Uncovering Plaque Erosion: A Distinct Pathway in Acute Coronary Syndromes and a Gateway to Personalized Therapy
Abstract
1. Introduction
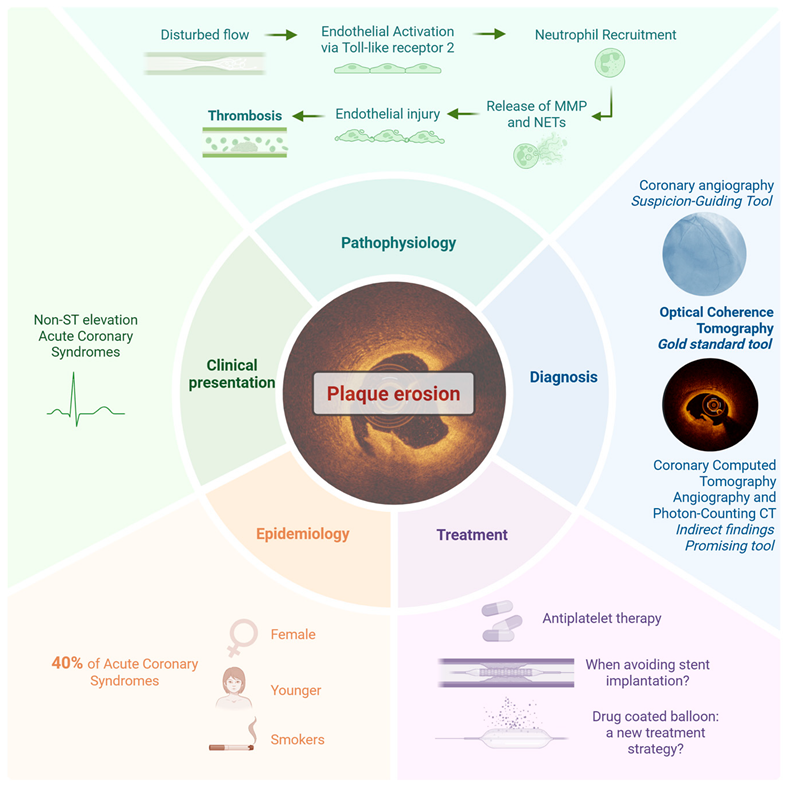
2. Plaque Erosion: An Autopsy-Driven Shift in the Understanding of Acute Coronary Syndromes
3. Epidemiology of Plaque Erosion: Prevalence and Patient Profiles
4. Clinical Presentation of Plaque Erosion: How Does It Differ from Plaque Rupture?
5. Pathophysiological Substrate of Plaque Erosion and Rupture: Two Distinct Mechanisms in ACS
6. Diagnosis of Plaque Erosion: The Role of Invasive and Non-Invasive Imaging
6.1. Plaque Erosion and Coronary Angiography: A Non-Diagnostic but Suspicion-Guiding Tool
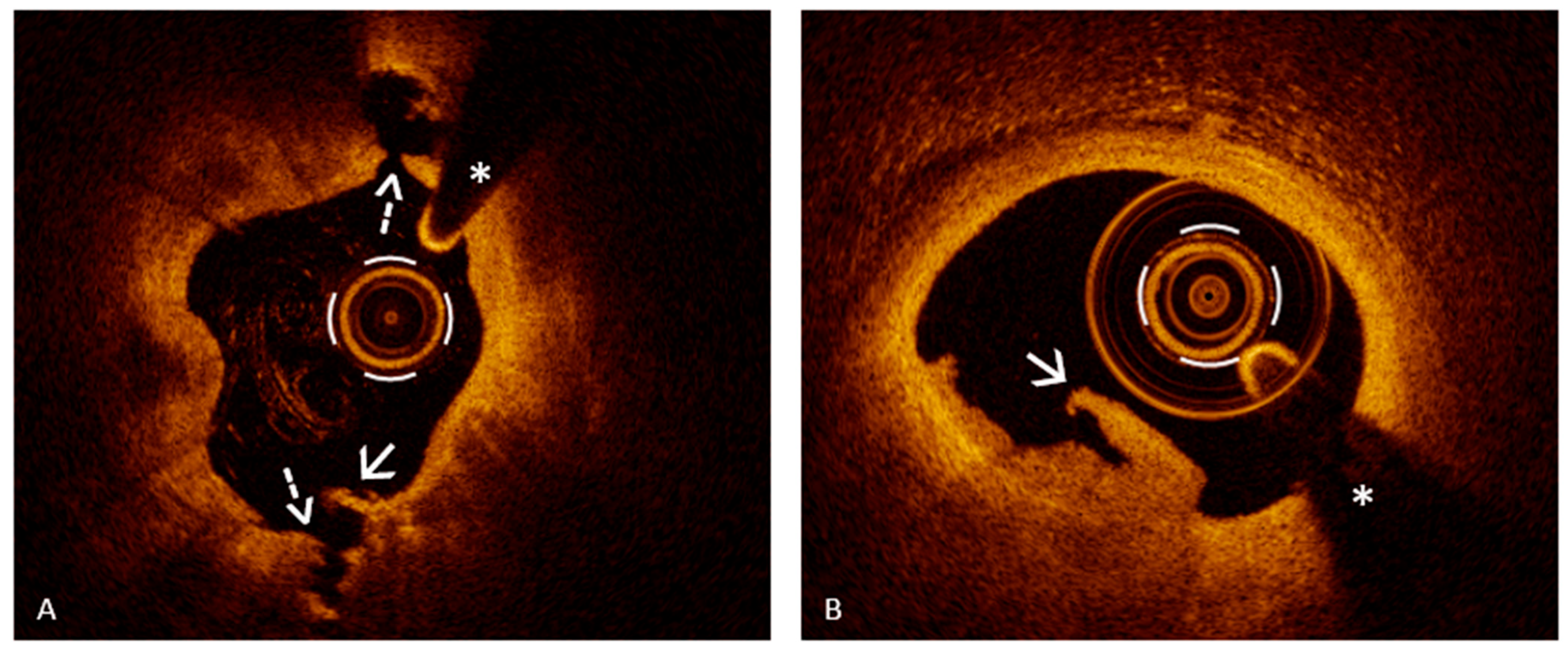
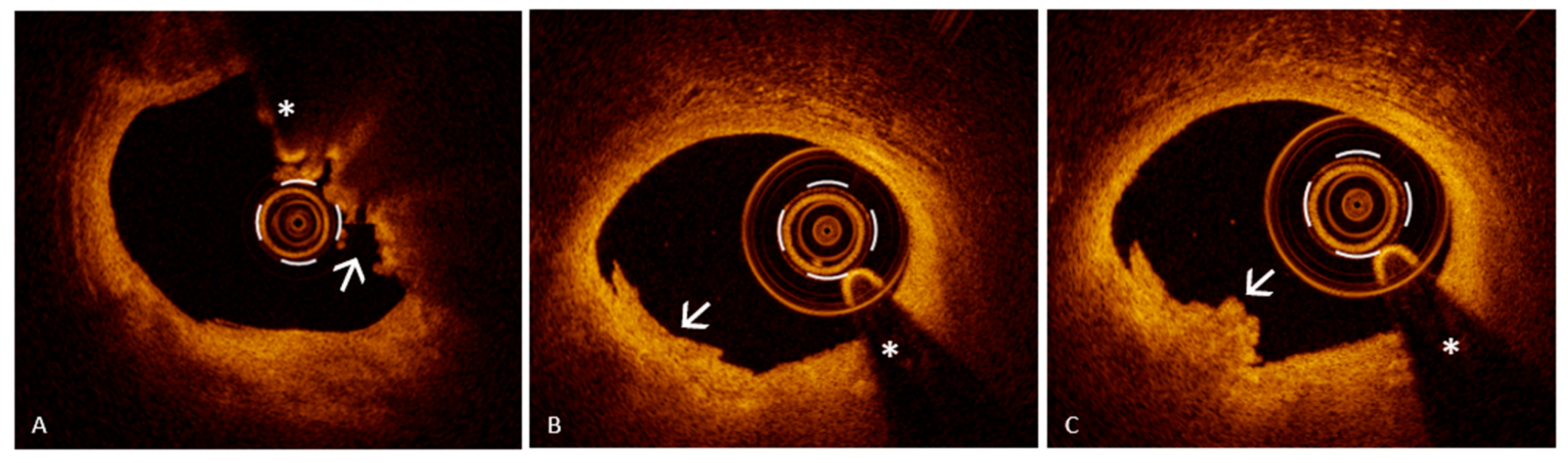
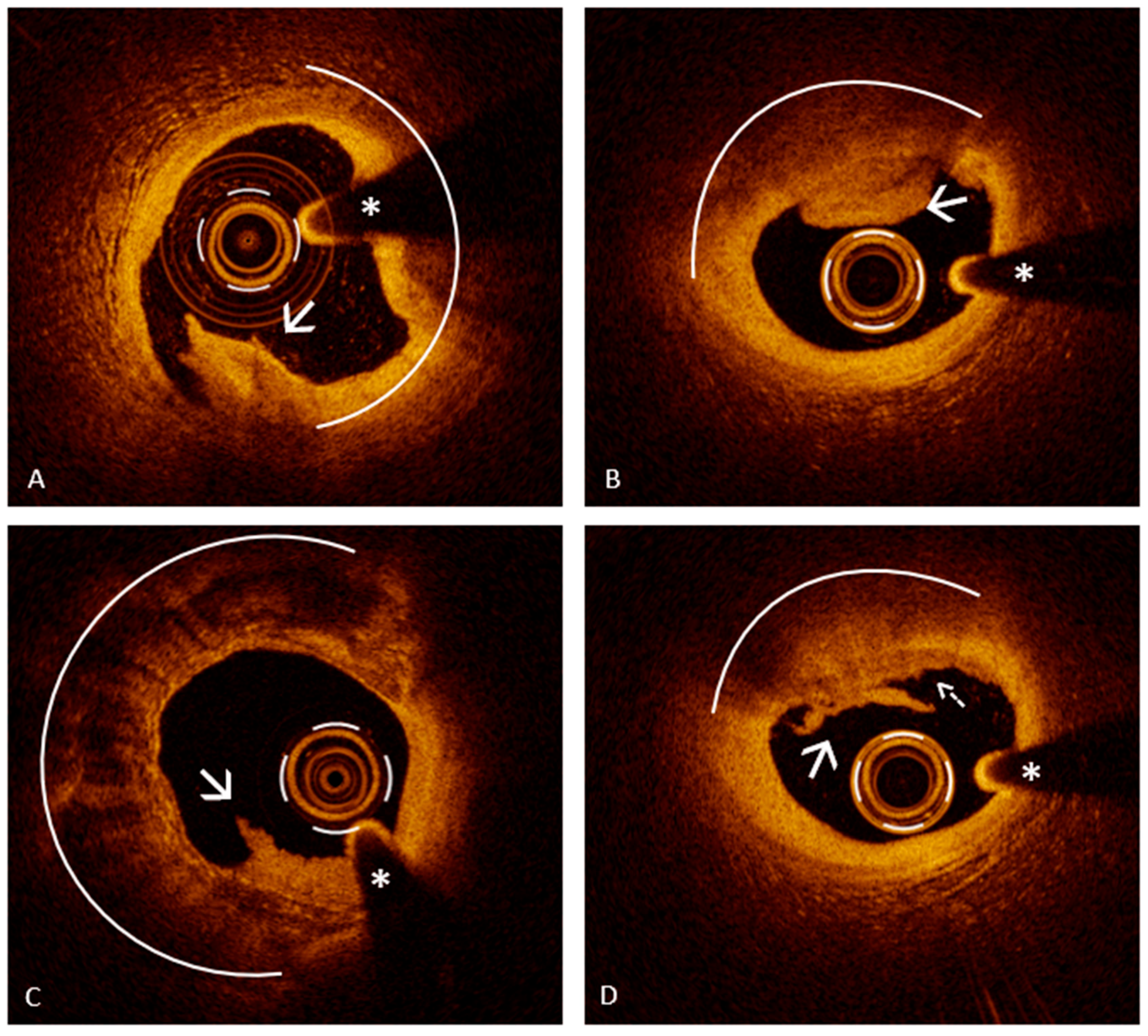
6.2. Assessing Plaque Erosion Using Optical Coherence Tomography: A Diagnostic Game Changer
6.3. Diagnostic Strategies with Intravascular Ultrasound and Near-Infrared Spectroscopy
6.4. Diagnostic Strategies with Coronary Computed Tomography Angiography
7. OCT-Guided Strategy in Plaque Erosion: A New Frontier in Personalized ACS Management
7.1. The EROSION Trial
7.2. Who Should Avoid a Stent? Patient Selection in Plaque Erosion Through the Lens of OCT Evidence
7.3. Next-Generation Management of Plaque Erosion
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| PR | Plaque rupture |
| PE | Plaque erosion |
| MI | Myocardial infarction |
| OCT | Optical coherence tomography |
| CAD | Coronary artery disease |
| NSTE-ACS | Non-ST elevation ACS |
| CN | Calcified nodule |
| VSMC | Vascular smooth muscle cells |
| AMI | Acute myocardial infarction |
| TCFA | Thin-cap fibroatheroma |
| CCs | Cholesterol crystals |
| IVI | Intravascular imaging |
| STEMI | ST-elevation myocardial infarction |
| TLR2 | Toll-like receptor 2 |
| NET | Neutrophil extracellular traps |
| MPO | Myeloperoxidase |
| RFC | Rupture fibrous cap |
| IFC | Intact fibrous cap |
| MMP | Matrix metalloproteinase |
| MACE | Major adverse cardiac events |
| EndMT | Endothelial-to-mesenchymal transition |
| QCA | Quantitative coronary angiography |
| IVUS | Intravascular ultrasound |
| NIRS | Near-infrared spectroscopy |
| CCTA | Coronary computed tomography angiography |
| LAD | Left anterior descending artery |
| MLA | Minimal lumen area |
| HD-IVUS | High-definition intravascular ultrasound |
| VH-IVUS | Virtual histology intravascular ultrasound |
| LCBI | Lipid core burden index |
| CT | Computed tomography |
| PVAT | Perivascular adipose tissue |
| FAI | Fatty attenuation index |
| PCCT | Photon-counting computed tomography |
| TLR | Target lesion revascularization |
| DCB | Drug-coated balloon |
