; Magidson, J. How to Perform Three-Step Latent Class Analysis in the Presence of Measurement Non-Invariance or Differential Item Functioning. Struct. Equ. Model. A Multidiscip. J. 2020, 28, 356–364. [Google Scholar] [CrossRef]
- Alagöz, Ö.E.C.; Vermunt, J.K. Stepwise Latent Class Analysis in the Presence of Missing Values on the Class Indicators. Struct. Equ. Model. A Multidiscip. J. 2022, 29, 784–790. [Google Scholar] [CrossRef]
- Janssen, J.H.M.; van Laar, S.; de Rooij, M.J.; Kuha, J.; Bakk, Z. The Detection and Modeling of Direct Effects in Latent Class Analysis. Struct. Equ. Model. A Multidiscip. J. 2018, 26, 280–290. [Google Scholar] [CrossRef]
- Nylund, K.L.; Asparouhov, T.; Muthén, B.O. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Struct. Equ. Model. A Multidiscip. J. 2007, 14, 535–569. [Google Scholar] [CrossRef]
- Weller, B.E.; Bowen, N.K.; Faubert, S.J. Latent Class Analysis: A Guide to Best Practice. J. Black Psychol. 2020, 46, 287–311. [Google Scholar] [CrossRef]
- Edwards, S.L.; Berzofsky, M.E.; Biemer, P.P. RTI Press Methods Report Series. In Addressing Nonresponse for Categorical Data Items Using Full Information Maximum Likelihood with Latent GOLD 5.0; RTI Press: Research Triangle Park, NC, USA, 2018. [Google Scholar]
- Vermunt, J.K.; Magidson, J. Upgrade Manual for Latent GOLD Basic, Advanced, Syntax, and Choice Version 6.0; Statistical Innovations Inc.: Arlington, MA, USA, 2021. [Google Scholar]
- McLachlan, G.J.; Lee, S.X.; Rathnayake, S.I. Finite mixture models. Annu. Rev. Stat. Its Appl. 2019, 6, 355–378. [Google Scholar] [CrossRef]
- Ottenbacher, K.J.; Hsu, Y.; Granger, C.V.; Fiedler, R.C. The reliability of the functional independence measure: A quantitative review. Arch. Phys. Med. Rehabil. 1996, 77, 1226–1232. [Google Scholar] [CrossRef]
- Furuta, H.; Mizuno, K.; Unai, K.; Ebata, H.; Yamauchi, K.; Watanabe, M. Functional Independence Measure Subtypes Among Inpatients with Subacute Stroke: Classification via Latent Class Analysis. Prog. Rehabil. Med. 2022, 7, 20220021. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.R.; Severinsen, K.; Nielsen, J.F. The Effect of Age on Rehabilitation Outcome After Traumatic Brain Injury Assessed by the Functional Independence Measure (FIM). Neurorehabilit. Neural Repair 2014, 29, 299–307. [Google Scholar] [CrossRef]
- Aoki, K.; Iguchi, A.; Watabe, T. Evaluation of Functional Independence Measure item scores for predicting home discharge after acute stroke rehabilitation. Jpn. J. Compr. Rehabil. Sci. 2020, 11, 17–20. [Google Scholar] [CrossRef]
- González-Fernández, M.; Christian, A.B.; Davis, C.; Hillis, A.E. Role of Aphasia in Discharge Location After Stroke. Arch. Phys. Med. Rehabil. 2013, 94, 851–855. [Google Scholar] [CrossRef]
- Lyden, P. Using the National Institutes of Health Stroke Scale. Stroke 2017, 48, 513–519. [Google Scholar] [CrossRef]
- Mauthe, R.W.; Haaf, D.C.; Haya, P.; Krall, J.M. Predicting discharge destination of stroke patients using a mathematical model based on six items from the functional independence measure. Arch. Phys. Med. Rehabil. 1996, 77, 10–13. [Google Scholar] [CrossRef]
- Kimura, T. Interaction Between Locomotion and Self-Care for Patients with Stroke in Severity Classification Based on the Motor Functional Independence Measure upon Admission to the Recovery Ward. Open J. Ther. Rehabil. 2020, 08, 164–182. [Google Scholar] [CrossRef]
