1. Introduction
In 2023 there were 371 adult patients with end-stage liver disease from primary sclerosing cholangitis (PSC) listed for liver transplantation and 385 adult liver transplants performed for PSC in the United States [
1]. PSC is an indication for liver transplantation worldwide. The crude incidence of liver transplantation in the United Kingdom was 10.9 per 1000-person-years while in the Netherlands 94 of 590 transplants were performed for end-stage liver disease from PSC over a median follow-up of 92 months [
2]. Liver transplantation for PSC is associated with excellent 1-year patient and graft survival exceeding 95%; however, recurrent PSC occurs in up to 20% of recipients within five years of transplant [
3]. Studies of liver transplantation and autoimmune or cholestatic liver diseases typically combine results of PSC, primary biliary cholangitis and autoimmune hepatitis or do not include diverse patient populations [
3,
4,
5,
6,
7].
The development of effective medical therapy for PSC before or after liver transplantation has been challenging. Effective therapy is needed because recurrent PSC may progress to graft failure after liver transplantation. Factors associated with graft failure or recurrent disease after liver transplantation include recipient age, donor age, rejection episodes, donor after circulatory death, ulcerative colitis, and history of colectomy prior to transplants [
4,
5,
6,
7]. Adult liver recipients of living and deceased donor liver transplants with PSC have a similar risk of recurrent disease after liver transplantation [
8]. Donor–recipient sex mismatch has not been associated with recurrent PSC or survival after liver transplantation for PSC [
9].
Donor–recipient race mismatch has been associated with outcomes after liver transplant [
10,
11,
12]. An analysis of the Organ Procurement and Transplantation Network (OPTN) database reported that race matched Caucasian recipients experienced a 1–3% improvement in mortality in lung, liver, and pancreas transplants [
10]. Among recipients transplanted for a variety of indications, matched Black donor–recipients experienced a 4–6% improvement in patient and graft survival [
10]. Black recipients of White donors have a 1.39-fold increase in graft loss compared to donor–recipient matched liver transplants [
13]. These studies have not reported outcomes in patients transplanted for end-stage liver disease from PSC. Because studies have demonstrated differences in survival after liver transplantation in donor-race mismatched recipients for indications other than PSC, we sought to report outcomes after liver transplantation for by donor and recipient race.
2. Methods
We analyzed the OPTN database for adult (≥18 years old) recipients of deceased donor liver transplants with diagnosis of end-stage liver disease and cirrhosis from PSC between 27 February 2002 (implementation of Model for End-Stage Liver Disease (MELD) and 26 February 2020 (prior to COVID pandemic) who were alive with a functioning graft at discharge. Recipients that had a diagnosis of PSC with or without inflammatory bowel disease were included. Multiorgan transplants, retransplants, and individuals with a prior transplant were excluded. Two transplant eras (2002–2011 and 2012–2020) were used to evaluate changes in characteristics of all PSC transplants over time while still maintaining an adequate number of recipients in each era, including for subgroup analyses. Our study complied with the strengthening the reporting of observational studies in epidemiology (STROBE) guidelines [
14] (
Supplementary Table S1).
The primary outcomes were defined as graft failure and death within 5 years after transplantation, by donor–recipient race. Follow-up time was measured as the duration from the transplant date to either the occurrence of the primary outcomes or the last known follow-up date. For 5-year graft and patient survival outcomes were assessed for adult deceased donor liver transplants through September 2018, to allow for 5 years of follow-up data plus an additional 3 months due to data reporting lags. We only considered graft failure and death within 5 years of transplant; any transplant recipient who had not experienced either event by the end of the five-year period was administratively censored.
Primary outcomes of graft failure and death within 5 years of transplant were analyzed from 2012 to 2018 as well as stratified by era (era 1: 2002–2011 vs. era 2: 2012–2018). Secondary outcomes included 1-year graft and patient survival by recipient race and ethnicity were analyzed from 2012 to 2020. Race and ethnicity were self-identified. Donor and recipient race was classified as White, Black, Hispanic, Asian, and ‘Other” in the univariate tabulation. For the outcomes analysis, the Hispanic and Asian categories were combined into ‘Other’ due to sample size. Additionally, donor-to-recipient race was categorized as White–White, White–Black, Black–White, and ‘Other’, with ‘Other’ encompassing any combinations outside the first four. A donor–recipient mismatch refers to transplant where the race of the donor differs from that of the recipient. The use antibody induction drug at transplant was categorized as IL (interleukin)-2 receptor antibody (basiliximab, daclizumab), monoclonal (alemtuzumab), polyclonal (anti-thymocyte globulin), or none. Maintenance immunosuppressive drugs were summarized as the usage of tacrolimus and mycophenolate mofetil at discharge.