| ACE | Angiotensin-converting enzyme |
| ARBs | Angiotensin receptor blockers |
References
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Burke, A.P.; Morbini, P.; Dal Bello, B.; Bocciarelli, M.; Virmani, R.; Arbustini, E.; Specchia, G. Plaque Erosion Is a Major Substrate for Coronary Thrombosis in Acute Myocardial Infarction. Heart 1999, 82, 269–272. [Google Scholar] [CrossRef]
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.; Smialek, J.; Virmani, R. Coronary Risk Factors and Plaque Morphology in Men with Coronary Disease Who Died Suddenly. N. Engl. J. Med. 1997, 336, 1276–1282. [Google Scholar] [CrossRef]
- Farb, A.; Burke, A.P.; Tang, A.L.; Liang, T.Y.; Mannan, P.; Smialek, J.; Virmani, R. Coronary Plaque Erosion without Rupture into a Lipid Core. A Frequent Cause of Coronary Thrombosis in Sudden Coronary Death. Circulation 1996, 93, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Burke, A.; dal Bello, B.; Morbini, P.; Specchia, G.; Virmani, R. Plaque Erosion Is a Major Substrate for Coronary Thrombosis in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 1998, 31, 379. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, E.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; Takano, M.; Niccoli, G.; Higuma, T.; et al. Clinical and Laboratory Predictors for Plaque Erosion in Patients with Acute Coronary Syndromes. J. Am. Heart Assoc. 2019, 8, e012322. [Google Scholar] [CrossRef] [PubMed]
- Seegers, L.M.; Araki, M.; Nakajima, A.; Yonetsu, T.; Minami, Y.; Ako, J.; Soeda, T.; Kurihara, O.; Higuma, T.; Kimura, S.; et al. Sex Differences in Culprit Plaque Characteristics Among Different Age Groups in Patients with Acute Coronary Syndromes. Circ. Cardiovasc. Interv. 2022, 15, e011612. [Google Scholar] [CrossRef]
- Araki, M.; Yonetsu, T.; Kurihara, O.; Nakajima, A.; Lee, H.; Soeda, T.; Minami, Y.; Higuma, T.; Kimura, S.; Takano, M.; et al. Age and Phenotype of Patients with Plaque Erosion. J. Am. Heart Assoc. 2021, 10, e020691. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, C.J.; Kim, W.; Cho, J.-M.; Soeda, T.; Takano, M.; Yan, B.P.; Crea, F.; Niccoli, G.; Vergallo, R.; et al. Relative Risk of Plaque Erosion among Different Age and Sex Groups in Patients with Acute Coronary Syndrome. J. Thromb. Thrombolysis 2020, 49, 352–359. [Google Scholar] [CrossRef]
