Comparative Outcomes of Intra-Aortic Balloon Pump Versus Percutaneous Left Ventricular Assist Device in High-Risk Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
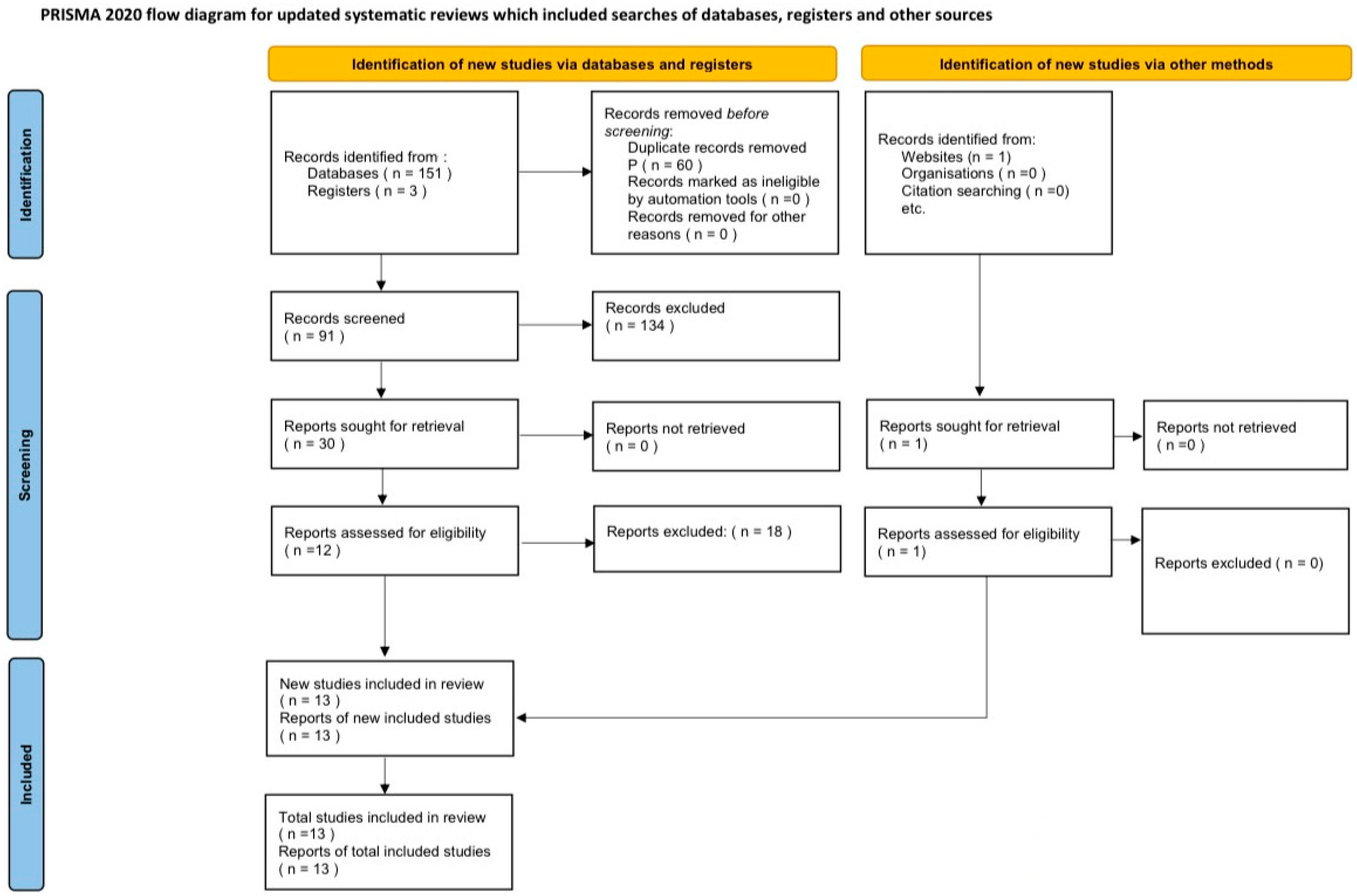
3. Results
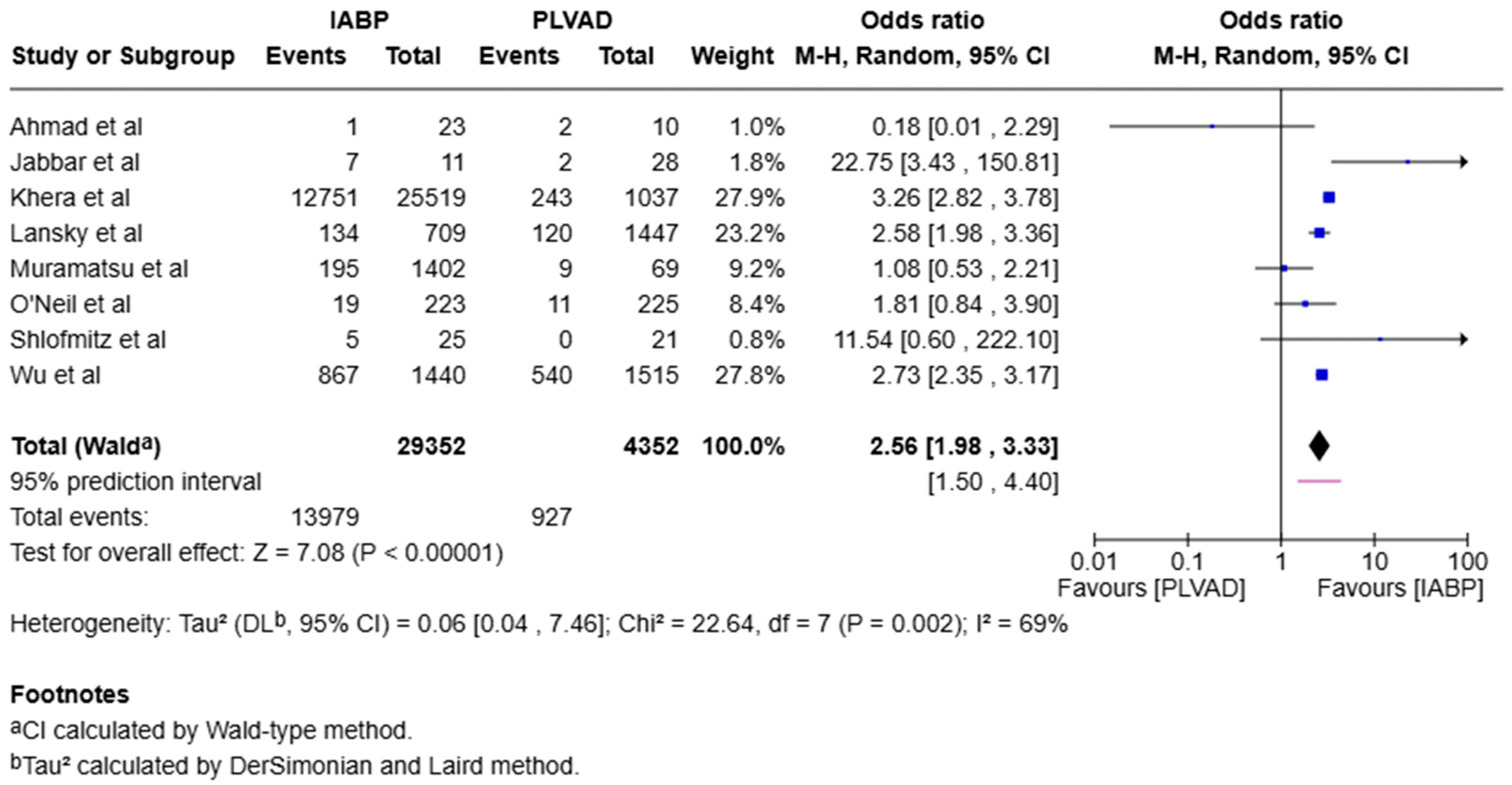
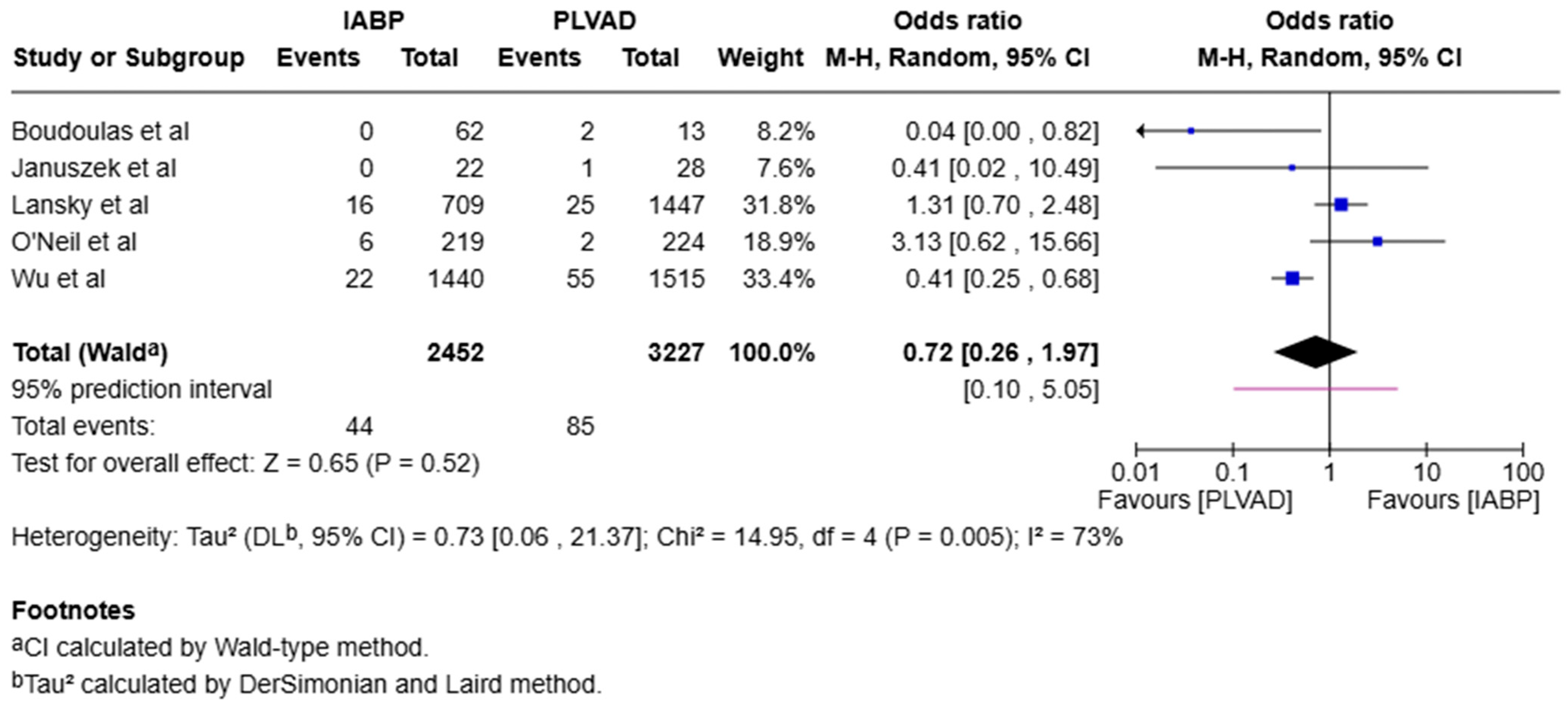
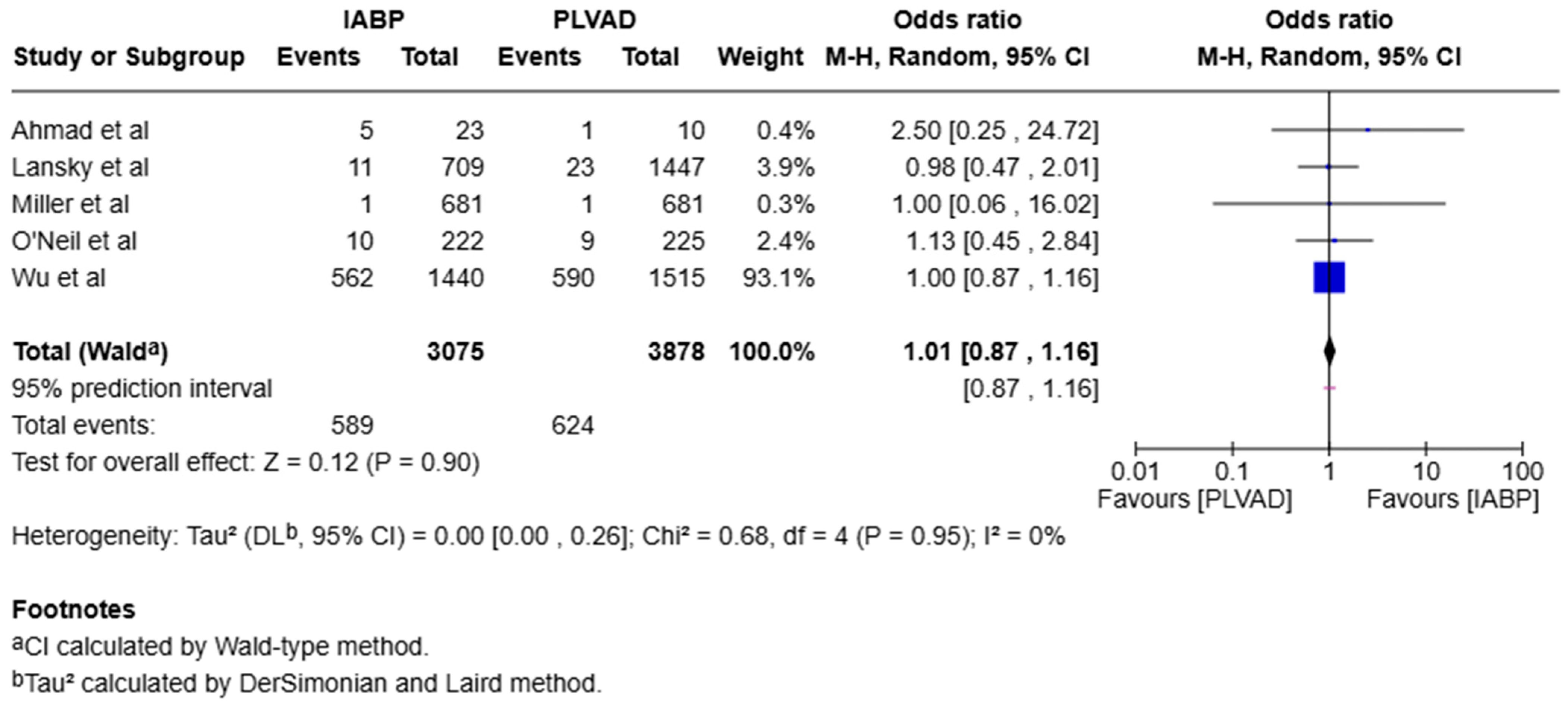
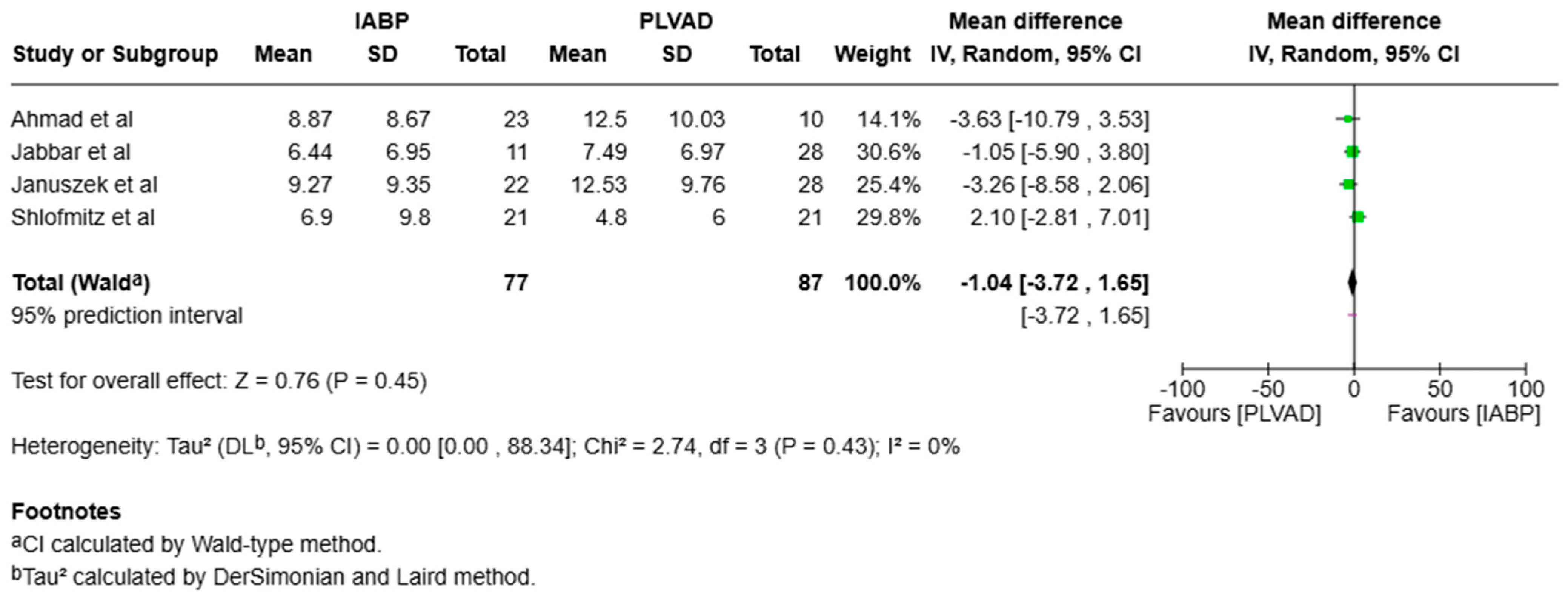
3.1. Mortality
3.2. Subgroup Analysis
3.3. Heterogeneity
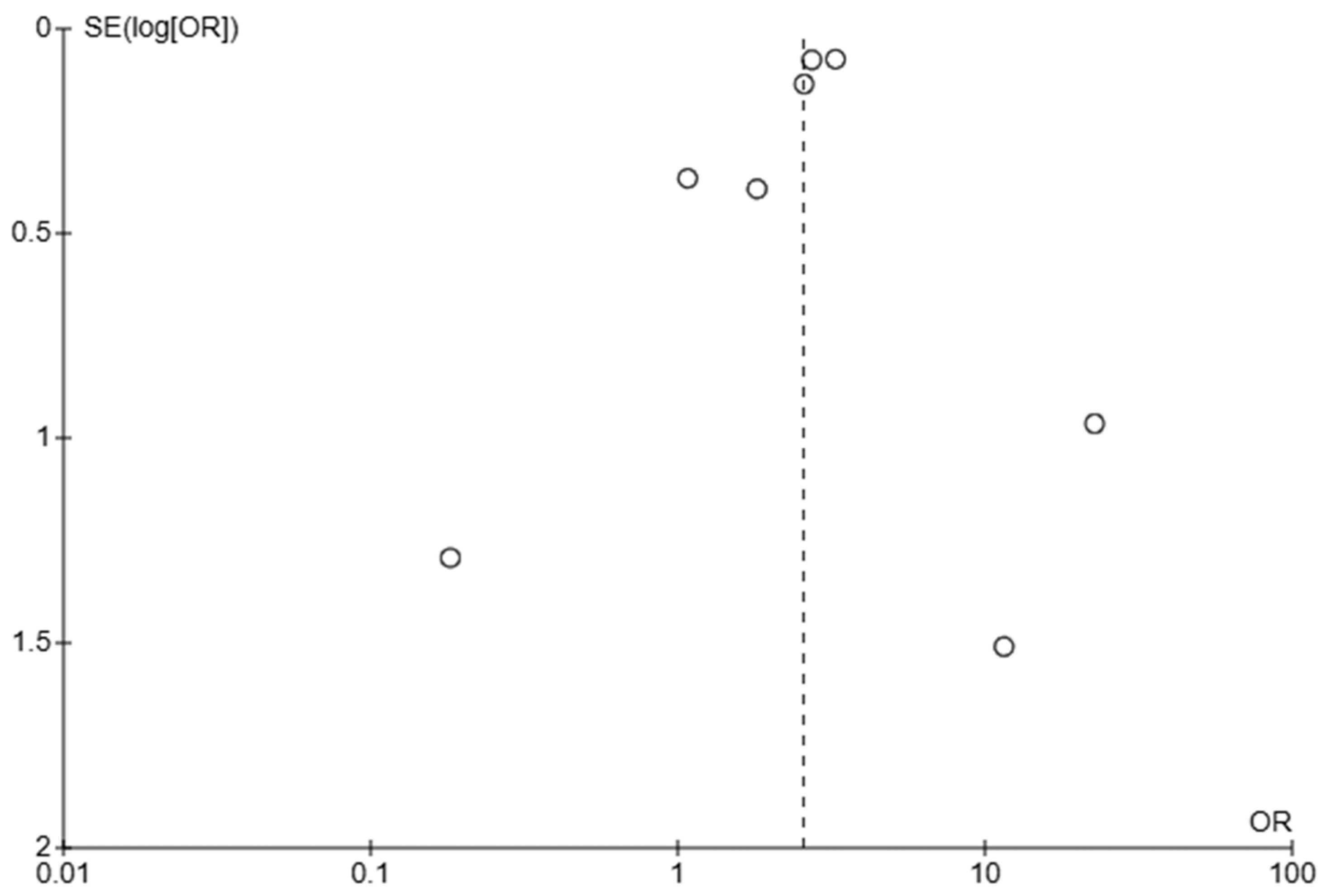
3.4. Assessment of Publication Bias
4. Discussion
4.1. Outcomes
4.1.1. Early Mortality
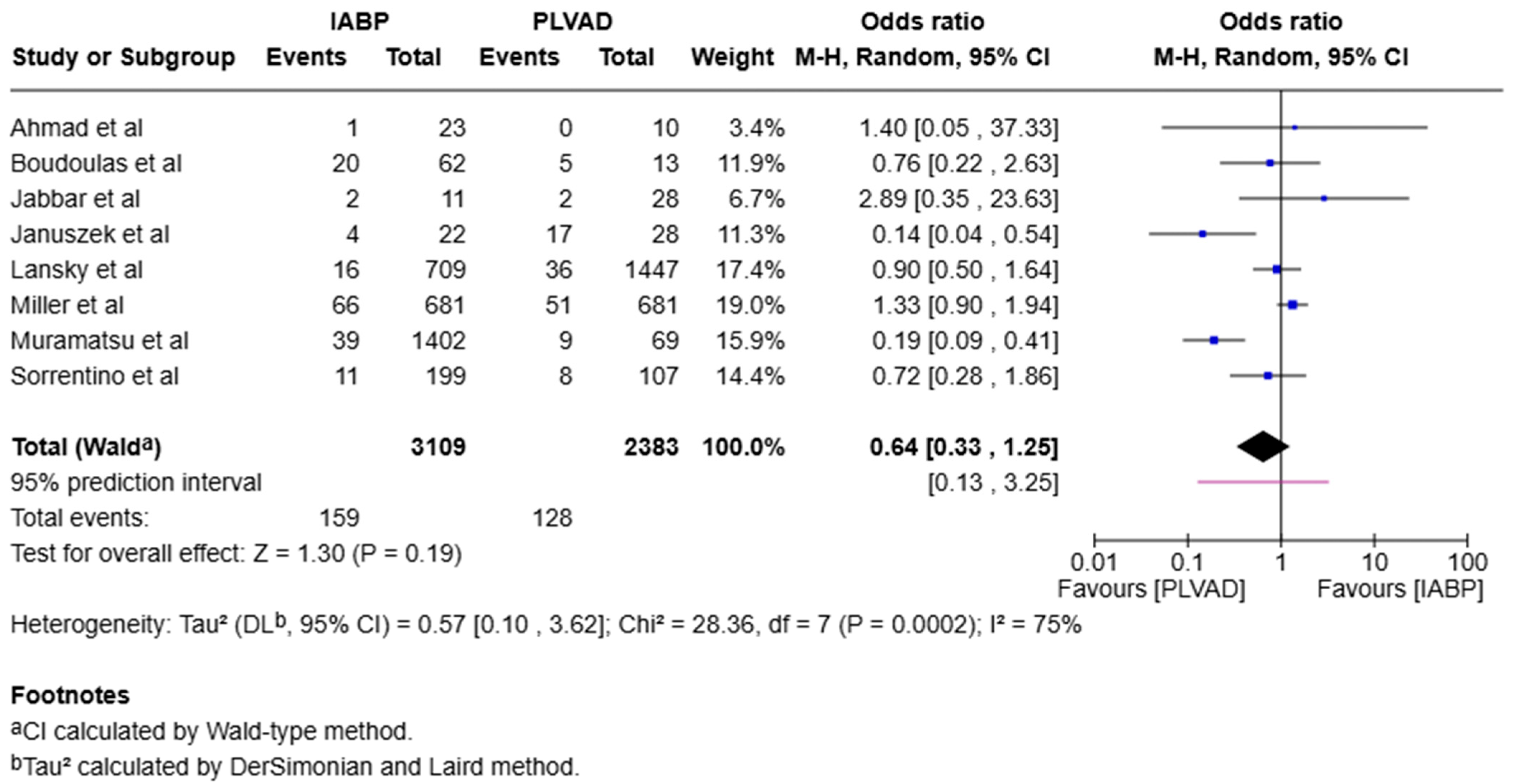
4.1.2. Cardiogenic Shock
4.1.3. Stroke
4.1.4. Major Bleeding
4.1.5. Acute Kidney Injury
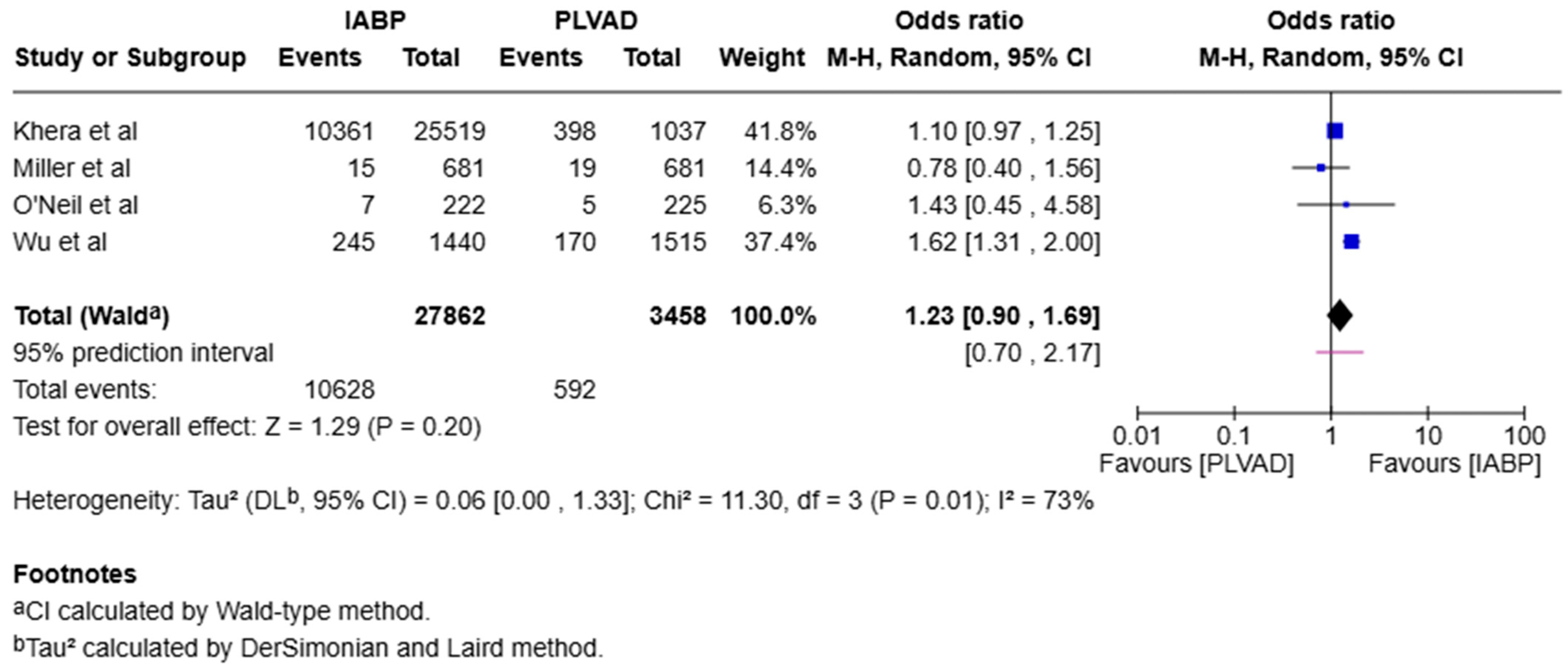
4.1.6. Arrhythmia
4.1.7. Length of Stay and Cost Effectiveness
4.2. Strengths and Limitations
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HR-PCIs | High-risk percutaneous coronary interventions |
| MCS | Mechanical circulatory support |
| IABP | Intra-aortic balloon pump |
| PLVAD | Percutaneous left ventricular assist device |
| MACEs | Major adverse cardiovascular events |
| AKI | Acute kidney injury |
| RCT | Randomized clinical trial (RCT) |
| OR | Odds ratio |
| CI | Confidence interval |
| MD | Mean difference |
| TIA | Transient ischemic attack |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| MI | Myocardial infarction |
References
- Bass, T.A. High-Risk Percutaneous Coronary Interventions in Modern Day Clinical Practice: Current Concepts and Challenges. Circ. Cardiovasc. Interv. 2015, 8, e003405. [Google Scholar] [CrossRef]
- Simonton, C.; Thompson, C.; Wollmuth, J.R.; Morris, D.L.; Dahle, T.G. The Role of Hemodynamic Support in High-risk Percutaneous Coronary Intervention. US Cardiol. Rev. 2020, 14, e13. [Google Scholar] [CrossRef]
- Kahaly, O.; Boudoulas, K.D. Percutaneous left ventricular assist device in high risk percutaneous coronary intervention. J. Thorac. Dis. 2016, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Takagi, H.; Ando, T.; Kodaira, M.; Numasawa, Y.; Fox, J.; Bangalore, S. Safety and efficacy of mechanical circulatory support with Impella or intra-aortic balloon pump for high-risk percutaneous coronary intervention and/or cardiogenic shock: Insights from a network meta-analysis of randomized trials. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 97, E636–E645. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Cui, L.Q. Percutaneous left ventricular assist device vs. intra-aortic balloon pump in patients with severe left ventricular dysfunction undergoing cardiovascular intervention: A meta-analysis. Chronic Dis. Transl. Med. 2018, 4, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Rios, S.A.; Bravo, C.A.; Weinreich, M.; Olmedo, W.; Villablanca, P.; Villela, M.A.; Ramakrishna, H.; Hirji, S.; Robles, O.A.; Mahato, P.; et al. Meta-Analysis and Trial Sequential Analysis Comparing Percutaneous Ventricular Assist Devices Versus Intra-Aortic Balloon Pump During High-Risk Percutaneous Coronary Intervention or Cardiogenic Shock. Am. J. Cardiol. 2018, 122, 1330–1338. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Kleiman, N.S.; Moses, J.; Henriques, J.P.; Dixon, S.; Massaro, J.; Palacios, I.; Maini, B.; Mulukutla, S.; Dzavík, V.; et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: The PROTECT II study. Circulation 2012, 126, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ Clin. Res. Ed. 2021, 372, n160. [Google Scholar] [CrossRef]
- Ahmad, S.; Tun, H.; Gaglia, M.; Mehra, A.; Matthews, R.V.; Shavelle, D.M.; Clavijo, L. CRT-128 Safety of Rotational Atherectomy in High Risk PCI with Hemodynamic Support. J. Am. Coll. Cardiol. Intv. 2015, 8, S16. [Google Scholar] [CrossRef][Green Version]
- Boudoulas, K.D.; Pederzolli, A.; Saini, U.; Gumina, R.J.; Mazzaferri, E.L.; Davis, M., Jr.; Bush, C.A.; Capers, Q.; Magorien, R., 4th; Pompili, V.J. Comparison of Impella and intra-aortic balloon pump in high-risk percutaneous coronary intervention: Vascular complications and incidence of bleeding. Acute Card. Care 2012, 14, 120–124. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Jbara, Y.; Ebrahimi, A.J.; Mufti, O.; Ali, O.; Markert, R.; Joffe, D.; Fishbein, G. Left Ventricular Support for Unprotected Left Main Coronary Artery Interventions (The Dayton Heart and Vascular Impella Registry). Heart Views Off. J. Gulf Heart Assoc. 2022, 23, 150–156. [Google Scholar] [CrossRef]
- Januszek, R.; Pawlik, A.; Rzeszutko, Ł.; Bartuś, K.; Bartuś, S. Clinical outcomes in patients undergoing complex, high-risk percutaneous coronary intervention and haemodynamic support with intra-aortic balloon versus Impella pump: Real-life single-centre preliminary results. Kardiol. Pol. 2022, 80, 1224–1231. [Google Scholar] [CrossRef]
- Khera, R.; Cram, P.; Vaughan-Sarrazin, M.; Horwitz, P.A.; Girotra, S. Use of Mechanical Circulatory Support in Percutaneous Coronary Intervention in the United States. Am. J. Cardiol. 2016, 117, 10–16. [Google Scholar] [CrossRef]