The current analysis was performed using OPTN data as of 5 April 2024. The data were analyzed using R version 4.3.3, R Project, Vienna, Austria.
3. Statistical Methods
Continuous variables were summarized as median and interquartile range (IQR). Distributions of continuous variables were compared by group using a Kruskal–Wallis test. Categorical variables were reported as number and percent, and the distribution was compared using a Chi-square test. Unadjusted Kaplan–Meier models were used to estimate patient and graft survival within 5 years and compared by transplant group using log-rank tests. p-values were adjusted for multiple comparisons of survival using the Benjamini–Hochberg procedure. Risk adjustment variables used in the Cox models were identified a priori to reduce bias.
The variables were selected based on clinical knowledge and the results of the univariate analyses. The primary covariate of interest in the Cox model was donor–recipient race mismatch, and the additional risk adjustment variables were transplant era, recipient age, donor age, liver donor risk index (LDRI), and treatment for acute rejection at discharge. Five-year patient and graft survival are reported for adult deceased donor liver transplants from 2002 to 2018. Our multivariable cohort had 10.9% missingness for these covariates of interest. Missing values for covariates were imputed using multiple imputation by chained equations via the aregImpute function from the Hmisc package version 5.1–3 and pooled across 11 imputation datasets for the cohort of deceased donor transplants. The results from each imputed dataset were combined to produce final estimates, including hazard ratios and p-values. Cox models for patient and graft survival were fit among deceased donor transplants, with administrative censoring at 5 years applied. The model with donor–recipient race mismatch utilized 14 degrees of freedom.
4. Results
During 2002–2020, 3790 adults underwent liver transplantation for end-stage liver disease from PSC. Characteristics of recipients included a median age of 48 years old, 31.6% female, 15.2% Black, 4.2% Hispanic or Latino, 84.4% deceased donor recipients, 15.6% adult living donor recipients, 4.4% donor after circulatory death recipients, and median MELD allocation score of 23 (
Table 1). Tacrolimus and mycophenolate mofetil were the most common discharge maintenance immunosuppressants, in 3641 (96.1%) and 3053 (80.6%) recipients, respectively. Overall 1-year graft and patient survival were 94.6% [95% CI 93.8–95.4%] and 97.2% [95% CI 96.6–97.8%], respectively.
Five-year patient and graft survival was available for 2907 adult deceased donor recipients who underwent liver transplant from 2002 to 2018. Overall 5-year graft and patient survival were 83.2%, [95% CI 81.9–84.6%] and 89.0% [95%CI 87.9–90.2%], respectively. The most common identified causes of death within 5 years of transplant in descending order were malignancy, infection, graft failure, and multiorgan system failure. Recurrent disease as a cause of graft failure within 5 years of transplant was reported in 13.7% of cases.