- Buonpane, A.; Trimarchi, G.; Ciardetti, M.; Coceani, M.A.; Alagna, G.; Benedetti, G.; Berti, S.; Andò, G.; Burzotta, F.; De Caterina, A.R. Optical Coherence Tomography in Myocardial Infarction Management: Enhancing Precision in Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 5791. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In Vivo Diagnosis of Plaque Erosion and Calcified Nodule in Patients with Acute Coronary Syndrome by Intravascular Optical Coherence Tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Yonetsu, T.; Kakuta, T.; Lee, T.; Takahashi, K.; Kawaguchi, N.; Yamamoto, G.; Koura, K.; Hishikari, K.; Iesaka, Y.; Fujiwara, H.; et al. In Vivo Critical Fibrous Cap Thickness for Rupture-Prone Coronary Plaques Assessed by Optical Coherence Tomography. Eur. Heart J. 2011, 32, 1251–1259. [Google Scholar] [CrossRef]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.K. Reassessing the Mechanisms of Acute Coronary Syndromes: The “Vulnerable Plaque” and Superficial Erosion. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Leistner, D.M.; Kränkel, N.; Meteva, D.; Abdelwahed, Y.S.; Seppelt, C.; Stähli, B.E.; Rai, H.; Skurk, C.; Lauten, A.; Mochmann, H.-C.; et al. Differential Immunological Signature at the Culprit Site Distinguishes Acute Coronary Syndrome with Intact from Acute Coronary Syndrome with Ruptured Fibrous Cap: Results from the Prospective Translational OPTICO-ACS Study. Eur. Heart J. 2020, 41, 3549–3560. [Google Scholar] [CrossRef]
- Meteva, D.; Vinci, R.; Seppelt, C.; Abdelwahed, Y.S.; Pedicino, D.; Nelles, G.; Skurk, C.; Haghikia, A.; Rauch-Kröhnert, U.; Gerhardt, T.; et al. Toll-like Receptor 2, Hyaluronan, and Neutrophils Play a Key Role in Plaque Erosion: The OPTICO–ACS Study. Eur. Heart J. 2023, 44, 3892–3907. [Google Scholar] [CrossRef]
- Gerhardt, T.; Seppelt, C.; Abdelwahed, Y.S.; Meteva, D.; Wolfram, C.; Stapmanns, P.; Erbay, A.; Zanders, L.; Nelles, G.; Musfeld, J.; et al. Culprit Plaque Morphology Determines Inflammatory Risk and Clinical Outcomes in Acute Coronary Syndrome. Eur. Heart J. 2023, 44, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Pedicino, D.; Vinci, R.; Giglio, A.F.; Pisano, E.; Porto, I.; Vergallo, R.; Russo, G.; Ruggio, A.; D’Aiello, A.; Flego, D.; et al. Alterations of Hyaluronan Metabolism in Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1490–1503. [Google Scholar] [CrossRef]
- Yamamoto, E.; Thondapu, V.; Poon, E.; Sugiyama, T.; Fracassi, F.; Dijkstra, J.; Lee, H.; Ooi, A.; Barlis, P.; Jang, I.-K. Endothelial Shear Stress and Plaque Erosion. JACC Cardiovasc. Imaging 2019, 12, 374–375. [Google Scholar] [CrossRef]
- Russo, G.; Pedicino, D.; Chiastra, C.; Vinci, R.; Lodi Rizzini, M.; Genuardi, L.; Sarraf, M.; d’Aiello, A.; Bologna, M.; Aurigemma, C.; et al. Coronary Artery Plaque Rupture and Erosion: Role of Wall Shear Stress Profiling and Biological Patterns in Acute Coronary Syndromes. Int. J. Cardiol. 