- Lansky, A.J.; Tirziu, D.; Moses, J.W.; Pietras, C.; Ohman, E.M.; O’Neill, W.W.; Ekono, M.M.; Grines, C.L.; Parise, H. Impella Versus Intra-Aortic Balloon Pump for High-Risk PCI: A Propensity-Adjusted Large-Scale Claims Dataset Analysis. Am. J. Cardiol. 2022, 185, 29–36. [Google Scholar] [CrossRef]
- Muramatsu, T.; Inohara, T.; Kohsaka, S.; Yamaji, K.; Ishii, H.; Shinke, T.; Toriya, T.; Yoshiki, Y.; Ozaki, Y.; Ando, H.; et al. Mechanical circulatory support devices for elective percutaneous coronary interventions: Novel insights from the Japanese nationwide J-PCI registry. Eur. Heart J. Open 2022, 2, oeac041. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Thomson, A.; Atianzar, K.; Somma, K.; Mehra, A.; Clavijo, L.; Matthews, R.V.; Shavelle, D.M. Percutaneous left ventricular support for high-risk PCI and cardiogenic shock: Who gets what? Cardiovasc. Revascularization Med. Incl. Mol. Interv. 2012, 13, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Shlofmitz, E.; Doshi, R.; Patel, A.; Meraj, P. TCT-611 Impact of Mechanical Support on Patients Undergoing Rotational Atherectomy. J. Am. Coll. Cardiol. 2017, 70, B252. [Google Scholar] [CrossRef]
- Sorrentino, S.; Baber, U.; Chandrasekhar, J.; Farhan, S.; Faggioni, M.; Vogel, B.; Sartori, S.; Kovacic, J.; Moreno, P.; Suleman, J.; et al. Clinical Outcomes Associated With Impella Versus Iabp Use in Stable Patients Undergoing High Risk Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2017, 69, 1117. [Google Scholar] [CrossRef]
- Wu, F.; Li, P.; Jha, A.; Krishnan, A.M.; Chakraborti, A. In-Hospital Outcomes Of Impella Versus Iabp in Multivessel Percutaneous Coronary Intervention: National Inpatient Database Analysis. J. Am. Coll. Cardiol. 2020, 75 (Suppl. S1), 1537. [Google Scholar] [CrossRef]
- Miller, P.E.; Gordon, A.S.; Liu, Y.; Ahmad, T.; Bromfield, S.G.; Girotra, S.; Davila, C.D.; Crawford, G.; Whitney, J.; Desai, N.R. Mechanical Circulatory Support in Patients Without Cardiogenic Shock Undergoing Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2025, 14, e037424. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Al-Khadra, Y.; Alraies, M.C.; Darmoch, F.; Pacha, H.M.; Soud, M.; Kaki, A.; Rab, T.; Grines, C.L.; Meraj, P.; Alaswad, K.; et al. Outcomes of nonemergent percutaneous coronary intervention requiring mechanical circulatory support in patients without cardiogenic shock. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2020, 95, 503–512. [Google Scholar] [CrossRef]
