Radial Head Prosthesis with Interconnected Porosity Showing Low Bone Resorption Around the Stem
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Prosthesis Features
2.4. Data Collection and Storage
2.5. Radiological Evaluation
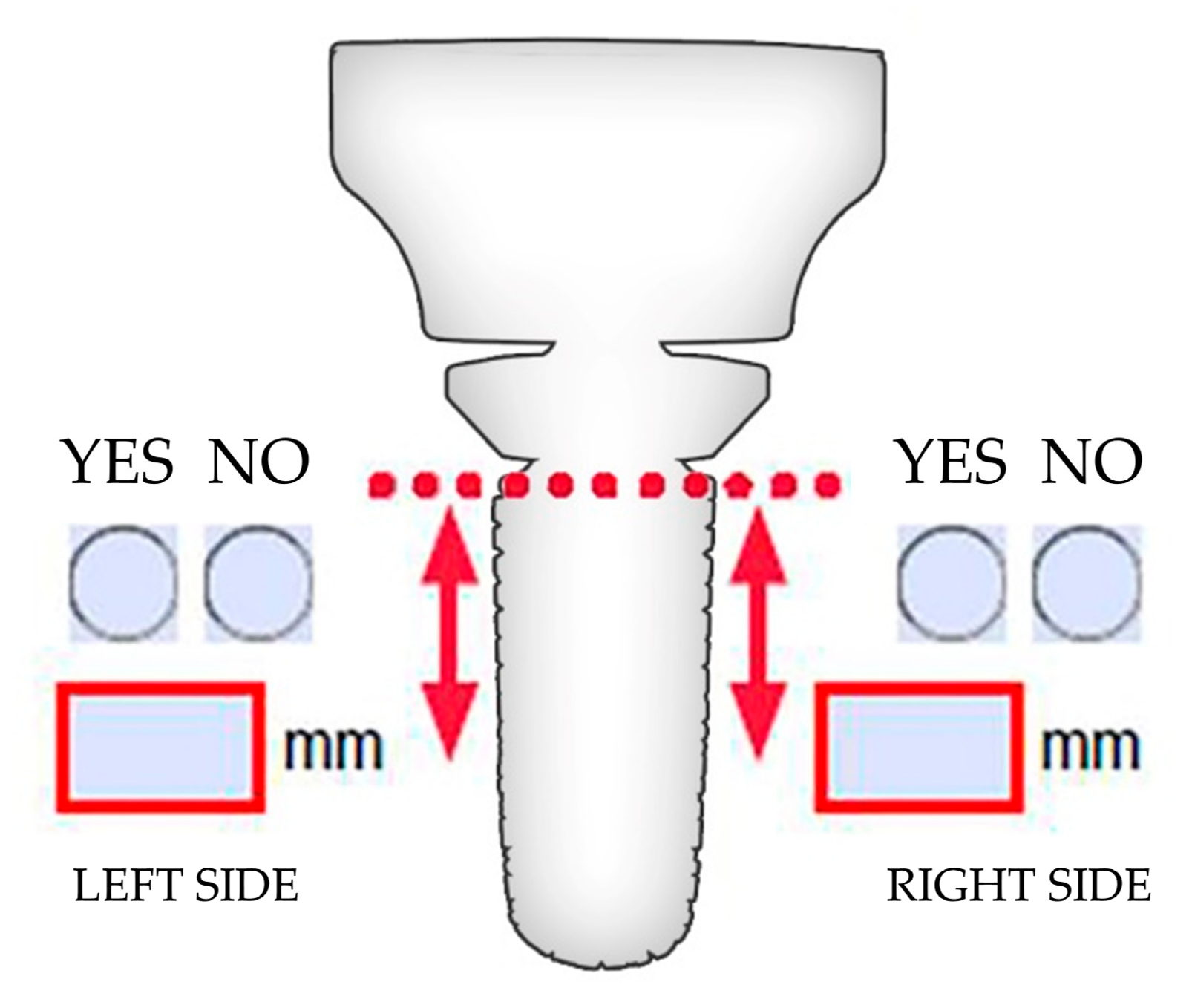
2.6. Evaluation of Resorption
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaas, L.; van Riet, R.P.; Vroemen, J.P.; Eygendaal, D. The epidemiology of radial head fractures. J. Shoulder Elbow Surg. 2010, 19, 520–523. [Google Scholar] [CrossRef] [PubMed]
- van Riet, R.P.; Morrey, B.F. Documentation of associated injuries occurring with radial head fracture. Clin. Orthop. Relat. Res. 2008, 466, 130–134. [Google Scholar] [CrossRef]
- van Riet, R.P.; Morrey, B.F.; O’Driscoll, S.W.; Van Glabbeek, F. Associated injuries complicating radial head fractures: A demographic study. Clin. Orthop. Relat. Res. 2005, 441, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, N.P.; Leopold, S.S. In brief: The Mason classification of radial head fractures. Clin. Orthop. Relat. Res. 2012, 470, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.D.; Wickramasinghe, N.R.; Clement, N.D.; Court-Brown, C.M.; McQueen, M.M. Long-term outcomes of isolated stable radial head fractures. J. Bone Joint Surg. Am. 2014, 96, 1716–1723. [Google Scholar] [CrossRef]
- Heijink, A.; Kodde, I.F.; Mulder, P.G.H.; Veltman, E.S.; Kaas, L.; van den Bekerom, M.P.J.; Eygendaal, D. Radial head arthroplasty: A systematic review. JBJS Rev. 2016, 4, e3. [Google Scholar] [CrossRef]
- Kodde, I.F.; Viveen, J.; The, B.; van Riet, R.P.; Eygendaal, D. Management of failed radial head arthroplasty. EFORT Open Rev. 2020, 5, 398–407. [Google Scholar] [CrossRef]
- Giannicola, G.; Amura, A.; Prigent, S.; Zoccali, C.; Sessa, P. Stress shielding around press-fit radial head arthroplasty: Proposal for a new classification system based on the analysis of 97 patients with a mid-term follow-up and a review of the literature. Healthcare 2024, 12, 396. [Google Scholar] [CrossRef]
- Hackl, M.; Wegmann, K.; Kahmann, S.L.; Heinze, N.; Staat, M.; Neiss, W.F.; Scaal, M.; Müller, L.P. Radial shortening osteotomy reduces radiocapitellar contact pressures while preserving valgus stability of the elbow. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2280–2288. [Google Scholar] [CrossRef]