- Gialanella, B.; Bertolinelli, M.; Lissi, M.; Prometti, P. Predicting outcome after stroke: The role of aphasia. Disabil. Rehabil. 2011, 33, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.Q.; PrvuBettger, J.; Guerrier, T.; Hirsch, M.A.; Thomas, J.G.; Pugh, T.M.; Rhoads, C.F., 3rd. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch. Phys. Med. Rehabil. 2015, 96, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, D.; Kolb, S.J.; Luciano, J.M.; Tovar, J.M.; Cucchiara, B.L.; Liebeskind, D.S.; Kasner, S.E. Utility of the NIH Stroke Scale as a Predictor of Hospital Disposition. Stroke 2003, 34, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Saito, J.; Koyama, T.; Domen, K. Long-Term Outcomes of FIM Motor Items Predicted from Acute Stage NIHSS of Patients with Middle Cerebral Artery Infarct. Ann. Rehabil. Med. 2018, 42, 670–681. [Google Scholar] [CrossRef]
- Doron, N.; Rand, D. Is Unilateral Spatial Neglect Associated with Motor Recovery of the Affected Upper Extremity Poststroke? A Systematic Review. Neurorehabilit. Neural Repair 2019, 33, 179–187. [Google Scholar] [CrossRef]
- Ten Brink, A.F.; Verwer, J.H.; Biesbroek, J.M.; Visser-Meily, J.M.A.; Nijboer, T.C.W. Differences between left- and right-sided neglect revisited: A large cohort study across multiple domains. J. Clin. Exp. Neuropsychol. 2017, 39, 707–723. [Google Scholar] [CrossRef]
- Gialanella, B.; Ferlucci, C. Functional Outcome After Stroke in Patients with Aphasia and Neglect: Assessment by the Motor and Cognitive Functional Independence Measure Instrument. Cerebrovasc. Dis. 2010, 30, 440–447. [Google Scholar] [CrossRef]
- Bernhardt, J.; Churilov, L.; Ellery, F.; Collier, J.; Chamberlain, J.; Langhorne, P.; Lindley, R.I.; Moodie, M.; Dewey, H.; Thrift, A.G.; et al. Prespecified dose-response analysis for A Very Early Rehabilitation Trial (AVERT). Neurology 2016, 86, 2138–2145. [Google Scholar] [CrossRef]
- Manocchio, N.; Ljoka, C.; Ferdinandi, V.; Cicchi, L.; Foti, C. Commentary on “The learning rehabilitation system: Strengthening an intersectoral strategy to improve functioning of an ageing population” by Bickenbach et al. Health Policy 2025, 155, 105303. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability and Health (ICF); World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
| Overall n = 6801 | ||
|---|---|---|
| Category variable | n (%) | |
| Sex, Female | 3046 (44.3%) | |
| Type of stroke | ||
| Lacunar infarction | 1091 (15.9%) | |
| Atherothrombotic cerebral infarction | 1697 (24.7%) | |
| Cardiogenic embolism | 1138 (16.5%) | |
| Cerebral infarction (others/unknown) | 614 (8.9%) | |
| Hypertensive cerebral hemorrhage | 1169 (17.0%) | |
| Cerebral hemorrhage (others/unknown) | 400 (5.8%) | |
| Subarachnoid hemorrhage | 339 (4.9%) | |
| Others/Unknown | 60 (0.9%) | |
| Missing | 373 (5.4%) | |
| Amount of rehabilitation per day | ||
| Less than 2 units (less than 40 min) | 2277 (33.1%) | |
| 2 or more to less than 4 units (40–80 min) | 2329 (33.8%) | |
| 4 or more to less than 6 units (80–120 min) | 990 (14.4%) | |
| 6 or more units (more than 120 min) | 1285 (18.7%) | |
| Continuous variable | Mean (SD) | |
| Age | 73.7 (12.7) | |
| Length of stay, days | 29.5 (21.4) | |
| Admission | Discharge | |