- Riley, R.F.; Henry, T.D.; Mahmud, E.; Kirtane, A.J.; Brilakis, E.S.; Goyal, A.; Grines, C.L.; Lombardi, W.L.; Maran, A.; Rab, T.; et al. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2020, 96, 346–362. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Nguyen, H.T.; Karajgikar, R.; Sharma, S.K.; Kini, A.S. The Impella Recover 2.5 and TandemHeart ventricular assist devices are safe and associated with equivalent clinical outcomes in patients undergoing high-risk percutaneous coronary intervention. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2013, 82, E28–E37. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Subramaniam, A.V.; Murphree Jr, D.H.; Patlolla, S.H.; Ya’Qoub, L.; Kumar, V.; Verghese, D.; Cheungpasitporn, W.; Jentzer, J.C.; Sandhu, G.S.; et al. Complications from percutaneous-left ventricular assist devices versus intra-aortic balloon pump in acute myocardial infarction-cardiogenic shock. PLoS ONE 2020, 15, e0238046. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Hanna, B.; Singh, S.; Gupta, S.; Deshmukh, A.; Kumar, G.; Sachdeva, R.; Berman, A.E. Percutaneous Ventricular Assist Device vs. Intra-Aortic Balloon Pump for Hemodynamic Support in Acute Myocardial Infarction-Related Cardiogenic Shock and Coexistent Atrial Fibrillation: A Nationwide Propensity-Matched Analysis’. Am. J. Med. Sci. 2021, 361, 55–62. [Google Scholar] [CrossRef]
- Siraw, B.B.; Isha, S.; Mehadi, A.Y.; Tafesse, Y.T. In-hospital outcomes of cardiogenic shock patients: A propensity score-matched nationwide comparative analysis between intra-aortic balloon pump and percutaneous ventricular assist devices. Int. J. Cardiol. 2025, 427, 133093. [Google Scholar] [CrossRef]
- Shi, W.; Wang, W.; Wang, K.; Huang, W. Percutaneous mechanical circulatory support devices in high-risk patients undergoing percutaneous coronary intervention. Medicine 2019, 98, e17107. [Google Scholar] [CrossRef]
- Cheng, J.M.; den Uil, C.A.; Hoeks, S.E.; van der Ent, M.; Jewbali, L.S.; van Domburg, R.T.; Serruys, P.W. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: A meta-analysis of controlled trials. Eur. Heart J. 2009, 30, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Mohebi, R.; Karimi Galougahi, K.; Garcia, J.J.; Horst, J.; Ben-Yehuda, O.; Radhakrishnan, J.; Chertow, G.M.; Jeremias, A.; Cohen, D.J.; Cohen, D.J.; et al. Long-Term Clinical Impact of Contrast-Associated Acute Kidney Injury Following PCI: An ADAPT-DES substudy. Cardiovasc. Interv. 2022, 15, 753–766. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734–745. [Google Scholar] [CrossRef]