- Chanlalit, C.; Shukla, D.R.; Fitzsimmons, J.S.; An, K.N.; O’Driscoll, S.W. Stress shielding around radial head prostheses. J. Hand Surg. Am. 2012, 37, 2118–2125. [Google Scholar] [CrossRef]
- Duckworth, A.D.; Wickramasinghe, N.R.; Clement, N.D.; Court-Brown, C.M.; McQueen, M.M. Radial head replacement for acute complex fractures: What are the rate and risks factors for revision or removal? Clin. Orthop. Relat. Res. 2014, 472, 2136–2143. [Google Scholar] [CrossRef]
- Maghen, Y.; Leo, A.J.; Hsu, J.W.; Hausman, M.R. Is a silastic radial head still a reasonable option? Clin. Orthop. Relat. Res. 2011, 469, 1061–1070. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castagnini, F.; Bordini, B.; Stea, S.; Calderoni, P.P.; Masetti, C.; Busanelli, L. Highly porous titanium cup in cementless total hip arthroplasty: Registry results at eight years. Int. Orthop. 2019, 43, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, M.; Bondi, M.; Renzi Brivio, L. Femoral neck preservation with a short hip stem produced with powder manufacturing: Mid-term results of a consecutive case series. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Kachooei, A.R.; Baradaran, A.; Ebrahimzadeh, M.H.; van Dijk, C.N.; Chen, N. The rate of radial head prosthesis removal or revision: A systematic review and meta-analysis. J. Hand Surg. Am. 2018, 43, 39–53.e1. [Google Scholar] [CrossRef]
- Shore, B.J.; Mozzon, J.B.; MacDermid, J.C.; Faber, K.J.; King, G.J. Chronic posttraumatic elbow disorders treated with metallic radial head arthroplasty. J. Bone Joint Surg. Am. 2008, 90, 271–280. [Google Scholar] [CrossRef]
- Laumonerie, P.; Reina, N.; Ancelin, D.; Delclaux, S.; Tibbo, M.E.; Bonnevialle, N.; Mansat, P. Mid-term outcomes of 77 modular radial head prostheses. Bone Joint J. 2017, 99, 1197–1203. [Google Scholar] [CrossRef]
- Sershon, R.A.; Luchetti, T.J.; Cohen, M.S.; Wysocki, R.W. Radial head replacement with a bipolar system: An average 10-year follow-up. J. Shoulder Elb. Surg. 2018, 27, e38–e44. [Google Scholar] [CrossRef]