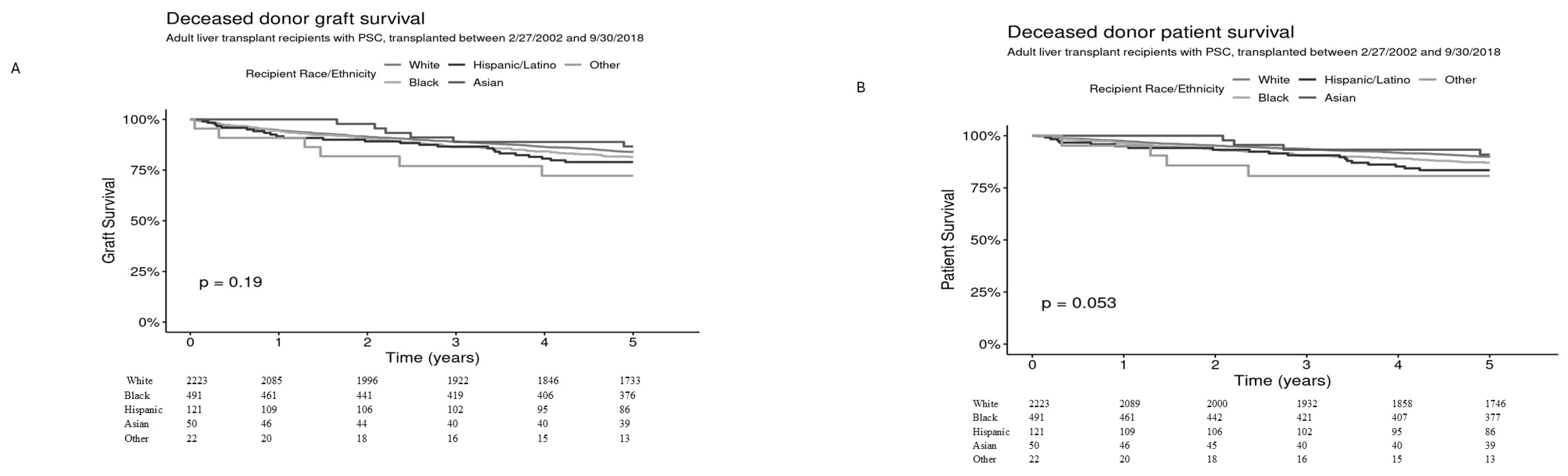
Graft and patient survival within 5 years was assessed by recipient race and ethnicity among 2907 deceased donor transplants. Five-year graft survival for White (
n = 2223), Black (
n = 491), Hispanic/Latino (
n = 121), Asian (
n = 50), and Other (
n = 22) was 83.9% [95% CI 82.3–85.4%], 81.5% [95% CI 78.1–85.0%], 79.0% [95% CI 72.0–86.7%], 86.7% [95% CI 77.3–97.2%], and 72.2% [95% CI 55.5–93.9%], respectively (overall
p = 0.19) (
Figure 1A) and 5 year-patient survival was 89.8% [95% CI 88.5–91.1%], 87.1% [95% CI 84.1–90.1%], 83.5% [95% CI 76.9–90.6%], 90.9% [95% CI 82.8–99.8%], and 80.7% [95% CI 65.3–99.6%], respectively,
p = 0.053 (
Figure 1B).
4.1. Donor and Recipient Race
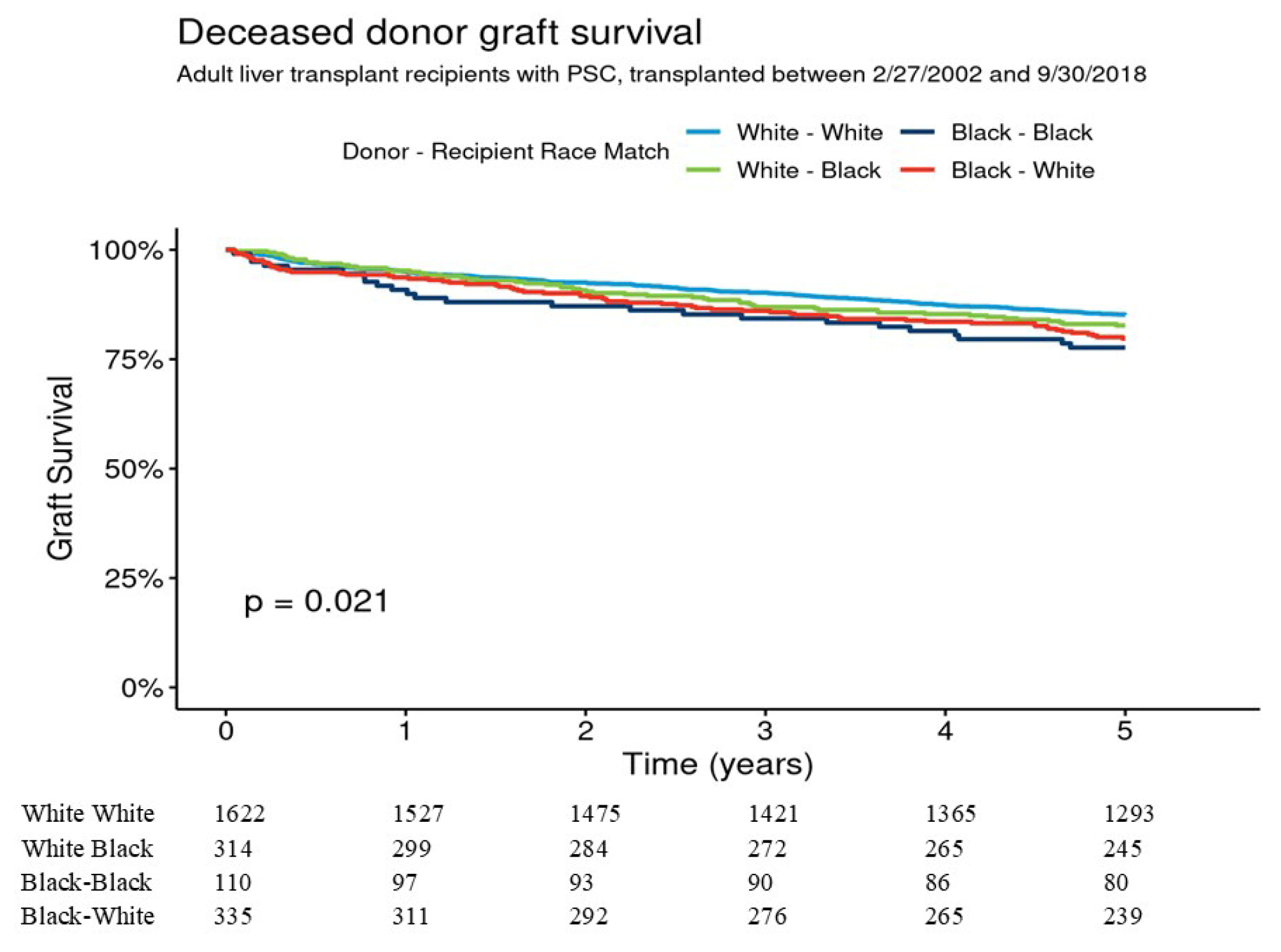
For deceased donor transplants donor–recipient race was White–White (W-W) (
n = 1622), Black–Black (B-B) (
n = 110), Black–White (B-W) (
n = 335), and White–Black (W-B) (
n = 314). Donor age was youngest in Black donors to Black recipients and allocation MELD score was highest in White donors to Black recipients (
