Cardiovascular Risk in Rheumatic Patients Treated with JAK Inhibitors: The Role of Traditional and Emerging Biomarkers in a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Cohort Selection
2.3. Clinical and Paraclinical Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics, Clinical and Paraclinical Assessments
3.2. Biological Assessment and Correlations with Cardiovascular Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van der Woude, D.; Van der Helm-Van Mil, A.H.M. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, Regional and National Burden of RheumaToid Arthritis 1990–2017: A Systematic analysis of The Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Song, G.G.; Choi, S.J.; Seok, H.; Jung, J.H. Relationship of rheumatoid arthritis and coronary artery disease in the Korean population: A nationwide cross-sectional study. Adv. Rheumatol. 2019, 59, 40. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Boavida, J.M.; Capodanno, D.; Crawford, C.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Hansildaar, R.; Vedder, D.; Baniaamam, M.; Tausche, A.K.; Gerritsen, M.; Nurmohamed, M.T. Cardiovascular risk in inflammatory arthritis: Rheumatoid arthritis and gout. Lancet Rheumatol. 2020, 3, E58–E70. [Google Scholar] [CrossRef]
- Restivo, V.; Candiloro, S.; Daidone, M.; Norrito, R.; Cataldi, M.; Minutolo, G.; Carraci, F.; Fasano, S.; Ciccia, F.; Casuccio, A.; et al. Systematic review and meta-analysis of cardiovascular risk in rheumatological disease: Symptomatic and non-symptomatic events in rheumatoid arthritis and systemic lupus erythematosus. Autoimmun. Rev. 2022, 21, 102925. [Google Scholar] [CrossRef]
- Kerola, A.M.; Rollefstad, S.; Semb, A.G. Atherosclerotic cardiovascular disease in rheumatoid arthritis: Impact of inflammation and antirheumatic treatment. Eur. Cardiol. 2021, 16, e18. [Google Scholar] [CrossRef]
- Graham, I.M.; Di Angelantonio, E.; Visseren, F.; De Bacquer, D.; Ference, B.A.; Timmis, A.; Halle, M.; Vardas, P.; Huculeci, R.; Cooney, M.T.; et al. Systematic Coronary Risk Evaluation (SCORE): JACC Focus Seminar 4/8. J. Am. Coll. Cardiol. 2021, 77, 3046–3057. [Google Scholar] [CrossRef]