- Vannabouathong, C.; Venugopal, N.; Athwal, G.S.; Moro, J.; Bhandari, M. Radial head arthroplasty: Fixed-stem implants are not all equal—A systematic review and meta-analysis. JSES Int. 2020, 4, 30–38. [Google Scholar] [CrossRef]
- Yang, G.; Li, S.; Zhang, H.; Lu, Y. A systematic review and meta-analysis on different stem fixation methods of radial head prostheses during long-term follow-up. Front. Bioeng. Biotechnol. 2022, 10, 1041531. [Google Scholar] [CrossRef]
- Morrey, B.F.; Askew, L.; Chao, E.Y. Silastic prosthetic replacement for the radial head. J. Bone Joint Surg. Am. 1981, 63, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, D.S. Radial head replacement—A comprehensive review. J. Orthop. 2022, 36, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.V.C.; Reis, A.C.D.; Valentem, M.L.D.C. Osseointegration in additive-manufactured titanium implants: A systematic review of animal studies on the need for surface treatment. Heliyon 2023, 9, e17105. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, X.; Zhang, S.; Wang, L.; Ding, F.; Song, S.; Chen, X.; Deng, B.; Song, Y. Selective laser melted titanium implants play a positive role in early osseointegration in type 2 diabetes mellitus rats. Dent. Mater. J. 2020, 39, 214–221. [Google Scholar] [CrossRef]
- Ragni, E.; Perucca Orfei, C.; Bidossi, A.; De Vecchi, E.; Francaviglia, N.; Romano, A.; Maestretti, G.; Tartara, F.; de Girolamo, L. Superior osteo-inductive and osteo-conductive properties of trabecular titanium vs. PEEK scaffolds on human mesenchymal stem cells: A proof of concept for the use of fusion cages. Int. J. Mol. Sci. 2021, 22, 2379. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Y.; Li, N. Powder metallurgy of titanium alloys: A brief review. J. Alloys Compd. 2023, 965, 25. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Shivaram, A.; Tarafder, S.; Sahasrabudhe, H.; Banerjee, D.; Bose, S. In vivo response of laser-processed porous titanium implants for load-bearing implants. Ann. Biomed. Eng. 2017, 45, 249–260. [Google Scholar] [CrossRef]
