Constipation in Ulcerative Colitis: An Underestimated Problem
Abstract
1. Introduction
2. Fecal Stasis and Constipation in UC
3. Constipation in UC: A Challenging Definition
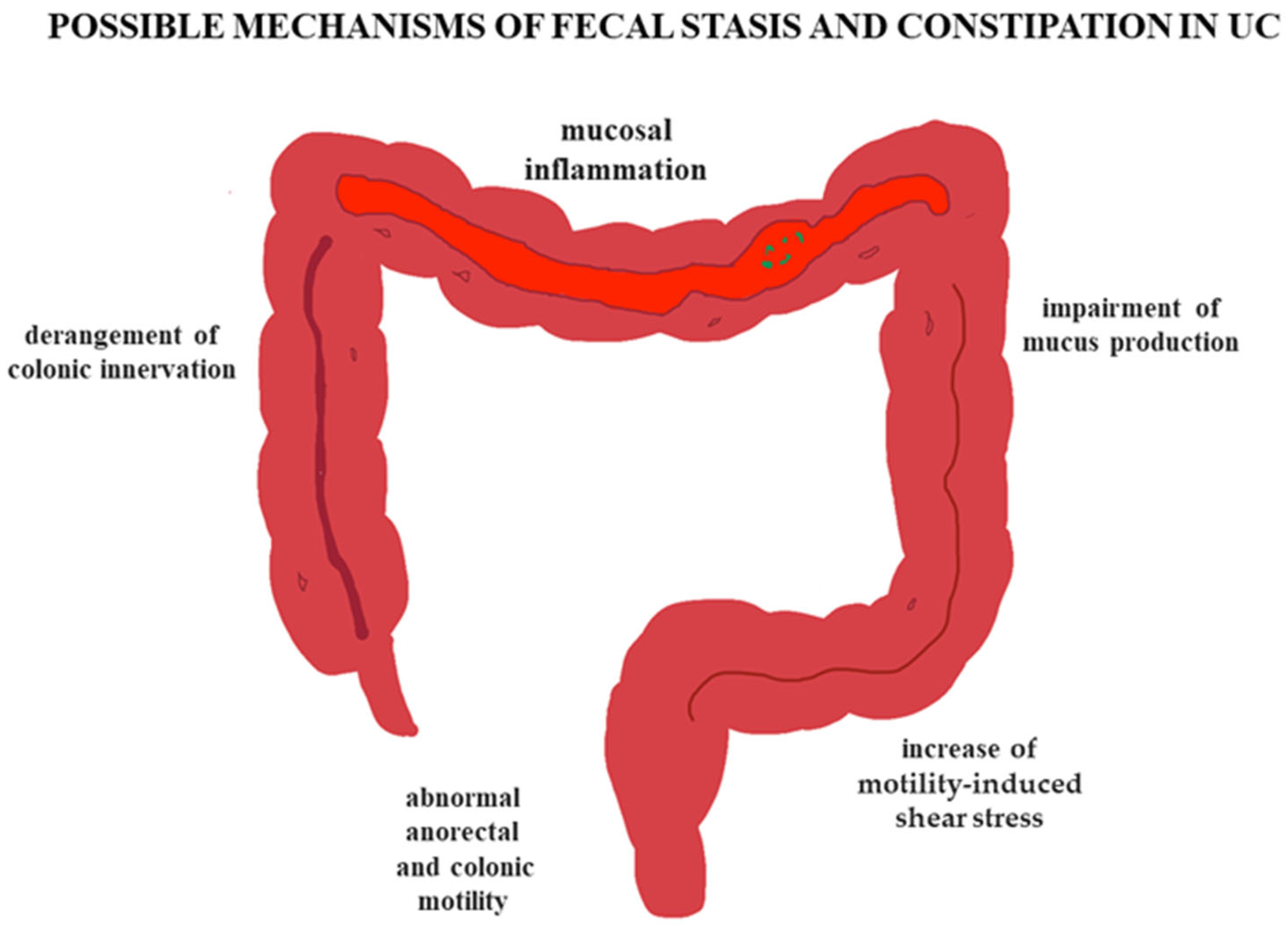
4. Pathophysiological Considerations
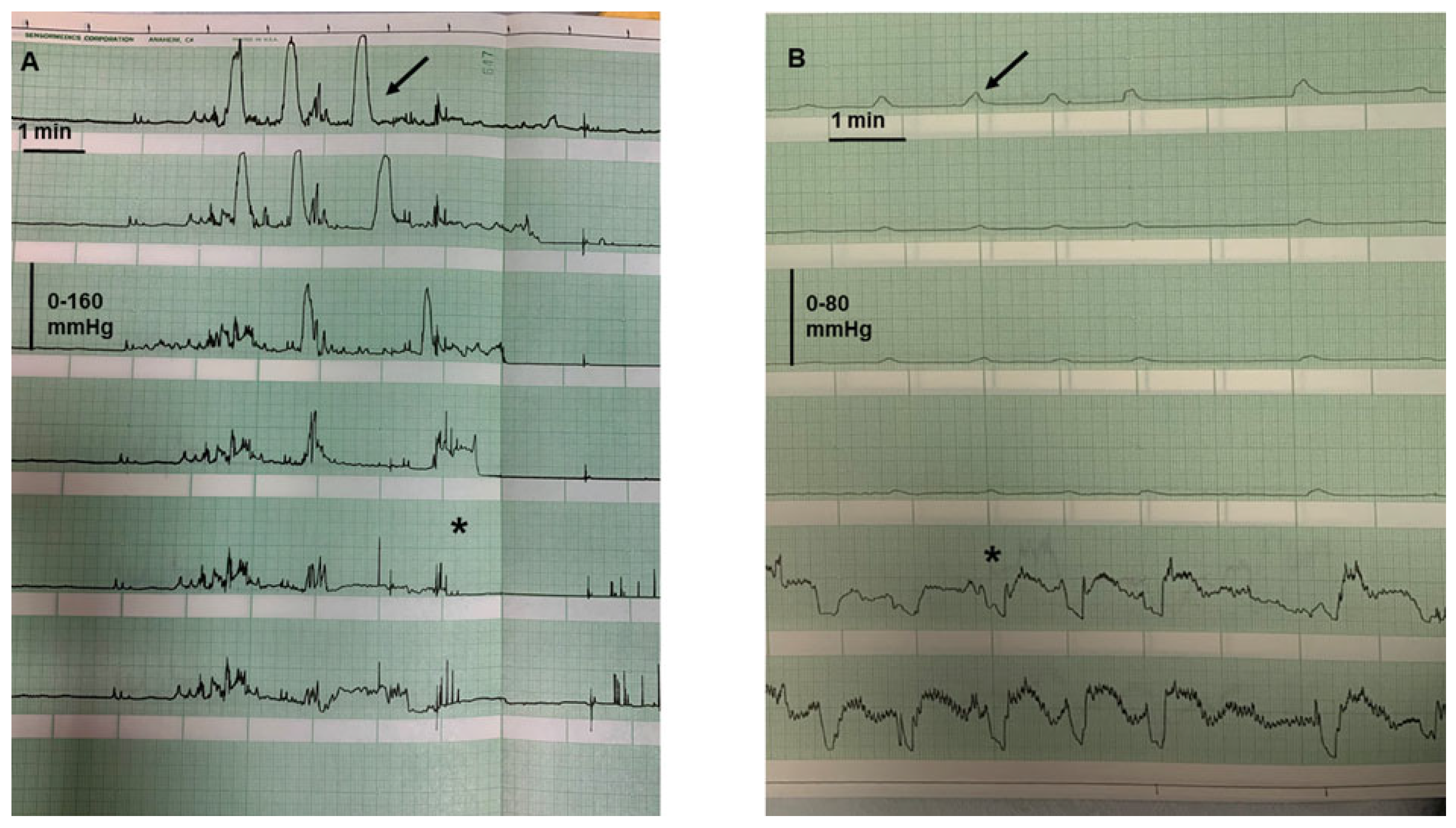
4.1. Colonic Motility
4.2. Effects of Inflammation on the Colon
5. Why Are Some Patients with UC Constipated?
6. Clinical Consequences
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HAPC | High-amplitude propagated contractions |
| UCAC | Ulcerative-colitis-associated constipation |
| IBD | Inflammatory bowel disease |
| LAPC | Low-amplitude propagated contraction |
| UC | Ulcerative colitis |
References
- Nguyen, L.; Parra, V.V. Surgical management of ulcerative colitis. Surg. Clin. N. Am. 2025, 105, 289–299. [Google Scholar] [CrossRef]
- Villanacci, V.; Maconi, G.; Laschi, L.; Bassotti, G. Diagnosing ulcerative colitis: Should we go beyond the surface? J. Clin. Med. 2025, 14, 3690. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E.; Langman, M.J.; Jones, F.A. Faecal stasis in proctocolitis. Gut 1962, 3, 301–305. [Google Scholar] [CrossRef]
- Jalan, K.N.; Walker, R.J.; Prescott, R.J.; Butterworth, S.T.; Smith, A.N.; Sircus, W. Faecal stasis and diverticular disease in ulcerative colitis. Gut 1970, 11, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Emmanuel, A.; Zarate-Lopez, N.; Taylor, S.; Bloom, S. Constipation in ulcerative colitis: Pathophysiology and practical management. Frontline Gastroenterol. 2020, 12, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Hart, A.L.; Lomer, M.; Verjee, A.; Kemp, K.; Faiz, O.; Daly, A.; Solomon, J.; McLaughlin, J. What are the top 10 research questions in the treatment of inflammatory bowel disease? A priority setting partnership with the James Lind Alliance. J. Crohn’s Colitis 2017, 11, 204–211. [Google Scholar] [CrossRef]
- Rao, S.S.; Holdsworth, C.D.; Read, N.W. Symptoms and stool patterns in patients with ulcerative colitis. Gut 1988, 29, 342–345. [Google Scholar] [CrossRef]
- Allison, M.C.; Vallance, R. Prevalence of proximal faecal stasis in active ulcerative colitis. Gut 1991, 32, 179–182. [Google Scholar] [CrossRef]
- Perera, L.P.; Ananthakrishnan, A.N.; Guilday, C.; Remshak, K.; Zadvornova, Y.; Naik, A.S.; Stein, D.J.; Massey, B.T. Dyssynergic defecation: A treatable cause of persistent symptoms when inflammatory bowel disease is in remission. Dig. Dis. Sci. 2013, 58, 3600–3605. [Google Scholar] [CrossRef]