- Sharma, A.; Christodorescu, R.; Agbariah, A.; Duda-Seiman, D.; Dahdal, D.; Man, D.; Kundnani, N.R.; Cretu, O.M.; Dragan, S. Cardiovascular risk prediction parameters for better management of rheumatic diseases. Heathcare 2022, 10, 312. [Google Scholar] [CrossRef]
- Cacciapaglia, F.; Spinelli, F.R.; Piga, M.; Erre, G.L.; Sakellariou, G.; Manfredi, A.; Viapiana, O.; Fornaro, M.; Colella, S.; Floris, A.; et al. Estimated 10-year cardiovascular risk in a large Italian cohort of rheumatoid arthritis patients: Data from the cardiovascular obesity and rheumatic disease (CORDIS) study group. Eur. J. Intern. Med. 2022, 96, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Drosos, G.C.; Vedder, D.; Houben, E.; Boekel, L.; Atzeni, F.; Badreh, S.; Boumpas, D.T.; Brodin, N.; Bruce, I.N.; Gonzalez-Gay, M.A.; et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systematic lupus erythematosus and antiphospholipid syndrome. Ann. Rheum. Dis. 2022, 81, 768–779. [Google Scholar] [CrossRef]
- Giles, J.T.; Sattar, N.; Gabriel, S.; Ridker, P.M.; Gay, S.; Warne, C.; Musselman, D.; Brockwell, L.; Shittu, E.; Klearman, M.; et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: A randomized controlled trial. Arthritis Rheumatol. 2019, 72, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Charles-Schoeman, C.; Buch, D.H.; Dougados, M.; Bhatt, D.L.; Giles, J.T.; Ytterberg, S.R.; Koch, G.G.; Vranic, I.; Wu, J.; Wang, C.; et al. Risk of major adverse cardiovascular events with tofacitinib versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: A post hoc analysis from ORAL Surveillance. Ann. Rheum. Dis. 2022, 81, 119–129. [Google Scholar] [CrossRef]
- Singht, S.; Fumery, M.; Singh, A.G.; Singh, N.; Prokop, L.J.; Dulai, P.S.; Sandborn, W.J.; Curtis, J.R. Comparative risk of cardiovascular events with biologic and synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: A systematic review and meta-analysis. Arthritis Care Res. 2020, 72, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Valanti, E.K.; Dalakoura-Karagkouni, K.; Siasos, G.; Kardassis, D.; Eliopoulos, A.G.; Sanoudou, D. Advances in biologic therapy for dyslipidemias and atherosclerosis. Metab. Clin. Exp. 2020, 116, 154461. [Google Scholar] [CrossRef]