| Motor-FIM (admission: n = 6751, discharge: n = 6652) | 33.4 (23.5) | 57.0 (29.7) |
| Cognitive-FIM (admission: n = 6774, discharge: n = 6676) | 20.8 (11.4) | 24.1 (10.7) |
| FIM (total) (admission: n = 6821, discharge: n = 6726) | 54.2 (32.6) | 80.9 (39.1) |
| NIHSS (total) (admission: n = 6575, discharge: n = 6544) | 8.3 (8.9) | 6.2 (8.2) |
| LL | BIC | ΔBIC | AIC | ΔAIC | df | VLMR | Entropy R2 | Smallest Class Size | |
|---|---|---|---|---|---|---|---|---|---|
| 1-Class | −82,978 | 166,230 | - | 166,018 | - | 6858 | 1.00 | - | |
| 2-Class | −62,502 | 125,561 | 40,669 | 125,130 | 40,888 | 6826 | 40,952 | 0.94 | 46% |
| 3-Class | −56,534 | 113,907 | 11,654 | 113,257 | 11,873 | 6794 | 11,937 | 0.94 | 25% |
| 4-Class | −54,963 | 111,048 | 2859 | 110,180 | 3078 | 6762 | 3142 | 0.92 | 16% |
| 5-Class | −53,875 | 109,155 | 1893 | 108,068 | 2111 | 6730 | 2175 | 0.92 | 13% |
| 6-Class | −53,000 | 107,688 | 1467 | 106,383 | 1685 | 6698 | 1749 | 0.92 | 6.8% |
| 7-Class | −52,566 | 107,102 | 586 | 105,578 | 805 | 6666 | 869 | 0.91 | 6.0% |
| 8-Class | −52,218 | 106,688 | 414 | 104,945 | 633 | 6634 | 697 | 0.88 | 5.6% |
| 9-Class | −51,933 | 106,403 | 286 | 104,440 | 505 | 6602 | 569 | 0.88 | 4.5% |
| 10-Class | −51,763 | 106,345 | 58 | 104,164 | 277 | 6570 | 341 | 0.87 | 1.5% |
| 11-Class | −51,620 | 106,341 | 3 | 103,941 | 222 | 6538 | 286 | 0.87 | 1.5% |
| 12-Class | −51,499 | 106,380 | −39 | 103,763 | 178 | 6498 | 242 | 0.86 | 1.4% |
| Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | Class 6 | Class 7 | Class 8 | Class 9 | Overall | |
|---|---|---|---|---|---|---|---|---|---|---|
| Class size | 29% | 15% | 11% | 10% | 10% | 9% | 6% | 6% | 4% | |
| Discharge destination | ||||||||||
| Discharged home | 97% | 4% | 46% | 30% | 35% | 7% | 64% | 5% | 9% | 46% |
| Discharged to another hospital | 1% | 77% | 52% | 61% | 60% | 82% | 33% | 91% | 81% | 48% |
| Discharged to a care facility | 1% | 7% | 1% | 6% | 4% | 7% | 1% | 3% | 7% | 4% |
| In-hospital death | 0% | 7% | 0% | 0% | 0% | 1% | 0% | 0% | 0% | 1% |
| Others | 1% | 5% | 1% | 3% | 1% | 3% | 2% | 1% | 3% | 2% |
| Length of stay | ||||||||||
| Less than 2 weeks (1 to 14 days) | 55% | 8% | 6% | 16% | 19% | 9% | 21% | 5% | 6% | 24% |
| 2–4 weeks (15–28 days) | 35% | 19% | 50% | 40% | 40% | 28% | 42% | 33% | 34% | 35% |
| 4–6 weeks (29–42 days) | 7% | 28% | 25% | 24% | 27% | 29% | 21% | 36% | 32% | 21% |
| More than 6 weeks (more than 42 days) | 3% | 44% | 19% | 21% | 14% | 35% | 16% | 27% | 28% | 20% |
| NIHSS score | ||||||||||
| Right Motor Arm | ||||||||||
| 0 | 97% | 20% | 85% | 74% | 79% | 54% | 91% | 77% | 40% | 72% |
| 1 | 3% | 9% | 11% | 17% | 13% | 17% | 5% | 5% | 15% | 9% |
| 2 | 0% | 16% | 2% | 4% | 4% | 13% | 1% | 5% | 14% | 6% |
| 3 | 0% | 17% | 2% | 4% | 3% | 9% | 1% | 9% | 16% | 6% |
| 4 | 0% | 37% | 1% | 1% | 1% | 7% | 1% | 4% | 15% | 8% |
| Left Motor Arm | ||||||||||
| 0 | 96% | 24% | 81% | 77% | 72% | 37% | 96% | 35% | 84% | 70% |
| 1 | 3% | 11% | 12% | 15% | 14% | 12% | 2% | 17% | 11% | 9% |
| 2 | 0% | 15% | 3% | 4% | 6% | 14% | 1% | 16% | 4% | 6% |
| 3 | 0% | 16% | 2% | 3% | 6% | 16% | 0% | 19% | 0% | 6% |
| 4 | 0% | 35% | 1% | 1% | 2% | 20% | 0% | 14% | 0% | 8% |
| FIM item | ||||||||||
| Eating | ||||||||||
| Independence | 100% | 1% | 96% | 51% | 83% | 9% | 93% | 44% | 22% | 62% |
| Modified dependence | 0% | 5% | 3% | 42% | 16% | 45% | 7% | 39% | 45% | 16% |