- Petryszyn, P.W.; Paradowski, L. Stool patterns and symptoms of disordered anorectal function in patients with inflammatory bowel diseases. Adv. Clin. Exp. Med. 2018, 27, 813–818. [Google Scholar] [CrossRef]
- James, S.L.; van Langenberg, D.R.; Taylor, K.M.; Gibson, P.R. Characterization of ulcerative colitis-associated constipation syndrome (proximal constipation). JGH Open 2018, 2, 217–222. [Google Scholar] [CrossRef]
- Khera, A.J.; Chase, J.W.; Salzberg, M.; Thompson, A.J.V.; Kamm, M.A. Gut-directed pelvic floor behavioral treatment for fecal incontinence and constipation in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2019, 25, 620–626. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Furukawa, S.; Miyake, T.; Yoshida, O.; Shiraishi, K.; Hashimoto, Y.; Tange, K.; Hanayama, M.; Kitahata, S.; Ninomiya, T.; et al. Severity of nocturia and constipation in patients with ulcerative colitis. Urology 2023, 181, 119–123. [Google Scholar] [CrossRef]
- Yagi, S.; Furukawa, S.; Miyake, T.; Yoshida, O.; Shiraishi, K.; Tange, K.; Hashimoto, Y.; Kitahata, S.; Ninomiya, T.; Hanayama, M.; et al. Aging is associated with constipation in Japanese patients with ulcerative colitis: A post hoc analysis. Gerontol. Geriatr. Med. 2023, 9, 23337214231215637. [Google Scholar] [CrossRef]
- Yagi, S.; Furukawa, S.; Suzuki, S.; Ohashi, K.; Tomida, H.; Yamamoto, Y.; Takeshita, E.; Ikeda, Y.; Hiasa, Y. No association between allergic diseases and constipation in Japanese ulcerative colitis patients: A cross-sectional study. Cureus 2024, 16, e55912. [Google Scholar] [CrossRef]
- Khan, R.; Kuenzig, M.E.; Benchimol, E.I. Epidemiology of pediatric inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2023, 52, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.; Kern, I.; Reinecke, I.; Bathelt, F.; Manuwald, U.; Henke, E.; Zoch, M.; Rothe, U.; Kugler, J. A systematic review and meta-regression on international trends in the incidence of ulcerative colitis in children and adolescents associated with socioeconomic and geographic factors. Eur. J. Pediatr. 2024, 183, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Cenni, S.; Colucci, A.; Salomone, S.; Pacella, D.; Casertano, M.; Buono, P.; Martinelli, M.; Miele, E.; Staiano, A.; Strisciuglio, C. The prevalence of constipation in children with new diagnosis of inflammatory bowel disease: A retrospective study. J. Pediatr. Gastroenterol. Nutr. 2025, 80, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Gaburri, M. Manometric investigation of high-amplitude propagated contractile activity of the human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 1988, 255, G660–G664. [Google Scholar] [CrossRef]