2023, 370, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Franck, G.; Mawson, T.; Sausen, G.; Salinas, M.; Masson, G.S.; Cole, A.; Beltrami-Moreira, M.; Chatzizisis, Y.; Quillard, T.; Tesmenitsky, Y.; et al. Flow Perturbation Mediates Neutrophil Recruitment and Potentiates Endothelial Injury via TLR2 in Mice: Implications for Superficial Erosion. Circ. Res. 2017, 121, 31–42. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, C.-J.; Kurihara, O.; Thondapu, V.; Russo, M.; Yamamoto, E.; Sugiyama, T.; Fracassi, F.; Lee, H.; Yonetsu, T.; et al. Angiographic Features of Patients with Coronary Plaque Erosion. Int. J. Cardiol. 2019, 288, 12–16. [Google Scholar] [CrossRef]
- Vergallo, R.; Lombardi, M.; Besis, G.; Migliaro, S.; Ricchiuto, A.; Maino, A.; Buonpane, A.; Bianchini, E.; Annibali, G.; Galli, M.; et al. Pre-Stenting Residual Thrombotic Volume Assessed by Dual Quantitative Coronary Angiography Predicts Microvascular Obstruction in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Minerva Cardiol. Angiol. 2023, 71, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wu, T.; Zhao, J.; Du, Z.; Wang, Z.; Li, L.; Wei, G.; Tian, J.; Jia, H.; Mintz, G.S.; et al. Focal Geometry and Characteristics of Erosion-Prone Coronary Plaques In Vivo Angiography and Optical Coherence Tomography Study. Front. Cardiovasc. Med. 2021, 8, 709480. [Google Scholar] [CrossRef]
- Jang, I.K. Cardiovascular OCT Imaging; Springer: Berlin/Heidelberg, Germany, 2015; p. 216. ISBN 978-3-319-10801-8. [Google Scholar]
- Araki, M.; Park, S.J.; Dauerman, H.L.; Uemura, S.; Kim, J.S.; Di Mario, C.; Johnson, T.W.; Guagliumi, G.; Kastrati, A.; Joner, M.; et al. Optical Coherence Tomography in Coronary Atherosclerosis Assessment and Intervention. Nat. Rev. Cardiol. 2022, 19, 684–703. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus Standards for Acquisition, Measurement, and Reporting of Intravascular Optical Coherence Tomography Studies: A Report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef]
- Higuma, T.; Soeda, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Vergallo, R.; Minami, Y.; Ong, D.S.; Lee, H.; et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients with ST-Segment Elevation Myocardial Infarction Incidence, Morphologic Characteristics, and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2015, 8, 1166–1176. [Google Scholar]
- Kramer, M.C.A.; Rittersma, S.Z.H.; de Winter, R.J.; Ladich, E.R.; Fowler, D.R.; Liang, Y.-H.; Kutys, R.; Carter-Monroe, N.; Kolodgie, F.D.; van der Wal, A.C.; et al. Relationship of Thrombus Healing to Underlying Plaque Morphology in Sudden Coronary Death. J. Am. Coll. Cardiol. 2010, 55, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Conte, E.; Mushtaq, S.; Brouwers, S.; Shinke, T.; Coskun, A.U.; Pu, Z.; Hakim, D.; Stone, P.H.; Andreini, D. Reviewing Imaging Modalities for the Assessment of Plaque Erosion. Atherosclerosis 2021, 318, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, J.; Antuña, P.; Jiménez, C.; Rivero, F.; Bastante, T.; García-Guimaraes, M.; Alfonso, F. Can Plaque Erosion Be Visualized by High-Definition Intravascular Ultrasound? JACC Cardiovasc. Interv. 2020, 13, e57–e61. [Google Scholar] [CrossRef]