- Cohen, D.J.; Cheng, A.; Sahingur, K.; Clohessy, R.M.; Hopkins, L.B.; Boyan, B.D.; Schwartz, Z. Performance of laser sintered Ti-6Al-4V implants with bone-inspired porosity and micro/nanoscale surface roughness in the rabbit femur. Biomed. Mater. 2017, 12, 025021. [Google Scholar] [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D-printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Ring, D.; King, G. Radial head arthroplasty with a modular metal spacer to treat acute traumatic elbow instability: Surgical technique. J. Bone Jt. Surg. Am. 2008, 90 Pt 1 (Suppl. S2), 63–73. [Google Scholar] [CrossRef][Green Version]
- Van Riet, R.P.; Van Glabbeek, F.; Verborgt, O.; Gielen, J. Capitellar erosion caused by a metal radial head prosthesis A case report. J. Bone Joint Surg. Am. 2004, 86, 1061–1064. [Google Scholar] [CrossRef]
- Ricón, F.J.; Lajara, F.; Fuentes, A.; Aguilar, M.L.; Boix, A.; Lozano, J.A. Pyrocarbon arthroplasty in acute unreconstructable radial head fractures: Mid-term to long-term results. J. Orthop. Traumatol. 2018, 19, 13. [Google Scholar] [CrossRef]
- Samra, I.; Kwaees, T.A.; Mati, W.; Blundell, C.; Lane, S.; Harrison, J.W.; Charalambous, C.P. Anatomic monopolar press-fit radial head arthroplasty: High rate of loosening at mid-term follow up. Shoulder Elb. 2023, 15, 207–217. [Google Scholar] [CrossRef]

| Patient | Implant Prosthesis | |||||
|---|---|---|---|---|---|---|
| Case | Gender | Age | Radial Stem Size Ø/L (mm) | Spacer/Collar Offset L (mm) | Radial Head Ø (mm) | Follow-Up Month |
| 1 | F | 48 | 8/19.4 | 13/1 | 19 | 8 |
| 2 | M | 56 | 12/20.9 | 13/1 | 20.5 | 6/12 |
| 3 | F | 62 | 9/19.7 | 13/1 | 19 | 9 |
| 4 | F | 48 | 7/19.1 | 13/1 | 19 | 6 |
| 5 | F | 48 | 7/19.1 | 17/5 | 17.5 | 10 |
| 6 | F | 80 | 9/19.7 | 13/1 | 20.5 | 6 |
| 7 | M | 47 | 10/20 | 13/1 | 22 | 15 |
| 8 | F | 49 | 8/19.4 | 14.5/2.5 | 19 | 7 |
| 9 | M | 54 | 8/19.4 | 13/1 | 20.5 | 11 |
| 10 | F | 75 | 8/19.4 | 13/1 | 19 | 6 |
| 11 | M | 55 | 10/20 | 14.5/2.5 | 19 | 6 |
| 12 | F | 57 | 8/19.4 | 14.5/2.5 | 19 | 6 |
| 13 | F | 56 | 7/19.1 | 13/1 | 17.5 | 6 |
| 14 | F | 60 | 10/20 | 13/1 | 19 | 6 |
| 15 | F | 64 | 9/19.7 | 14.5/2.5 | 20.5 | 6 |
| 16 | M | 56 | 11/20.3 | 13/1 | 20.5 | 6 |
| 17 | M | 67 | 8/19.4 | 17/5 | 22 | 6 |
| 18 | F | 78 | 8/19.4 | 13/1 | 19 | 6 |
| 19 | F | 39 | 7.5/19.2 | 13/1 | 20.5 | 11 |
| 20 | F | 55 | 7.5/19.2 | 13/1 | 19 | 9 |
| 21 | F | 50 | 8/19.4 | 14.5/2.5 | 22 | 11 |
| 22 | M | 48 | 10/20 | 14.5/2.5 | 23.5 | 14 |