- Bassotti, G.; Clementi, M.; Antonelli, E.; Pelli, M.A.; Tonini, M. Low-amplitude propagated contractile waves: A relevant propulsive mechanism of human colon. Dig. Liver Dis. 2001, 33, 36–40. [Google Scholar] [CrossRef]
- Dinning, P.G.; Wiklendt, L.; Maslen, L.; Gibbins, I.; Patton, V.; Arkwright, J.W.; Lubowski, D.Z.; O’Grady, G.; Bampton, P.A.; Brookes, S.J.; et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol. Motil. 2014, 26, 1443–1457. [Google Scholar] [CrossRef]
- Chen, J.H.; Yu, Y.; Yang, Z.; Yu, W.Z.; Chen, W.L.; Yu, H.; Kim, M.J.; Huang, M.; Tan, S.; Luo, H.; et al. Intraluminal pressure patterns in the human colon assessed by high-resolution manometry. Sci. Rep. 2017, 7, 41436. [Google Scholar] [CrossRef]
- Furukawa, Y.; Cook, I.J.; Panagopoulos, V.; McEvoy, R.D.; Sharp, D.J.; Simula, M. Relationship between sleep patterns and human colonic motor patterns. Gastroenterology 1994, 107, 1372–1381. [Google Scholar] [CrossRef]
- Bassotti, G.; Betti, C.; Imbimbo, B.P.; Pelli, M.A.; Morelli, A. Colonic motor response to eating: A manometric investigation in proximal and distal portions of the viscus in man. Am. J. Gastroenterol. 1989, 84, 118–122. [Google Scholar]
- Bassotti, G.; Iantorno, G.; Fiorella, S.; Bustos-Fernandez, L.; Bilder, C.R. Colonic motility in man: Features in normal subjects and in patients with chronic idiopathic constipation. Am. J. Gastroenterol. 1999, 94, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Johnson, K.; Yin, J.; Lee, S.; Lin, R.; Yu, H.; In, J.; Foulke-Abel, J.; Zachos, N.C.; Donowitz, M.; et al. Chronic inflammation in ulcerative colitis causes long-term changes in goblet cell function. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Erbay, I.H.; Alexiadis, A.; Rochev, Y. Computational insights into colonic motility: Mechanical role of mucus in homeostasis and inflammation. Comput. Biol. Med. 2024, 176, 108540. [Google Scholar] [CrossRef] [PubMed]
- Moynes, D.M.; Lucas, G.H.; Beyak, M.J.; Lomax, A.E. Effects of inflammation on the innervation of the colon. Toxicol. Pathol. 2014, 42, 111–117. [Google Scholar] [CrossRef]
- Rumessen, J.J.; Vanderwinden, J.M.; Horn, T. Ulcerative colitis: Ultrastructure of interstitial cells in myenteric plexus. Ultrastruct. Pathol. 2010, 34, 279–287. [Google Scholar] [CrossRef]
- Aulí, M.; Nasser, Y.; Ho, W.; Burgueño, J.F.; Keenan, C.M.; Romero, C.; Sharkey, K.A.; Fernández, E. Neuromuscular changes in a rat model of colitis. Auton. Neurosci. 2008, 141, 10–21. [Google Scholar] [CrossRef]