- Oemrawsingh, R.M.; Cheng, J.M.; García-García, H.M.; Van Geuns, R.-J.; De Boer, S.P.M.; Simsek, C.; Kardys, I.; Lenzen, M.J.; Van Domburg, R.T.; Regar, E.; et al. Near-Infrared Spectroscopy Predicts Cardiovascular Outcome in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 64, 2510–2518. [Google Scholar] [CrossRef]
- Terada, K.; Kubo, T.; Kameyama, T.; Matsuo, Y.; Ino, Y.; Emori, H.; Higashioka, D.; Katayama, Y.; Khalifa, A.K.M.; Takahata, M.; et al. NIRS-IVUS for Differentiating Coronary Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2021, 14, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Nurmohamed, N.S.; Van Rosendael, A.R.; Danad, I.; Ngo-Metzger, Q.; Taub, P.R.; Ray, K.K.; Figtree, G.; Bonaca, M.P.; Hsia, J.; Rodriguez, F.; et al. Atherosclerosis Evaluation and Cardiovascular Risk Estimation Using Coronary Computed Tomography Angiography. Eur. Heart J. 2024, 45, 1783–1800. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Tzolos, E.; Williams, M.C.; Dey, D.; Berman, D.; Slomka, P.; Newby, D.E.; Dweck, M.R. Noninvasive Coronary Atherosclerotic Plaque Imaging. JACC Cardiovasc. Imaging 2023, 16, 1608–1622. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the Management of Chronic Coronary Syndromes: Developed by the Task Force for the Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Angelopoulos, A.; Tsioufis, K.; Antoniades, C.; Tousoulis, D. Cardiovascular Risk Stratification by Coronary Computed Tomography Angiography Imaging: Current State-of-the-Art. Eur. J. Prev. Cardiol. 2022, 29, 608–624. [Google Scholar] [CrossRef]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.V.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated with Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef]
- Antoniades, C.; Tousoulis, D.; Vavlukis, M.; Fleming, I.; Duncker, D.J.; Eringa, E.; Manfrini, O.; Antonopoulos, A.S.; Oikonomou, E.; Padró, T.; et al. Perivascular Adipose Tissue as a Source of Therapeutic Targets and Clinical Biomarkers. Eur. Heart J. 2023, 44, 3827–3844. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-Invasive Detection of Coronary Inflammation Using Computed Tomography and Prediction of Residual Cardiovascular Risk (the CRISP CT Study): A Post-Hoc Analysis of Prospective Outcome Data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Suzuki, K.; Kinoshita, D.; Sugiyama, T.; Yuki, H.; Niida, T.; Dey, D.; Lee, H.; McNulty, I.; Ferencik, M.; Kakuta, T.; et al. Coronary Computed Tomography Angiography Findings of Plaque Erosion. Am. J. Cardiol. 2023, 196, 52–58. [Google Scholar] [CrossRef]