- Hoisnard, L.; Vegas, L.P.; Dray-Spira, R.; Weill, A.; Zureik, M.; Sbidian, E. Risk of major adverse cardiovascular and venous thromboembolism events in patients with rheumatoid arthritis exposed to JAK inhibitors versus adalimumab: A nationwide cohort study. Ann. Rheum. Dis. 2022, 74, 182–188. [Google Scholar] [CrossRef]
- Tesser, J.; Gul, A.; Olech, E.; Oelke, K.; Lukic, T.; Kwok, K.; Ebrahim, A. Efficacy and safety of tofacitinib in patients with rheumatoid arthritis by previous treatment: Post hoc analysis of phase II/III trials. Arthritis Res. Ther. 2023, 25, 214. [Google Scholar] [CrossRef]
- Caporali, R.; Taylor, P.C.; Aletaha, D.; Sanmarti, R.; Takeuchi, T.; Mo, D.; Haladyj, E.; Bello, N.; Zaremba-Pechmann, L.; Fang, Y.; et al. Efficacy of baricitinib in patients with moderate-to-severe rheumatoid arthritis up to 6.5 years of treatment: Results of a long-term study. Rheumatology 2024, 63, 2799–2809. [Google Scholar] [CrossRef]
- Sanmarti, R.; Coromias, H. Upadacitinib for patients with rheumatoid arthritis: A comprehensive review. J. Clin. Med. 2023, 12, 1734. [Google Scholar] [CrossRef]
- Harrington, R.; Harkins, P.; Conway, R. Janus kinase inhibitors in rheumatoid arthritis: An update on the efficacy and safety of tofacitinib, baricitinib and upadacitinib. J. Clin. Med. 2023, 12, 6690. [Google Scholar] [CrossRef]
- Calvo-Garcia, A.; Herraiz, E.R.; Cubas, I.M.L.; De Dios, B.V.; Gonzalez, J.B.; Baladron, A.M.; Garcia-Vicuna, R. The real-world effectiveness, persistence, adherence, and safety of Janus kinase inhibitor baricitinib in rheumatoid arthritis: A long-term study. J. Clin. Med. 2024, 13, 2517. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Molina, C.; Gich, I.; Diaz-Torne, C.; Park, H.S.; Feliu, A.; Vidal, S.; Coromias, H. Patient-related factors influencing the effectiveness and safety of Janus Kinase inhibitors in rheumatoid arthritis: A real-world study. Sci. Rep. 2024, 14, 172. [Google Scholar] [CrossRef]