- von Boyen, G.B.; Schulte, N.; Pflüger, C.; Spaniol, U.; Hartmann, C.; Steinkamp, M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 2011, 11, 3. [Google Scholar] [CrossRef]
- da Silva, M.V.; Marosti, A.R.; Mendes, C.E.; Palombit, K.; Castelucci, P. Differential effects of experimental ulcerative colitis on P2X7 receptor expression in enteric neurons. Histochem. Cell Biol. 2015, 143, 171–184. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ahuja, V.; Paul, J. Attenuated GABAergic signaling in intestinal epithelium contributes to pathogenesis of ulcerative colitis. Dig. Dis. Sci. 2017, 62, 2768–2779. [Google Scholar] [CrossRef]
- da Silva, M.D.V.; da Silva Bonassa, L.; Piva, M.; Basso, C.R.; Zaninelli, T.H.; Machado, C.C.A.; de Andrade, F.G.; Miqueloto, C.A.; Sant Ana, D.M.G.; Aktar, R.; et al. Perineuronal net in the extrinsic innervation of the distal colon of mice and its remodeling in ulcerative colitis. J. Neurochem. 2024, 168, 1937–1955. [Google Scholar] [CrossRef]
- Zhao, M.; Lei, Y.; Wang, M.; Chen, Y.; Hou, S.; Dai, X.; Gao, D.; Liu, Y.; Mazet, B.; Sha, L. Carbon monoxide produced by HO-1 upregulation is the main factor behind the abnormal motility seen in experimental ulcerative colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2025, 328, G311–G322. [Google Scholar] [CrossRef]
- Crispino, P.; Habib, F.I.; Badiali, D.; Pica, R.; Iacopini, F.; Bella, A.; Cassieri, C.; Anzini, F.; Paoluzi, P. Colorectal motor and sensitivity features in patients affected by ulcerative proctitis with constipation: A radiological and manometric controlled study. Inflamm. Bowel Dis. 2006, 12, 712–718. [Google Scholar] [CrossRef]
- da Silva Watanabe, P.; Cavichioli, A.M.; D’Arc de Lima Mendes, J.; Aktar, R.; Peiris, M.; Blackshaw, L.A.; de Almeida Araújo, E.J. Colonic motility adjustments in acute and chronic DSS-induced colitis. Life Sci. 2023, 321, 121642. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.N.; Bazzocchi, G.; Chan, S.; Akashi, K.; Villanueva-Meyer, J.; Yanni, G.; Mena, I.; Snape, W.J., Jr. Colonic motility and transit in health and ulcerative colitis. Gastroenterology 1991, 101, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Antonelli, E.; Villanacci, V.; Baldoni, M.; Dore, M.P. Colonic motility in ulcerative colitis. United Eur. Gastroenterol. J. 2014, 2, 457–462. [Google Scholar] [CrossRef]
- Snape, W.J., Jr.; Matarazzo, S.A.; Cohen, S. Abnormal gastrocolonic response in patients with ulcerative colitis. Gut 1980, 21, 392–396. [Google Scholar] [CrossRef][Green Version]