Table 2).
Five-year graft survival for W-W, B-B, B-W, and W-B was 85.2% [95% CI 83.4–86.9%], 77.7% [95% CI 70.2–85.9%], 79.7% [95% CI 75.4–84.2%], and 82.7% [78.6–87.0%], respectively (overall
p = 0.021) (
Figure 2). Pairwise comparisons indicated that the graft survival differences were not significant for W-W vs. B-B (
p = 0.09) and for W-W vs. B-W (
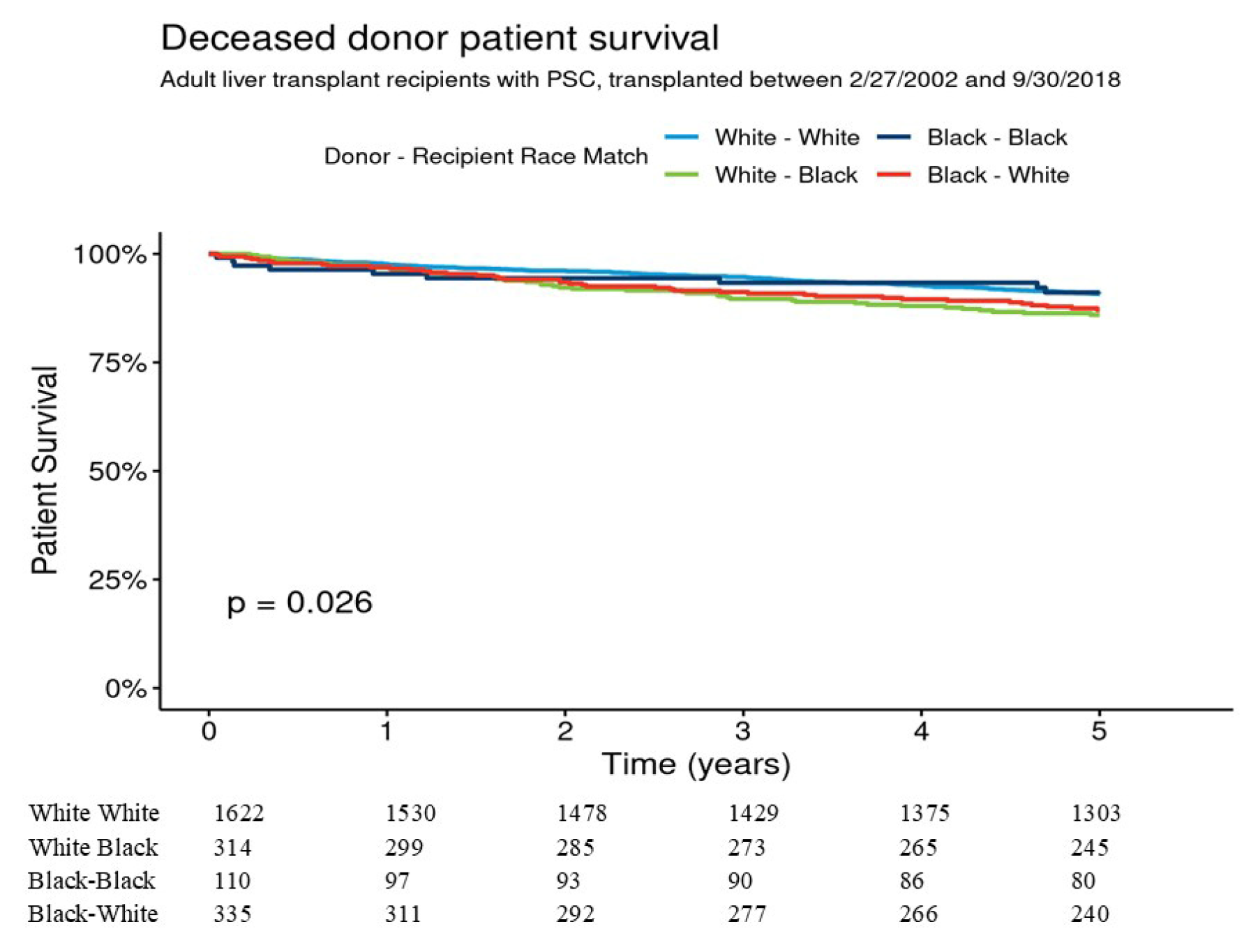
p = 0.08). In multivariable analysis 5-year graft survival was not significantly different between B-B, B-W, and W-B as compared to W-W donor–recipient group. Five-year patient survival for W-W, B-B, B-W, and W-B were 90.8% [95% CI 89.3–92.2%], 91.1% [95% CI 85.7–96.7%], 87.1% [95% CI 83.5–90.9%], and 86.0% [95% CI 82.2–89.9%], respectively,
p = 0.026 (
Figure 3). Pairwise comparisons showed that patient survival difference was marginally significant for W-W vs. W-B donor–recipient group (
p = 0.051). The adjusted 5-year risk of patient mortality was 1.69-fold higher in the W-B donor–recipient group compared to the W-W donor–recipient group (HR = 1.69 [95% CI 1.16–2.45], (
p = 0.006) (
Table 3).
4.2. Era 1 Vs. Era 2
The number of transplants from 2002 to 2011 (era 1) and 2012 to 2020 (era 2) were 1890 (49.9%) and 1900 (50.1%), respectively. From year to year the number of patients transplanted for PSC was similar ranging from 168 to 285 recipients or 3.6–5.6% of total liver transplants. Median recipient age was similar between the two eras while the proportion of female recipients increased from 29.8% to 33.4% (
p = 0.02). Allocation MELD score increased from era 1 to era 2, 21 and 25, respectively (
p < 0.001). More recipients received induction therapy and MMF discharge maintenance for immunosuppression in era 2 than era 1, 89.1% and 72.0%, respectively (
p < 0.001) (
Table 1). Overall graft and patient survival rates within 5 years of transplant among deceased donor transplants between 2002 and 2018 were not significantly different between era 1, 88.6% [95% CI 87.0–90.2%] and era 2, 89.6% [95% CI 87.9–91.3%],
p = 0.31.