| 23 | F | 65 | 7.5/19.2 | 14.5/2.5 | 22 | 6 |
| 24 | M | 62 | 10/20 | 13/1 | 23 | 9 |
| 25 | F | 57 | 9/19.7 | 13/1 | 19 | 6/8 |
| 26 | F | 64 | 9/19.7 | 13/1 | 19 | 6 |
| 27 | M | 63 | 8.5/19.5 | 14.5/2.5 | 20.5 | 9 |
| 28 | F | 73 | 8/19.4 | 13/1 | 17.5 | 12 |
| 29 | F | 70 | 9/19.7 | 14.5/2.5 | 23.5 | 14 |
| 30 | M | 44 | 9/19.7 | 14.5/2.5 | 22 | 8 |
| 31 | F | 55 | 7/19.1 | 13/1 | 19 | 12/20 |
| 32 | F | 80 | 10/20 | 13/1 | 20.5 | 6/12/24 |
| 33 | F | 56 | 9/19.7 | 13/1 | 19 | 15/32 |
| 34 | F | 61 | 9/19.7 | 13/1 | 22 | 8 |
| 35 | F | 80 | 8/19.4 | 13/1 | 20.5 | 6 |
| 36 | M | 44 | 10/20 | 13/1 | 23.5 | 6 |
| 37 | F | 55 | 7.5/19.2 | 13/1 | 19 | 6 |
| 38 | F | 63 | 8.5/19.5 | 13/1 | 19 | 9 |
| 39-right | M | 47 | 8/19.4 | 14.5/2.5 | 20.5 | 6 |
| 40-left | M | 47 | 8/19.4 | 14.5/2.5 | 20.5 | 6 |
| 41 | M | 45 | 9/19.7 | 13/1 | 20.5 | 6 |
| 42 | M | 52 | 10/20 | 17/5 | 22 | 6 |
| 43 | F | 53 | 10/20 | 13/1 | 20.5 | 6 |
| 44 | F | 70 | 10/20 | 13/1 | 20.5 | 8 |
| Panel A | B | |||||
|---|---|---|---|---|---|---|
| AP (mm) | LL (mm) | Mean (mm) | ||||
| Case | Month | Left | Right | Left | Right | |
| 1 | 8 | 3 | 4 | 4 | 5 | 4 |
| 2 | 6 | 0 | 0 | 0 | 3 | 1 |
| 2 | 12 | 0 | 2 | 0 | 3 | 1 |
| 3 | 9 | 2 | 2 | 0 | 0 | 1 |
| 4 | 6 | 5 | 6 | 7 | 6 | 6 |
| 5 | 10 | 11 | 7 | 4 | 7 | 7 |
| 6 | 6 | 0 | 0 | 2 | 0 | 1 |
| 7 | 15 | 1 | 3 | 5 | 0 | 2 |
| 8 | 7 | 8 | 7 | 7 | 7 | 7 |
| 9 | 11 | 5 | 6 | 6 | 6 | 6 |
| 10 | 6 | 5 | 4 | 5 | 4 | 5 |
| 11 | 6 | 4 | 2 | 2 | 2 | 3 |
| 12 | 6 | 6 | 9 | 6 | 9 | 8 |
| 13 | 6 | 9 | 9 | 9 | 8 | 9 |
| 14 | 6 | 1 | 3 | 1 | 3 | 2 |
| 15 | 6 | 3 | 3 | 2 | 3 | 3 |
| 16 | 6 | 1 | 1 | 2 | 2 | 2 |
| 17 | 6 | 2 | 1 | 2 | 1 | 2 |
| 18 | 6 | 2 | 6 | 1 | 1 | 3 |
| 19 | 11 | 0 | 1 | 1 | 1 | 1 |
| 20 | 9 | 2 | 3 | 5 | 2 | 3 |
| 21 | 11 | 2 | 5 | 3 | 2 | 3 |
| 22 | 14 | 5 | 5 | 5 | 2 | 4 |
| 23 | 6 | 6 | 5 | 5 | 4 | 5 |
| 24 | 9 | 8 | 8 | - | - | 8 |
| 25 | 6 | 7 | 6 | 7 | 6 | 7 |
| 25 | 8 | 7 | 6 | 7 | 6 | 7 |
| 26 | 6 | 0 | 0 | 3 | 0 | 1 |
| 27 | 9 | 0 | 0 | 0 | 0 | 0 |
| 28 | 12 | 0 | 2 | 3 | 3 | 2 |
| 29 | 14 | 4 | 4 | 5 | 5 | 5 |
| 30 | 8 | 1 | 1 | 2 | 1 | 1 |
| 31 | 12 | 7 | 7 | 8 | 7 | 7 |
| 31 | 20 | 6 | 7 | 7 | 7 | 7 |
| 32 | 6 | 2 | 0 | 0 | 2 | 1 |