- Yan, J.; Yang, S.; Han, L.; Ba, X.; Shen, P.; Lin, W.; Li, T.; Zhang, R.; Huang, Y.; Huang, Y.; et al. Dyslipidemia in rheumatoid arthritis: The possible mechanisms. Front. Immunol. 2023, 14, 1254753. [Google Scholar] [CrossRef] [PubMed]
- Kotyla, P.J.; Islam, A.; Engelmann, M. Clinical aspects of Janus kinase (JAK) inhibitors in the cardiovascular system in patients with rheumatoid arthritis. Int. J. Mol. Sci. 2020, 21, 7390. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, H.; Zhao, J.; Luo, Z.; Wang, C.; Zhu, C.; Su, N.; Zhang, S. Cardiovascular safety of Janus kinase inhibitors in patients with rheumatoid arthritis: Systematic review and network meta-analysis. Front. Pharmacol. 2023, 14, 1237234. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.W.; Wu, T.; Li, Q.H.; Ma, J.D.; Pan, J.; Lu, Y.; Lin, Y.Z.; Jia, P.W.; Zheng, H.W.; Gao, J.W.; et al. Association of serum concentration of remnant cholesterol with incident cardiovascular disease in patients with rheumatoid arthritis: A real-world data from 2001 to 2022. Int. J. Cardiol. 2024, 405, 1311947. [Google Scholar] [CrossRef]
- Rogriguez-Gonzalez, D.; Garcia-Gonzalez, M.; Gomez-Bernal, F.; Quevedo-Abeledo, J.C.; Gonzalez-Rivero, A.F.; Jimenez-Sosa, A.; Gonzalez-Lopez, E.; Heras-Recuero, E.; Ocejo-Vinyls, J.G.; Gonzalez-Gay, M.A.; et al. Relationship between the complement system and serum lipid profile in patients with rheumatoid arthritis. Front. Immunol. 2024, 15, 1420292. [Google Scholar] [CrossRef]
- Giles, J.T.; Wasko, C.M.; Chung, C.P.; Szklo, M.; Blumenthal, R.S.; Kao, A.; Bokhari, S.; Zartoshti, A.; Stein, C.M.; Bathon, J.M. Exploring the lipid paradox theory in rheumatoid arthritis: Associations of low circulating low-density lipoprotein concentration with subclinical coronary atherosclerosis. Arthritis Rheumatol. 2019, 71, 1426–1436. [Google Scholar] [CrossRef]
- Chang, C.K.; Li, Y.C.; Chen, P.K.; Chang, S.H.; Chen, D.Y. Elevated remnant cholesterol as a potential predictor for cardiovascular events in rheumatoid arthritis patients. Front. Cardiovasc. Med. 2024, 11, 1449219. [Google Scholar] [CrossRef]