The proportion of White patients decreased from era 1 to era 2, 1536 (81.3%) to 1423 (74.9%), respectively, while the proportion of Black, Asian, and Hispanic/Latino patients transplanted for PSC increased, 255 (13.5%) to 321 (16.9%), 26 (1.4%) to 42 (2.2%), and 64 (3.4%) to 97 (5.1%), respectively (p < 0.001).
5. Discussion
Liver transplantation for end-stage liver disease from primary sclerosing cholangitis is associated with excellent outcomes at 1-year, but recurrent PSC occurs in 15–20% of recipients within 5 years of transplantation. Studies have reported factors associated with recurrent PSC, however they have not reported outcomes by race or ethnicity or by donor–recipient race which has impacted transplant outcomes for other diseases [
3,
4,
5,
6,
7,
8]. Our study was conducted across two decades that provides outcomes five years after liver transplant in a diverse patient population. While the scientific registry for transplant recipients report provides national data for liver transplantation, it combines PSC with other autoimmune or cholestatic diseases including autoimmune hepatitis and primary biliary cholangitis and does not report outcomes by donor and recipient race.
The higher patient mortality in donor–recipient race mismatched patients with PSC is a novel finding. Five-year patient mortality for deceased donor transplants was 1.69-fold higher in Black recipients from White donors compared to White recipients from White donors. The higher mortality is not explained by older donor age, which was adjusted for in multivariable analysis. Others have reported worse outcomes in race mismatched liver transplants, including hepatitis C where one explanation was differences in expression of the IL-28B polymorphism. Human leukocyte antigen mismatching has not been associated with rejection or graft survival after liver transplantation for autoimmune liver diseases [
15,
16]. Our study did not find a significant difference in 5-year graft survival in donor-race mismatched recipients. The discrepancy between a difference in patient survival but not graft survival may be due to unrecognized confounding factors. Due to limitations in data available in the organ procurement and transplantation network database (OPTN) there may be other variables not accounted for, such as nutritional status or patient compliance with immunosuppression and follow-up, which explain the association between higher patient mortality in Black recipients of White donors. Nevertheless, our findings warrant further study to uncover the reasons for higher mortality in Black recipients from White donors.
Our study demonstrates the changing racial and ethnic makeup of patients transplanted for PSC in the United States. The relative proportion of White patients transplanted for PSC decreased from era 1 to era 2 by 7.9%, while the relative proportion of Black, Asian, and Hispanic or Latino patients increased by 25.2%, 57.1%, and 50%, respectively. These changes are not completely explained by changes in demographics of the U.S. population. From 2010 to 2020 there was a 5.6%, 35.5%, and 23% increase in the Black, Asian, and Hispanic or Latino populations, respectively [
17]. A possible explanation for the increase in liver transplantation for PSC in non-White patients is the implementation of the Affordable Care Act (ACA) and Medicaid expansion that occurred during and after 2010 which may have resulted in increased access to care. The greatest reductions in uninsured rates occurred in Black, Asian and Hispanic populations. From 2010 to 2022 the percent of uninsured White, Black, Asian, and Hispanic individuals decreased from 13.1% to 6.6%, 19.9% to 10.0%, 16.7% to 6.0%, and 32.6% to 18.0%, respectively [
18]. It is possible that the increase in liver transplants in patients with PSC is partly attributed to increased access to health care and liver transplantation after introduction of the ACA. While this may be an encouraging finding from our work, addressing the structural racism that exists in access to liver transplant remains critically important [
19]. We can only speculate on other explanations for the increase in liver transplants for PSC in Black, Asian and Latino populations including better awareness of autoimmune liver diseases, an increase in access and referrals to gastroenterologists and hepatologists, or an increase in the number of hepatologists in the United States
Factors other than donor–recipient race that are associated with recurrent PSC or graft survival have been reported. In a meta-analysis that included 14 studies and 2481 recipients transplanted for PSC variables associated with recurrent disease included colectomy prior to liver transplant, inflammatory bowel disease, cholangiocarcinoma, donor age, acute cellular rejection, and MELD score [
20]. Colectomy prior to transplant was associated with a 35% reduced risk of recurrent PSC while acute cellular rejection, cholangiocarcinoma and inflammatory bowel disease were associated with an increased risk of recurrent PSC. The meta-analysis included studies from the U.S. as well as other countries where allocation systems may be different than the U.S. Additionally, the inclusion period for most of the studies started in the 1980s when tacrolimus may not have been widely available and cyclosporine use was more common which may have impacted the rate of rejection or graft loss. Studies included in the meta-analysis did not evaluate the association between survival and donor–recipient race.