- Niida, T.; Usui, E.; Suzuki, K.; Kinoshita, D.; Yuki, H.; Fujimoto, D.; Covani, M.; Dey, D.; Lee, H.; McNulty, I.; et al. Differences in Total Plaque Burden between Plaque Rupture and Plaque Erosion: A Combined Computed Tomography Angiography and Optical Coherence Tomography Study. J. Cardiovasc. Comput. Tomogr. 2024, 18, 568–574. [Google Scholar] [CrossRef]
- Nagamine, T.; Hoshino, M.; Yonetsu, T.; Sugiyama, T.; Kanaji, Y.; Matsuda, K.; Sayama, K.; Ueno, H.; Nogami, K.; Hanyu, Y.; et al. Identification of Optical Coherence Tomography-Defined Coronary Plaque Erosion by Preprocedural Computed Tomography Angiography. J. Am. Heart Assoc. 2023, 12, e029239. [Google Scholar] [CrossRef]
- Meloni, A.; Maffei, E.; Positano, V.; Clemente, A.; De Gori, C.; Berti, S.; La Grutta, L.; Saba, L.; Bossone, E.; Mantini, C.; et al. Technical Principles, Benefits, Challenges, and Applications of Photon Counting Computed Tomography in Coronary Imaging: A Narrative Review. Cardiovasc. Diagn. Ther. 2024, 14, 698–724. [Google Scholar] [CrossRef]
- Meloni, A.; Maffei, E.; Clemente, A.; De Gori, C.; Occhipinti, M.; Positano, V.; Berti, S.; La Grutta, L.; Saba, L.; Cau, R.; et al. Spectral Photon-Counting Computed Tomography: Technical Principles and Applications in the Assessment of Cardiovascular Diseases. J. Clin. Med. 2024, 13, 2359. [Google Scholar] [CrossRef]
- Kotronias, R.A.; Raman, B.; Ferreira, V.; Neubauer, S.; Antoniades, C. Photon-Counting Computed Tomography: ‘One-Stop Shop’ for Coronary Stenosis, Inflammation, and Myocardial Assessment in ST-Segment Elevation Acute Coronary Syndrome. Eur. Heart J.-Cardiovasc. Imaging 2024, 25, e165. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Niccoli, G.; Montone, R.A.; Di Vito, L.; Gramegna, M.; Refaat, H.; Scalone, G.; Leone, A.M.; Trani, C.; Burzotta, F.; Porto, I.; et al. Plaque Rupture and Intact Fibrous Cap Assessed by Optical Coherence Tomography Portend Different Outcomes in Patients with Acute Coronary Syndrome. Eur. Heart J. 2015, 36, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Uemura, S.; Souteyrand, G.; Virmani, R.; Motreff, P.; Di Vito, L.; Biondi-Zoccai, G.; Halperin, J.; Fuster, V.; Ozaki, Y.; et al. OCT-Based Diagnosis and Management of STEMI Associated with Intact Fibrous Cap. JACC Cardiovasc. Imaging 2013, 6, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Dai, J.; Hou, J.; Xing, L.; Ma, L.; Liu, H.; Xu, M.; Yao, Y.; Hu, S.; Yamamoto, E.; et al. Effective Anti-Thrombotic Therapy without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion (the EROSION Study). Eur. Heart J. 2017, 38, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Yamamoto, E.; Sugiyama, T.; Jia, H.; Ma, L.; Hu, S.; Wang, C.; Zhu, Y.; Li, L.; Xu, M.; et al. EROSION Study (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography–Based Management in Plaque Erosion): A 1-Year Follow-Up Report. Circ. Cardiovasc. Interv. 