- Choi, W.; Kang, J.H.; Park, J.Y.; Hong, A.R.; Yoon, J.H.; Kim, H.K.; Kang, H.C. Elevated triglycerides levels are associated with increased risk for major adverse cardiovascular events in statin-naïve rheumatoid arthritis patients: A nationwide cohort study. Semin. Arthritis Rheum. 2023, 63, 152274. [Google Scholar] [CrossRef]
- Vinci, P.; Di Girolamo, F.G.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Altamura, N.; Pirulli, A.; Zaccari, M.; Biasinutto, C.; et al. Lipoprotein(a) as a risk factors for cardiovascular diseases: Pathophysiology and treatment perspectives. Int. J. Environ. Res. Public Health 2023, 20, 6721. [Google Scholar] [CrossRef]
- Garcia-Gomez, C.; Martin-Martinez, M.A.; Costaneda, S.; Sanchez-Alonso, F.; Uriarte-Ecenarro, M.; Gonzalez-Juanatey, C.; Romera-Baures, M.; Santos-Rey, J.; Pinto-Tasende, J.A.; Quesada-Masachs, E.; et al. Lipoprotein(a) concentrations in rheumatoid arthritis on biologic therapy: Results from the CARdiovascular in rheuMAtology study project. J. Clin. Lipidol. 2017, 2, 749–756. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: Developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESR) and the European Stroke Organization (ESO). Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/AphA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Misra, D.P.; Pande, G.; Agarwal, V. Cardiovascular risks associated with Janus kinase inhibitors: Peering outside the black box. Clin. Rheumatol. 2023, 42, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Popa, C.D.; Nucera, V.; Nurmohamed, M.T. Safety of JAK inhibitors: Focus on cardiovascular and thromboembolic events. Exp. Rev. Clin. Immunol. 2022, 18, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- de Resende Guimaraes, M.F.B.; Rodrigues, C.E.M.; Gomes, K.W.P.; Machado, C.J.; Brenol, C.V.; Krampe, S.F.; de Andrade, N.P.B.; Kakehasi, A.M. High prevalence of obesity in rheumatoid arthritis patients: Association with disease activity, hypertension, dyslipidemia and diabetes, a multi-center study. Adv. Rheumatol. 2019, 59, 44. [Google Scholar] [CrossRef]