Limitations of our study include data availability in the OPTN database. Although our primary outcome was graft and patient survival, we could not reliably determine the cause of graft loss or patient death. The study was limited to five years and results may not capture grafts that would be potentially lost to recurrent PSC or how many recipients died from recurrent PSC or related conditions as well as those who may be too unwell to be re-transplanted. The OPTN database provides a large, population-based cohort for liver transplant recipients, but it does not provide granular detail for many variables including for precise causes of death or data may be missing or inaccurate. However, data for variables we were able to include in our model were missing in 10% or fewer cases. There were too few cases to stratify donor–recipient race in living donor liver transplant recipients with PSC and limited data on ethnicity so our results may not be generalizable to these populations. We did not validate our findings in an independent cohort of recipients and our findings of donor–recipient race mismatch on patient survival warrants validation prior to reaching definitive conclusions. However, the cohort included a large population of Black patients with PSC which has been a limitation of other studies.
In conclusion, our study provides useful data across two decades on outcomes for adults undergoing liver transplantation for PSC, by donor and recipient race. The results demonstrate an association between donor–recipient race mismatch and patient survival which warrants further study. In addition, over time more Black individuals have undergone liver transplantation for end-stage liver disease from PSC in the United States. With changing demographics and the challenges associated with managing recurrent PSC after liver transplant, it is important to pursue further research to validate our findings and investigate possible causes of lower patient survival in donor–recipient race mismatched liver transplant recipients.
Author Contributions
M.W.R.: Concept/design, Data interpretation, Drafting article, Critical revision of article, and Approval of article; W.W.: Concept/design, Data interpretation, Drafting article, Critical revision of article, and Approval of article; W.S.C.: Concept/design, Data analysis/interpretation, Drafting article, and Approval of article; A.E.T.: Research acquisition, Analysis of data, and Drafting the paper or approval of the submitted and final versions; A.T.L.: Research acquisition, Analysis of data, and Drafting the paper or approval of the submitted and final versions; A.S.d.: Research design, Data interpretation, Drafting article, Critical revision of article, and Approval of article. All authors have read and agreed to the published version of the manuscript.
Funding
Study supported by unrestricted funds from the Anne and Epes Robinson Liver Research Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available from organ procurement and transplant network.
Acknowledgments
Study supported by unrestricted funds from the Anne and Epes Robinson Liver Research Fund.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACA | affordable care act |
| D | donor |
| DCD | donor after circulatory death |
| MELD | model for end-stage liver disease |
| OPTN | Organ Procurement and Transplantation Network |
| PSC | primary sclerosing cholangitis |
| R | recipient |
| SRTR | Scientific Registry for Transplant Recipients |
| UNOS | United Network for Organ Sharing |
References
- Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced/ (accessed on 26 July 2024).