- Davidavicius, G.; Godino, C.; Shannon, J.; Takagi, K.; Bertoldi, L.; Mussardo, M.; Chieffo, A.; Arioli, F.; Ielasi, A.; Montorfano, M.; et al. Incidence of Overall Bleeding in Patients Treated With Intra-Aortic Balloon Pump During Percutaneous Coronary Intervention: 12-Year Milan Experience. JACC Cardiovasc. Interv. 2012, 5, 350–357. [Google Scholar] [CrossRef]
- Perera, D. Elective Intra-aortic Balloon Counterpulsation During High-Risk Percutaneous Coronary Intervention: A Randomized Controlled Trial. JAMA 2010, 304, 867. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, M.P.; Pant, S.; Patel, S.V.; Kilgore, T.; Dassanayaka, S.; Loughran, J.H.; Rawasia, W.; Dawn, B.; Cheng, A.; Bartoli, C.R. Hemodynamic Support With a Microaxial Percutaneous Left Ventricular Assist Device (Impella) Protects Against Acute Kidney Injury in Patients Undergoing High-Risk Percutaneous Coronary Intervention. Circ. Res. 2017, 120, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, V.; Rihal, C.S.; Reeder, G.; Nath, K.A.; Lewis, B.R.; Singh, M. Impact of Renal Function and Acute Kidney Injury on Long-term Outcomes After Percutaneous Coronary Intervention. Mayo Clin. Proc. 2025, in press. Available online: https://pubmed.ncbi.nlm.nih.gov/40481827 (accessed on 14 June 2025). [CrossRef] [PubMed]
- Lee, J.M.; Park, J.; Kang, J.; Jeon, K.H.; Jung, J.H.; Lee, S.E.; Han, J.K.; Kim, H.L.; Yang, H.M.; Park, K.W.; et al. The efficacy and safety of mechanical hemodynamic support in patients undergoing high-risk percutaneous coronary intervention with or without cardiogenic shock: Bayesian approach network meta-analysis of 13 randomized controlled trials. Int. J. Cardiol. 2015, 184, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Lennon, R.J.; Gulati, R.; Holmes, D.R. Risk scores for 30-day mortality after percutaneous coronary intervention: New insights into causes and risk of death. Mayo Clin. Proc. 2014, 89, 631–637. [Google Scholar] [CrossRef]
- Aggarwal, B.; Ellis, S.G.; Lincoff, A.M.; Kapadia, S.R.; Cacchione, J.; Raymond, R.E.; Cho, L.; Bajzer, C.; Nair, R.; Franco, I.; et al. Cause of death within 30 days of percutaneous coronary intervention in an era of mandatory outcome reporting. J. Am. Coll. Cardiol. 2013, 62, 409–415. [Google Scholar] [CrossRef]
- Agoritsas, T.; Merglen, A.; Shah, N.D.; O’Donnell, M.; Guyatt, G.H. Adjusted Analyses in Studies Addressing Therapy and Harm: Users’ Guides to the Medical Literature. JAMA 2017, 317, 748–759. [Google Scholar] [CrossRef]