- Hadwen, B.; Stranges, S.; Barra, L. Risk factors for hypertension in rheumatoid arthritis patients: A systematic review. Autoimmun. Rev. 2021, 20, 102786. [Google Scholar] [CrossRef]
- Patrick, D.M.; Van Beusecum, J.P.; Kirabo, A. The role of inflammation in hypertension: Novel concepts. Curr. Opin. Physiol. 2021, 19, 92–98. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, S.C.; Lai, K.L.; Tang, K.T.; Lin, C.H.; Chen, Y.M.; Tseng, C.W.; Chang, Y.M.; Gotcher, D.F.; Chiou, C.C.; et al. Factors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: A nationwide, population-based, case-control study. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211030809. [Google Scholar] [CrossRef]
- Koh, J.H.; Lee, B.W.; Kim, W.U. Changes in the cholesterol profile of patients with rheumatoid arthritis treated with biologics or Janus kinase inhibitors. J. Rheumatol. Dis. 2023, 30, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gou, Z.P.; Du, S.Q.; Zhu, X.H.; Lin, H.; Liang, X.F.; Wang, Y.S.; Feng, P. Effect of JAK inhibitors on high- and low-density lipoprotein in patients with rheumatoid arthritis: A systematic review and network meta-analysis. Clin. Rheumatol. 2022, 41, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.N.; Shi, M.F.; Wang, S.I.; Feng, W.; Chen, S.L.; Zhong, X.Q.; Liu, Q.P.; Cheng-Chung Wei, J.; Lin, C.S.; Xu, Q. Risk of dyslipidemia and major adverse cardiac events with tofacitinib versus adalimumab in rheumatoid arthritis: A real-world cohort study from 7580 patients. Front. Pharmacol. 2024, 15, 1370661. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Kremer, J.M.; Emery, P.; Zuckerman, S.H.; Ruotolo, G.; Zhong, J.; Chen, L.; Witt, S.; Saifan, C.; Kurzawa, M. Lipid profile and effect of statin treatment in pooled phase II and phase III baricitinib studies. Ann. Rheum. Dis. 2018, 77, 988–995. [Google Scholar] [CrossRef]
- Kamstrup, P.R. Lipoprotein(a) and cardiovascular disease. Clin. Chem. 2021, 67, 154–166. [Google Scholar] [CrossRef]