| 32 | 12 | 1 | 0 | 2 | 0 | 1 |
| 32 | 24 | 5 | 1 | 3 | 0 | 2 |
| 33 | 15 | 3 | 3 | - | - | 3 |
| 33 | 32 | 5 | 4 | 4 | 4 | 4 |
| 34 | 8 | 4 | 4 | 5 | 5 | 5 |
| 35 | 6 | 5 | 8 | 6 | 5 | 6 |
| 36 | 6 | 1 | 3 | 2 | 2 | 2 |
| 37 | 6 | 3 | 0 | 0 | 0 | 1 |
| 38 | 9 | 5 | 3 | 5 | 3 | 4 |
| 39 | 6 | 5 | 3 | 4 | 4 | 4 |
| 40 | 6 | 1 | 1 | 4 | 3 | 2 |
| 41 | 6 | 0 | 4 | 4 | 3 | 3 |
| 42 | 6 | 3 | 3 | 3 | 3 | 3 |
| 43 | 6 | 0 | 3 | 2 | 3 | 2 |
| 44 | 8 | 0 | 0 | 8 | 0 | 2 |
| “r” | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| AP Left | AP Right | LL Left | LL Right | AP Left | AP Right | LL Left | LL Right | |
| AP left | 1.00 | 0.78 | 0.65 | 0.76 | <0.001 | <0.001 | <0.001 | <0.001 |
| AP right | 1.00 | 0.70 | 0.85 | <0.001 | <0.001 | <0.001 | ||
| LL left | 1.00 | 0.67 | <0.001 | <0.001 | ||||
| LL right | 1.00 | <0.001 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vismara, V.; Guerra, E.; Accetta, R.; Cardile, C.; Boero, E.; Aliprandi, A.; Porta, M.; Zaolino, C.; Marinelli, A.; Cazzaniga, C.; et al. Radial Head Prosthesis with Interconnected Porosity Showing Low Bone Resorption Around the Stem. J. Clin. Med. 2025, 14, 5439. https://doi.org/10.3390/jcm14155439
Vismara V, Guerra E, Accetta R, Cardile C, Boero E, Aliprandi A, Porta M, Zaolino C, Marinelli A, Cazzaniga C, et al. Radial Head Prosthesis with Interconnected Porosity Showing Low Bone Resorption Around the Stem. Journal of Clinical Medicine. 2025; 14(15):5439. https://doi.org/10.3390/jcm14155439
Chicago/Turabian StyleVismara, Valeria, Enrico Guerra, Riccardo Accetta, Carlo Cardile, Emanuele Boero, Alberto Aliprandi, Marco Porta, Carlo Zaolino, Alessandro Marinelli, Carlo Cazzaniga, and et al. 2025. "Radial Head Prosthesis with Interconnected Porosity Showing Low Bone Resorption Around the Stem" Journal of Clinical Medicine 14, no. 15: 5439. https://doi.org/10.3390/jcm14155439
APA StyleVismara, V., Guerra, E., Accetta, R., Cardile, C., Boero, E., Aliprandi, A., Porta, M., Zaolino, C., Marinelli, A., Cazzaniga, C., & Arrigoni, P. (2025). Radial Head Prosthesis with Interconnected Porosity Showing Low Bone Resorption Around the Stem. Journal of Clinical Medicine, 14(15), 5439. https://doi.org/10.3390/jcm14155439