- Rihal, C.S.; Naidu, S.S.; Givertz, M.M.; Szeto, W.Y.; Burke, J.A.; Kapur, N.K.; Kern, M.; Garratt, K.N.; Goldstein, J.A.; Dimas, V.; et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J. Am. Coll. Cardiol. 2015, 65, e7–e26. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar] [CrossRef]
- Briasoulis, A.; Telila, T.; Palla, M.; Mercado, N.; Kondur, A.; Grines, C.; Schreiber, T. Meta-Analysis of Usefulness of Percutaneous Left Ventricular Assist Devices for High-Risk Percutaneous Coronary Interventions. Am. J. Cardiol. 2016, 118, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, L.; Pastena, P.; Cacciatore, S.; Betti, M.; Biondi-Zoccai, G.; D’Ascenzo, F.; De Ferrari, G.M.; De Filippo, O.; Versaci, F.; Sciarretta, S.; et al. One-Year Prognostic Differences and Management Strategies between ST-Elevation and Non-ST-Elevation Myocardial Infarction: Insights from the PRAISE Registry. Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2025. [Google Scholar] [CrossRef] [PubMed]


| Studies | Selection | Comparability | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed | Selection of the Non-Exposed | Ascertainment of Exposure | Outcome of Interest Not Present at Study Beginning | Main Factor | Additional Factor | Assessment | Follow-Up Length | Adequacy of Follow-Up | Score | |
| Ahmed et al. [10] | * | * | * | * | * | * | * | * | * | 9 |
| Boudoulas et al. [11] | * | * | * | * | 0 | 0 | * | * | 0 | 6 |
| Jabbar et al. [12] | * | * | * | * | 0 | 0 | * | * | 0 | 6 |
| Januszek et al. [13] | * | * | * | * | 0 | 0 | * | * | * | 7 |
| Khera et al. [14] | * | * | * | * | * | * | * | * | * | 9 |
| Lansky et al. [15] | * | * | * | * | * | * | * | * | * | 9 |
| Muramatsu et al. [16] | * | * | * | * | 0 | 0 | * | * | * | 9 |
| Shah et al. [17] | * | * | * | * | * | 0 | * | * | * | 8 |
| Shlofmitz et al. [18] | * | * | * | * | * | * | * | * | * | 9 |
| Sorrentino et al. [19] | * | * | * | * | * | * | * | * | 0 | 8 |
| Wu et al. [20] | * | * | * | * | * | * | * | * | * | 9 |
| Miller et al. [21] | * | * | * | * | * | * | * | * | * | 9 |
| Risk-of-Bias Assessment—Protect II Trial (O’Neill et al. [7]) | ||
|---|---|---|
| Domain | Judgment | Justification |
| Random sequence generation (selection bias) | Low risk of bias | Randomization was performed using an automated interactive voice response system stratified by geography and angioplasty indication, indicating an adequate randomization process. |
| Allocation concealment (selection bias) | Unclear risk of bias | While randomization was automated, the method of allocation concealment (e.g., central allocation, opaque envelopes) was not explicitly stated in the methods section. |