- Tsioulos, G.; Kounatidis, D.; Vallianou, N.G.; Poulaki, A.; Kotsi, E.; Christodoulatos, G.S.; Tsilingiris, D.; Karampela, I.; Skourtis, A.; Dalamaga, M. Lipoprotein(a) and atherosclerotic cardiovascular disease: Where do we stand? Int. J. Mol. Sci. 2024, 25, 3537. [Google Scholar] [CrossRef]
- Wolk, R.; Armostrong, E.J.; Hansen, P.R.; Thiers, B.; Lan, S.; Tallman, A.M.; Kaur, M.; Tatulych, S. Effect of tofacitinib on lipid levels and lipid-related parameters in patients with moderate to severe psoriasis. J. Clin. Lipidol. 2017, 11, 1243–1256. [Google Scholar] [CrossRef]
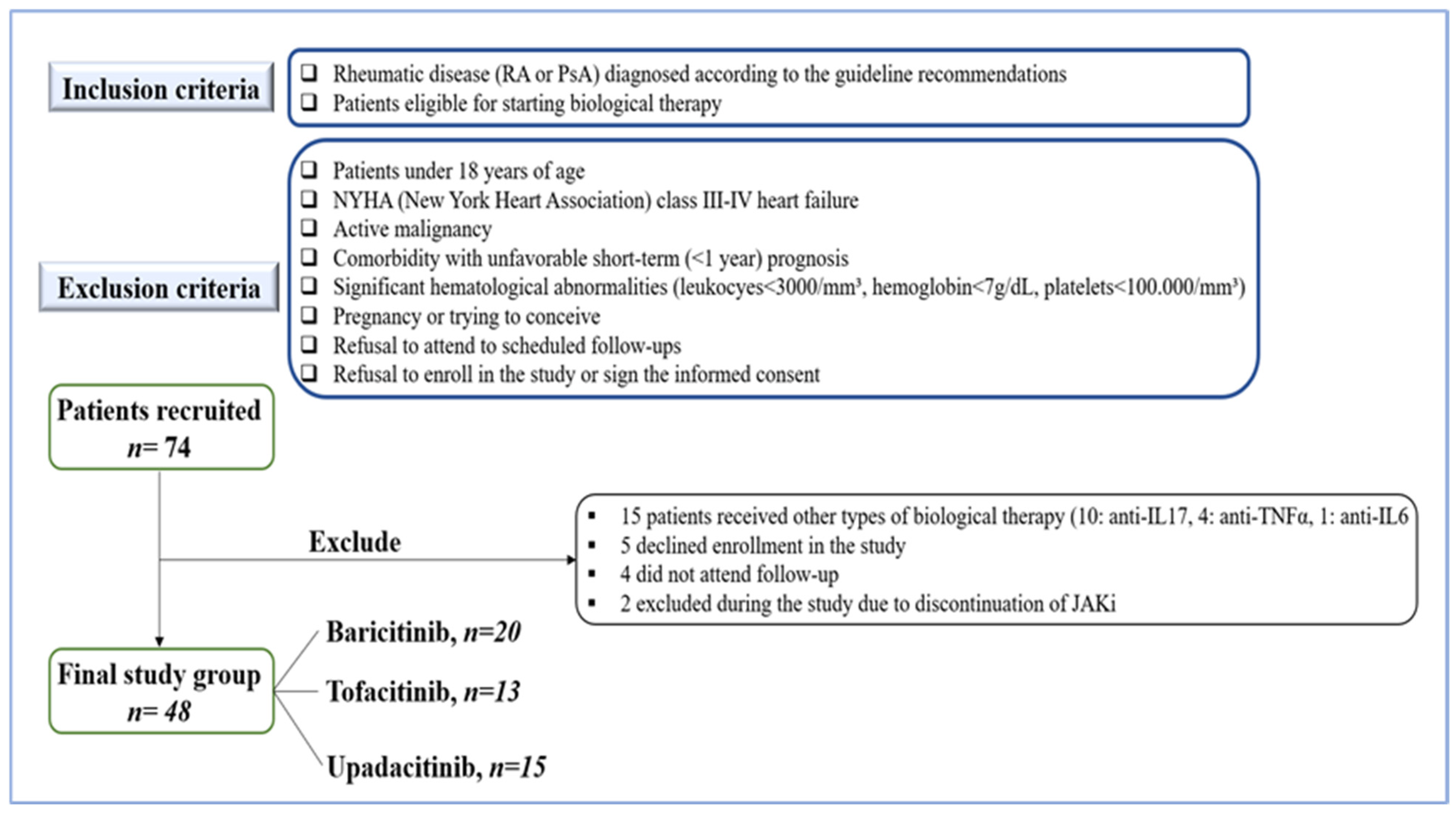
| Variable | n = 48 |
|---|---|
| Demographic parameters | |
| Mean age (years) | 56.83 |
| Female/male ratio | 4.3:1 |
| Cardiovascular risk factors | |
| Current smoking, n (%) | 8 (17%) |
| Increased BMI (kg/m2), n (%) | 36 (75%) |
| Arterial hypertension, n (%) | 28 (58%) |
| Dyslipidemia, n (%) | 17 (35.4%) |
| Statin use, n (%) | 12 (25%) |
| DM, n (%) | 9 (19%) |
| PAD, n (%) | 0 |
| Arrythmias, n (%) | 0 |
| Chronic coronary syndrome | 7 (14.6%) |
| History of stroke, n (%) | 1 (2%) |
| History of MI, n (%) | 0 |
| Rheumatic disease characteristics | |
| Positive RF, n (%) | 35 (76%) |
| Positive ACPA, n (%) | 36 (78%) |
| JAKi therapy | |
| Baricitinib, n (%) | 20 (42%) |
| Upadacitinib, n (%) | 15 (31%) |
| Tofacitinib, n (%) | 13 (27%) |
| Concomitant csDMARDs | |
| Methotrexate, n (%) | 21 (44%) |
| Leflunomide, n (%) | 16 (33%) |
| Sulfasalazine, n (%) | 1 (2%) |
| Biological parameters | |
| Increased TC, n (%) | 27 (56%) |
| Increased LDL-C, n (%) | 27 (56%) |
| Increased triglycerides, n (%) | 6 (12%) |
| Increased Lp(a), n (%) | 9 (19%) |
| Increased UA, n (%) | 7 (15%) |
| Type of Event | Number of Events (n) | Number of Patients Affected | Method of Ascertainment |
|---|---|---|---|
| Uncontrolled arterial hypertension | 18 | 18 | BP measurement (BP ≥ 140/90 mmHg) at any visit |
| Stable angina pectoris | 3 | 3 | Clinical symptoms, ECG changes |
| Hospitalization for heart failure | 0 | 0 | Clinical symptoms |
| New-onset arrhythmia (e.g., AF) | 2 | 2 | ECG confirmation at any visit |