2017, 10, e005860. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Qin, Y.; Xu, Y.; Hu, S.; Wang, Y.; Zeng, M.; Feng, X.; Liu, Q.; Syed, I.; Demuyakor, A.; et al. Predictors of Non-Stenting Strategy for Acute Coronary Syndrome Caused by Plaque Erosion: Four-Year Outcomes of the EROSION Study. EuroIntervention 2021, 17, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhu, Y.; Zhang, Y.; Dai, J.; Li, L.; Dauerman, H.; Soeda, T.; Wang, Z.; Lee, H.; Wang, C.; et al. Management and Outcome of Patients with Acute Coronary Syndrome Caused by Plaque Rupture Versus Plaque Erosion: An Intravascular Optical Coherence Tomography Study. J. Am. Heart Assoc. 2017, 6, e004730. [Google Scholar] [CrossRef]
- Jia, H.; Kubo, T.; Akasaka, T.; Yu, B. Optical Coherence Tomography Guidance in Management of Acute Coronary Syndrome Caused by Plaque Erosion. Circ. J. 2018, 82, 302–308. [Google Scholar] [CrossRef]
- Jia, H.; Dai, J.; He, L.; Xu, Y.; Shi, Y.; Zhao, L.; Sun, Z.; Liu, Y.; Weng, Z.; Feng, X.; et al. EROSION III. JACC Cardiovasc. Interv. 2022, 15, 846–856. [Google Scholar] [CrossRef]
- Yin, Y.; Fang, C.; Jiang, S.; Wang, J.; Wang, Y.; Guo, J.; Lei, F.; Sun, S.; Pei, X.; Jia, R.; et al. In Vivo Evidence of Atherosclerotic Plaque Erosion and Healing in Patients with Acute Coronary Syndrome Using Serial Optical Coherence Tomography Imaging. Am. Heart J. 2022, 243, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Lei, F.; Fang, C.; Jiang, S.; Xu, X.; Sun, S.; Pei, X.; Jia, R.; Tang, C.; Peng, C.; et al. Predictors of Adverse Prognosis in Patients with Acute Coronary Syndrome Caused by Plaque Erosion with a Nonstent Strategy. J. Am. Heart Assoc. 2022, 11, e026414. [Google Scholar] [CrossRef]
- Cortese, B.; Kalkat, H.; Bathia, G.; Basavarajaiah, S. The Evolution and Revolution of Drug Coated Balloons in Coronary Angioplasty: An up-to-Date Review of Literature Data. Catheter. Cardiovasc. Interv. 2023, 102, 1069–1077. [Google Scholar] [CrossRef]