- Trivedi, P.J.; Bowlus, C.L.; Yimam, K.K.; Razavi Estes, C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: A systematic review of population-based studies. Clin. Gastroenterol. Hepatol. 2022, 20, 1687–1700. [Google Scholar] [CrossRef] [PubMed]
- Visseren, T.; Erler, N.S.; Polak, W.G.; Adam, R.; Karam, V.; Vondran, F.W.R.; Ericzon, B.G.; Thorburn, D.; IJzermans, J.N.M.; Paul, A.; et al. Recurrence of primary sclerosing cholangitis after liver transplantation-analysing the European Liver Transplant Registry and beyond. Transpl. Int. 2021, 34, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Lord, J.D.; Yeh, M.M.; Cuevas, C.; Bakthavatsalam, R.; Kowdley, K.V. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008, 14, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, R.; Tsochatzis, E.; Jose, S.; Allison, M.; Athale, A.; Creamer, F.; Gunson, B.; Iyer, V.; Madanur, M.; Manas, D.; et al. Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. J. Hepatol. 2015, 63, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Vera, A.; Moledina, S.; Gunson, B.; Hubscher, S.; Mirza, D.; Olliff, S.; Neuberger, J. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet 2002, 360, 1943–1944. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Menegas, S.; Lee, K.J.; Pu, A.; Bhowmick, K.; Ponder, R.; Fan, G.H.; Chou, H.; Lee, K.; Urrunaga, N.H. Impact of inflammatory bowel disease subtypes on the post-liver transplant outcomes of patients with primary sclerosing cholangitis. Dig. Dis. Sci. 2023, 68, 3781–3800. [Google Scholar] [CrossRef] [PubMed]
- Gordon, F.D.; Goldberg, D.S.; Goodrich, N.P.; Lok, A.S.; Verna, E.C.; Selzner, N.; Stravitz, R.T.; Merion, R.M. Recurrent primary sclerosing cholangitis in the adult-to-adult living donor liver transplantation cohort study: Comparison of risk factors between living donor and deceased donor recipients. Liver Transpl. 2016, 22, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Harmacinski, A.; Kolachana, S.; Bahadur, A.; Lee, K.; Lee, K.J.; Pu, A.; Chou, H.; Fan, G.H.; Malik, R. The role of donor sex on the post-liver transplant outcomes in patients with primary sclerosing cholangitis. Eur. J. Gastroenterol. Hepatol. 2024, 36, 452–468. [Google Scholar] [CrossRef] [PubMed]
- LeClaire, J.M.; Smith, N.J.; Chandratre, S.; Rein, L.; Kamalia, M.A.; Kohmoto, T.; Joyce, L.D.; Joyce, D.L. Solid organ donor-recipient race-matching: Analysis of the United Network for Organ Sharing database. Transpl. Int. 2021, 34, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Maurina, M.N.; Tsai, S.; Christians, K.K.; Clarke, C.N.; Mogal, H.; Saeian, K.; Gamblin, T.C. Effect of Donor Race-Matching on Overall Survival for African-American Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma. J. Am. Coll. Surg. 2019, 228, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Lai, J.C.; O’Leary, J.G.; Verna, E.C.; Brown, R.S., Jr.; Stravitz, R.T.; Trotter, J.F.; Krishnan, K.; Terrault, N.A.; Consortium to Study Health Outcomes in HCV Liver Transplant Recipients. Recipient-donor race mismatch for African American liver transplant patients with chronic hepatitis C. Liver Transpl. 2012, 18, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Impact of Donor Recipient Gender and Race Mismatch on Graft Outcomes in Patients With End-Stage Liver Disease Undergoing Liver Transplantation. Prog. Transplant. 2017, 27, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Nabulsi, S.; Otunia, A.A.; Salciccioli, J.; Marshall, D.C.; Villani, V.; Shanmugarajah, K.; Shalhoub, J. HLA matching between donors and recipients improves clinical liver transplant graft survival. Liver Int. 2024, 44, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Laffey, M.; Ashwat, E.; Lui, H.; Zhang, X.; Kaltenmeier, C.; Packiaraj, G.; Crane, A.; Alshamery, S.; Gunabushanam, V.; Ganoza, A.; et al. Donor-recipient race-ethnicity concordance and patient survival after liver transplantation. HPB 2024, 26, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.census.gov/library/visualizations/interactive/race-and-ethnicity-in-the-united-state-2010-and-2020-census.html (accessed on 12 September 2024).
- Available online: https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity (accessed on 12 September 2024).
- Deutsch-Link, S.; Bittermann, T.; Nephew, L.; Ross-Driscoll, K.; Weinberg, E.M.; Weinrieb, R.M.; Olthoff, K.M.; Addis, S.; Serper, M. Racial and ethnic disparities in psychosocial evaluation and liver transplant waitlisting. Am. J. Transpl. 2023, 23, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Steenstraten, I.C.; Korkmaz, K.S.; Trivedi, P.J.; Inderson, A.; Hoek, B.V.; Girondo, M.D.M.R.; Malijaars, P.W.J. Systematic review with meta-analysis: Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment. Pharmacol. Ther. 2019, 49, 636–643. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).