| Blinding of participants and personnel (performance bias) | High risk of bias | This was an open-label trial with no blinding of clinicians or participants. Operator awareness may have influenced treatment decisions, such as more aggressive atherectomy in the Impella arm. |
| Blinding of outcome assessment (detection bias) | Low risk of bias | Outcome events were adjudicated by an independent Clinical Events Committee (CEC) blinded to treatment assignment, minimizing detection bias. |
| Incomplete outcome data (attrition bias) | Low risk of bias | Very low dropout rate reported: 448 out of 452 randomized patients were included in the final analysis. Both ITT and PP analyses were clearly defined. |
| Selective reporting (reporting bias) | Unclear risk of bias | Although the primary and secondary outcomes were pre-specified, the study was stopped early due to futility, and additional subgroup analyses were emphasized, raising the possibility of selective emphasis in reporting. |
| Other bias | High risk of bias | Potential learning curve effect was acknowledged, with safety improvements noted later in the trial. This introduces a possible performance or temporal bias in early vs. late enrollees. |
| Study ID | Study Center | Study Duration (Year–Year) | Study Design | PLVAD | Number of Patients | AGE | AGE | |
|---|---|---|---|---|---|---|---|---|
| IABP | PLVAD | IABP | PLVAD | |||||
| Sorrentino et al. (2017) [19] | Single-center prospective study | 2007 to 2016 | Prospective cohort | Impella 2.5 | 199 | 107 | 70 ± 10.9 | 70 ± 10.9 |
| Ahmed et al. (2015) [10] | Observational single-center study | 2008 to 2014 | Retrospective cohort | Impella 2.5 | 23 | 10 | 71 ± 14.47 | 71 ± 14.47 |
| O’Neil et al. (2015) [7] | Prospective multicenter randomized trial | 2007 to 2010 | Randomized controlled trial | Impella 2.5 | 223 | 225 | 67 ± 11 | 68 ± 11 |
| Khera et al. (2015) [14] | NIS database | 2004 to 2012 | Retrospective cohort | Not specified | 25519 | 1037 | 64.7 | 69 |
| Januszek et al. (2022) [13] | Single-center prospective study | 2018 to 2021 | Prospective cohort | Impella 2.5 | 22 | 28 | 74.6 ± 9.6 | 7.6 ± 9.6 |
| Boudoulas et al. (2012) [11] | Observational single-center study | 2008 to 2010 | Retrospective analysis | Impella 2.5 | 62 | 13 | 60.8 ± 12.6 | 62.5 ± 9.7 |
| Shah et al. (2012) [17] | Observational single-center study | 2007 to 2009 | Prospective analysis | Tandem Heart 35 and Impella | 35 | 22 | 60 ± 9.9 | 69 ± 9 |