- Mangner, N.; Farah, A.; Ohlow, M.-A.; Möbius-Winkler, S.; Weilenmann, D.; Wöhrle, J.; Linke, A.; Stachel, G.; Markovic, S.; Leibundgut, G.; et al. Safety and Efficacy of Drug-Coated Balloons Versus Drug-Eluting Stents in Acute Coronary Syndromes: A Prespecified Analysis of BASKET-SMALL 2. Circ. Cardiovasc. Interv. 2022, 15, e011325. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawamori, H.; Toba, T.; Kakizaki, S.; Nakamura, K.; Fujimoto, D.; Sasaki, S.; Fujii, H.; Hamana, T.; Osumi, Y.; et al. Clinical Impact of Optical Coherence Tomography Findings after Drug-Coated Balloon Treatment for Patients with Acute Coronary Syndromes. Int. J. Cardiol. 2023, 387, 131149. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yang, Y.; Dong, S.L.; Zhao, C.; Yang, F.; Yuan, Y.F.; Liao, Y.H.; He, S.L.; Liu, K.; Wei, F.; et al. Effect of Colchicine on Coronary Plaque Stability in Acute Coronary Syndrome as Assessed by Optical Coherence Tomography: The COLOCT Randomized Clinical Trial. Circulation 2024, 150, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, U.; De Caterina, A.R.; Trimarchi, G.; Pizzino, F.; Bastiani, L.; Dossi, F.; Raccis, M.; Bianchi, G.; Palmieri, C.; de Gregorio, C.; et al. The Enigma of the ‘Smoker’s Paradox’: Results from a Single-Center Registry of Patients with STEMI Undergoing Primary Percutaneous Coronary Intervention. Cardiovasc. Revasc. Med. 2024, 69, 42–49. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonpane, A.; De Caterina, A.R.; Trimarchi, G.; Pizzino, F.; Ciardetti, M.; Coceani, M.A.; Esposito, A.; Pastormerlo, L.E.; Monteleone, A.; Clemente, A.; et al. Uncovering Plaque Erosion: A Distinct Pathway in Acute Coronary Syndromes and a Gateway to Personalized Therapy. J. Clin. Med. 2025, 14, 5456. https://doi.org/10.3390/jcm14155456
Buonpane A, De Caterina AR, Trimarchi G, Pizzino F, Ciardetti M, Coceani MA, Esposito A, Pastormerlo LE, Monteleone A, Clemente A, et al. Uncovering Plaque Erosion: A Distinct Pathway in Acute Coronary Syndromes and a Gateway to Personalized Therapy. Journal of Clinical Medicine. 2025; 14(15):5456. https://doi.org/10.3390/jcm14155456
Chicago/Turabian StyleBuonpane, Angela, Alberto Ranieri De Caterina, Giancarlo Trimarchi, Fausto Pizzino, Marco Ciardetti, Michele Alessandro Coceani, Augusto Esposito, Luigi Emilio Pastormerlo, Angelo Monteleone, Alberto Clemente, and et al. 2025. "Uncovering Plaque Erosion: A Distinct Pathway in Acute Coronary Syndromes and a Gateway to Personalized Therapy" Journal of Clinical Medicine 14, no. 15: 5456. https://doi.org/10.3390/jcm14155456
APA StyleBuonpane, A., De Caterina, A. R., Trimarchi, G., Pizzino, F., Ciardetti, M., Coceani, M. A., Esposito, A., Pastormerlo, L. E., Monteleone, A., Clemente, A., Paradossi, U., Berti, S., Leone, A. M., Trani, C., Liuzzo, G., Burzotta, F., & Crea, F. (2025). Uncovering Plaque Erosion: A Distinct Pathway in Acute Coronary Syndromes and a Gateway to Personalized Therapy. Journal of Clinical Medicine, 14(15), 5456. https://doi.org/10.3390/jcm14155456