| Complete dependence | 0% | 95% | 1% | 7% | 1% | 47% | 0% | 17% | 34% | 22% |
| Transfers (bed/chair/wheelchair) | ||||||||||
| Independence | 99% | 0% | 98% | 3% | 12% | 0% | 93% | 0% | 1% | 47% |
| Modified dependence | 1% | 3% | 2% | 97% | 88% | 34% | 7% | 73% | 96% | 31% |
| Complete dependence | 0% | 97% | 0% | 0% | 0% | 66% | 0% | 27% | 3% | 22% |
| Toileting | ||||||||||
| Independence | 100% | 0% | 95% | 3% | 23% | 0% | 87% | 0% | 0% | 47% |
| Modified dependence | 0% | 0% | 5% | 86% | 73% | 4% | 13% | 31% | 46% | 21% |
| Complete dependence | 0% | 100% | 0% | 11% | 3% | 96% | 0% | 69% | 54% | 32% |
| Locomotion (walking/wheelchair) | ||||||||||
| Independence | 97% | 0% | 78% | 1% | 4% | 0% | 67% | 1% | 0% | 41% |
| Modified dependence | 3% | 1% | 22% | 72% | 81% | 3% | 32% | 13% | 44% | 23% |
| Complete dependence | 0% | 99% | 1% | 27% | 15% | 97% | 1% | 86% | 56% | 36% |
| Transfers (shower/bathtub) | ||||||||||
| Independence | 75% | 0% | 39% | 0% | 0% | 0% | 32% | 0% | 0% | 28% |
| Modified dependence | 18% | 0% | 56% | 43% | 68% | 1% | 57% | 10% | 24% | 27% |
| Complete dependence | 7% | 100% | 5% | 57% | 32% | 99% | 11% | 90% | 76% | 45% |
| Communication (comprehension) | ||||||||||
| Independence | 99% | 0% | 100% | 5% | 96% | 5% | 9% | 91% | 2% | 56% |
| Modified dependence | 1% | 8% | 0% | 92% | 4% | 91% | 87% | 9% | 43% | 27% |
| Complete dependence | 0% | 92% | 0% | 2% | 0% | 4% | 4% | 0% | 55% | 17% |
| Communication (expression) | ||||||||||
| Independence | 99% | 0% | 93% | 8% | 93% | 3% | 13% | 89% | 0% | 55% |
| Modified dependence | 1% | 1% | 6% | 90% | 6% | 84% | 77% | 11% | 20% | 25% |
| Complete dependence | 0% | 99% | 0% | 2% | 0% | 13% | 10% | 0% | 80% | 21% |
| Social interaction | ||||||||||
| Independence | 97% | 1% | 93% | 19% | 82% | 11% | 38% | 72% | 11% | 56% |
| Modified dependence | 3% | 4% | 7% | 65% | 17% | 56% | 54% | 25% | 35% | 22% |
| Complete dependence | 0% | 95% | 0% | 16% | 1% | 33% | 8% | 3% | 54% | 22% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, J.; Yamada, M.; Nagayama, H.; Tomori, K.; Ikeda, K.; Yamauchi, K. Classifying Patient Characteristics and Determining a Predictor in Acute Stroke Patients: Application of Latent Class Analysis in Rehabilitation Practice. J. Clin. Med. 2025, 14, 5466. https://doi.org/10.3390/jcm14155466
Uchida J, Yamada M, Nagayama H, Tomori K, Ikeda K, Yamauchi K. Classifying Patient Characteristics and Determining a Predictor in Acute Stroke Patients: Application of Latent Class Analysis in Rehabilitation Practice. Journal of Clinical Medicine. 2025; 14(15):5466. https://doi.org/10.3390/jcm14155466
Chicago/Turabian StyleUchida, Junya, Moeka Yamada, Hirofumi Nagayama, Kounosuke Tomori, Kohei Ikeda, and Keita Yamauchi. 2025. "Classifying Patient Characteristics and Determining a Predictor in Acute Stroke Patients: Application of Latent Class Analysis in Rehabilitation Practice" Journal of Clinical Medicine 14, no. 15: 5466. https://doi.org/10.3390/jcm14155466
APA StyleUchida, J., Yamada, M., Nagayama, H., Tomori, K., Ikeda, K., & Yamauchi, K. (2025). Classifying Patient Characteristics and Determining a Predictor in Acute Stroke Patients: Application of Latent Class Analysis in Rehabilitation Practice. Journal of Clinical Medicine, 14(15), 5466. https://doi.org/10.3390/jcm14155466