| Jabbar et al. (2022) [12] | Observational single-center study | 2010 to 2014 | retrospective cohort | Impella 2.5 | 11 | 28 | 73.73 ± 16.89 | 75.60 ± 10.78 |
| Muramatsu et al. (2022) [16] | Japanese percutaneous coronary intervention registry | 2018 | Prospective cohort | Impella 2.5 | 1402 | 69 | 74 | 74 |
| Lansky et al. (2022) [15] | Premier Healthcare Database | 2016–2019 | Retrospective cohort | Impella 2.5 | 709 | 1447 | 69.2 ± 10.9 | 71.4 ± 10.9 |
| Wu et al. (2020) [20] | NIS database | 2016 | retrospective cohort | Impella 2.5 | 1440 | 1515 | NA | NA |
| Shlofmitz et al. (2017) [18] | Observational multicenter study | 2011–2017 | Retrospective cohort | Impella 2.5 | 25 | 21 | NA | NA |
| Miller et al. (2025) [21] | Observational multicenter study | 2016–2022 | Retrospective cohort | Impella 2.5 | 681 | 681 | 65 ± 12.3 | 71 ± 11.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivasubramanian, D.; Senthilkumar, V.; Nanda Palanisamy, N.; Bilgaiyan, R.; Aravind, S.; Kumar, S.D.; Balasubramanian, A.; Sanil, S.; Balasubramanian, K.; Kamaladasan, D.; et al. Comparative Outcomes of Intra-Aortic Balloon Pump Versus Percutaneous Left Ventricular Assist Device in High-Risk Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 5430. https://doi.org/10.3390/jcm14155430
Sivasubramanian D, Senthilkumar V, Nanda Palanisamy N, Bilgaiyan R, Aravind S, Kumar SD, Balasubramanian A, Sanil S, Balasubramanian K, Kamaladasan D, et al. Comparative Outcomes of Intra-Aortic Balloon Pump Versus Percutaneous Left Ventricular Assist Device in High-Risk Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(15):5430. https://doi.org/10.3390/jcm14155430
Chicago/Turabian StyleSivasubramanian, Dhiran, Virushnee Senthilkumar, Nithish Nanda Palanisamy, Rashi Bilgaiyan, Smrti Aravind, Sri Drishaal Kumar, Aishwarya Balasubramanian, Sathwik Sanil, Karthick Balasubramanian, Dharssini Kamaladasan, and et al. 2025. "Comparative Outcomes of Intra-Aortic Balloon Pump Versus Percutaneous Left Ventricular Assist Device in High-Risk Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 15: 5430. https://doi.org/10.3390/jcm14155430
APA StyleSivasubramanian, D., Senthilkumar, V., Nanda Palanisamy, N., Bilgaiyan, R., Aravind, S., Kumar, S. D., Balasubramanian, A., Sanil, S., Balasubramanian, K., Kamaladasan, D., Pilathodan, H., & Shankar, K. (2025). Comparative Outcomes of Intra-Aortic Balloon Pump Versus Percutaneous Left Ventricular Assist Device in High-Risk Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(15), 5430. https://doi.org/10.3390/jcm14155430