| MI | 0 | 0 | Clinical symptoms/ECG/TTE/biomarkers at any visit |
| Stroke | 0 | 0 | Clinical symptoms |
| Total | 23 | 23 | - |
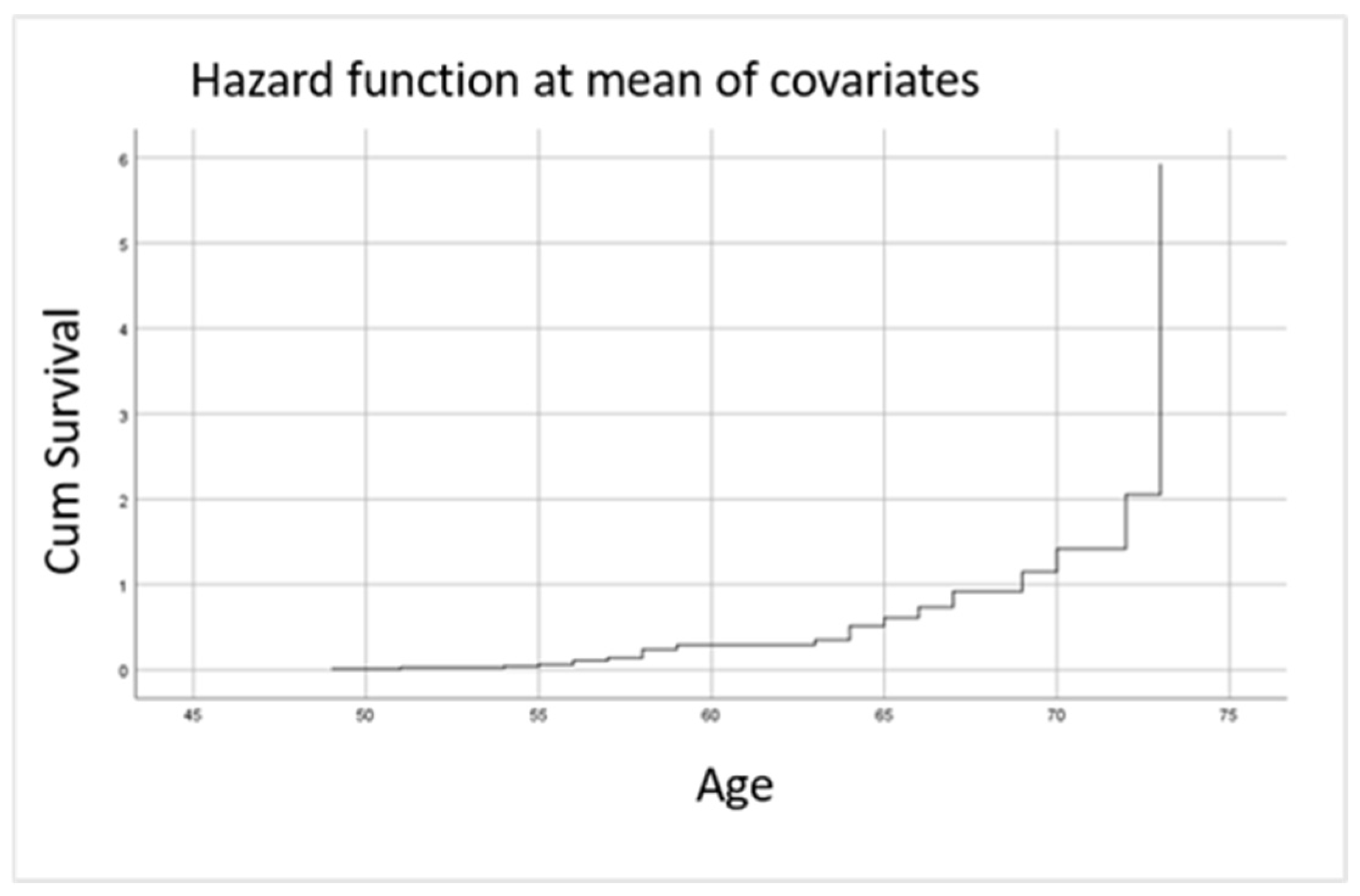
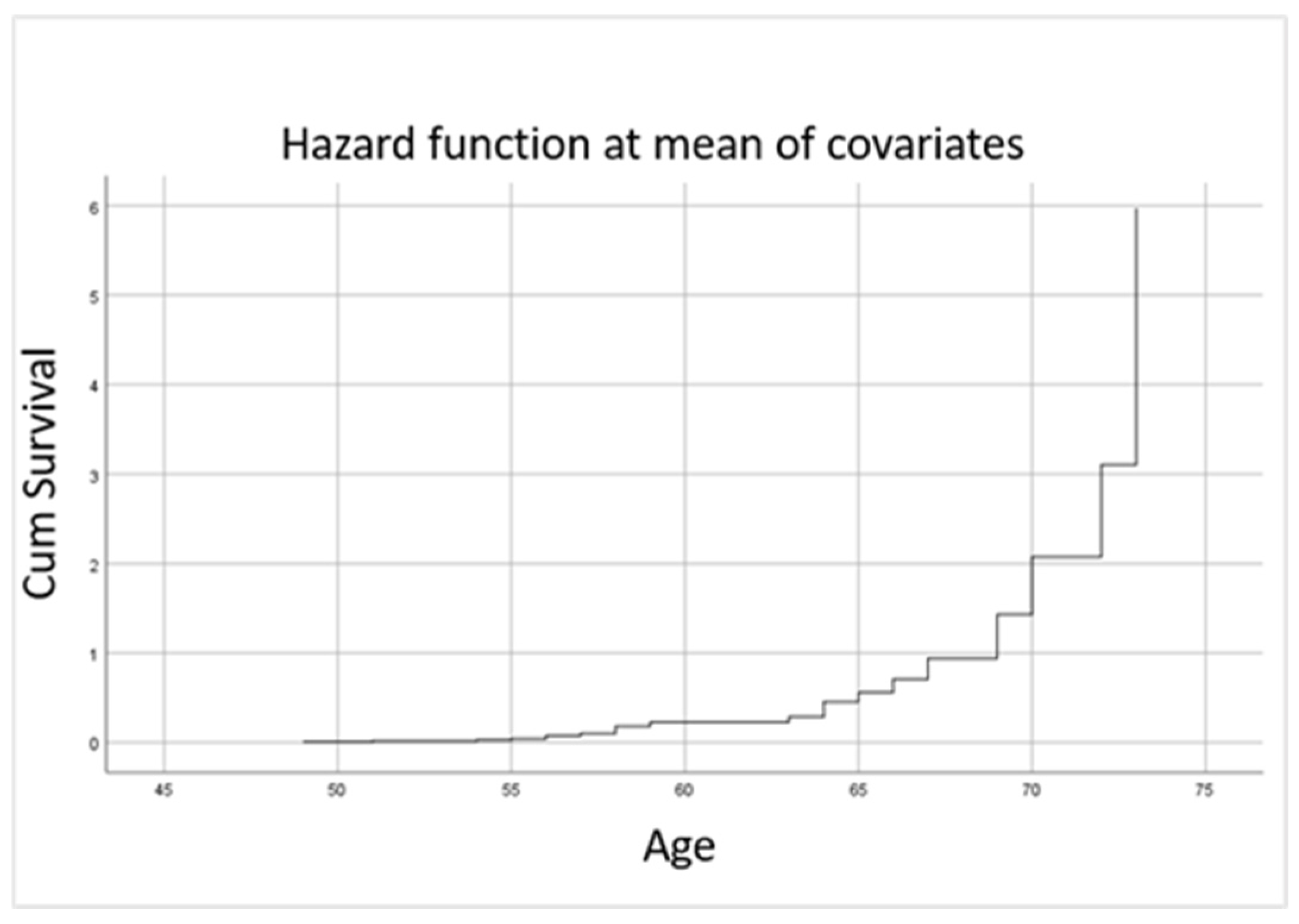
| Cardiovascular Risk Factor | HR | 95% Confidence Interval (CI) | p-Value |
|---|---|---|---|
| BMI ≥ 25 kg/m2 | 3.746 | 1.000–15.484 | 0.050 |
| Active smoking | 9.853 | 2.000–48.538 | 0.005 |
| Arterial hypertension | 1.219 | 1.061–1.793 | 0.021 |
| Biological Parameter | HR | 95% CI | p-Value |
|---|---|---|---|
| UA | 1.515 | 1.018–2.255 | 0.040 |
| TC | 1.019 | 1.001–1.036 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, D.; Badescu, M.C.; Rezus, E.; Tanase, D.M.; Ouatu, A.; Dima, N.; Buliga-Finis, O.-N.; Gosav, E.M.; Costin, D.; Rezus, C. Cardiovascular Risk in Rheumatic Patients Treated with JAK Inhibitors: The Role of Traditional and Emerging Biomarkers in a Pilot Study. J. Clin. Med. 2025, 14, 5433. https://doi.org/10.3390/jcm14155433
Popescu D, Badescu MC, Rezus E, Tanase DM, Ouatu A, Dima N, Buliga-Finis O-N, Gosav EM, Costin D, Rezus C. Cardiovascular Risk in Rheumatic Patients Treated with JAK Inhibitors: The Role of Traditional and Emerging Biomarkers in a Pilot Study. Journal of Clinical Medicine. 2025; 14(15):5433. https://doi.org/10.3390/jcm14155433
Chicago/Turabian StylePopescu, Diana, Minerva Codruta Badescu, Elena Rezus, Daniela Maria Tanase, Anca Ouatu, Nicoleta Dima, Oana-Nicoleta Buliga-Finis, Evelina Maria Gosav, Damiana Costin, and Ciprian Rezus. 2025. "Cardiovascular Risk in Rheumatic Patients Treated with JAK Inhibitors: The Role of Traditional and Emerging Biomarkers in a Pilot Study" Journal of Clinical Medicine 14, no. 15: 5433. https://doi.org/10.3390/jcm14155433
APA StylePopescu, D., Badescu, M. C., Rezus, E., Tanase, D. M., Ouatu, A., Dima, N., Buliga-Finis, O.-N., Gosav, E. M., Costin, D., & Rezus, C. (2025). Cardiovascular Risk in Rheumatic Patients Treated with JAK Inhibitors: The Role of Traditional and Emerging Biomarkers in a Pilot Study. Journal of Clinical Medicine, 14(15), 5433. https://doi.org/10.3390/jcm14155433