- Coulie, B.; Camilleri, M.; Bharucha, A.E.; Sandborn, W.J.; Burton, D. Colonic motility in chronic ulcerative proctosigmoiditis and the effects of nicotine on colonic motility in patients and healthy subjects. Aliment. Pharmacol. Ther. 2001, 15, 653–663. [Google Scholar] [CrossRef]
- Rao, S.S.; Read, N.W. Gastrointestinal motility in patients with ulcerative colitis. Scand. J. Gastroenterol. 1990, 25 (Suppl. S172), 22–28. [Google Scholar] [CrossRef]
- Haase, A.M.; Gregersen, T.; Christensen, L.A.; Agnholt, J.; Dahlerup, J.F.; Schlageter, V.; Krogh, K. Regional gastrointestinal transit times in severe ulcerative colitis. Neurogastroenterol. Motil. 2016, 28, 217–224. [Google Scholar] [CrossRef]
- Nullens, S.; Nelsen, T.; Camilleri, M.; Burton, D.; Eckert, D.; Iturrino, J.; Vazquez-Roque, M.; Zinsmeister, A.R. Regional colon transit in patients with dyssynergic defaecation or slow transit in patients with constipation. Gut 2012, 61, 1132–1139. [Google Scholar] [CrossRef]
- Cowan, G.O.; Das, K.M.; Eastwood, M.A. Further studies of sulphasalazine metabolism in the treatment of ulcerative colitis. Br. Med. J. 1977, 2, 1057–1059. [Google Scholar] [CrossRef] [PubMed]
- Hebden, J.M.; Blackshaw, P.E.; Perkins, A.C.; Wilson, C.G.; Spiller, R.C. Limited exposure of the healthy distal colon to orally-dosed formulation is further exaggerated in active left-sided ulcerative colitis. Aliment. Pharmacol. Ther. 2000, 14, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.J.; Chase, J.W.; Stillman, B.C.; Salzberg, M.; Thompson, A.J.V.; Basnayake, C.; Wilson-O’Brien, A.; Kamm, M.A. Pelvic floor behavioral treatment for fecal incontinence and constipation in quiescent inflammatory bowel disease. Scand. J. Gastroenterol. 2022, 57, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Sukoff Rizzo, S.J.; Finkel, T.; Greenspan, S.L.; Resnick, N.M.; Brach, J.S. Speaking the same language: Team science approaches in aging research for integrating basic and translational science with clinical practice. Innov. Aging 2023, 7, igad035. [Google Scholar] [CrossRef]
- Drossman, D.A.; Chang, L.; Deutsch, J.K.; Ford, A.C.; Halpert, A.; Kroenke, K.; Nurko, S.; Ruddy, J.; Snyder, J.; Sperber, A. A review of the evidence and recommendations on communication skills and the patient-provider relationship: A Rome Foundation Working Team Report. Gastroenterology 2021, 161, 1670–1688.e7. [Google Scholar] [CrossRef]
- Keller, J.; Bassotti, G.; Clarke, J.; Dinning, P.; Fox, M.; Grover, M.; Hellström, P.M.; Ke, M.; Layer, P.; Malagelada, C.; et al. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.H. Enhancing scintigraphy for evaluation of gastric, small bowel, and colonic motility. Gastroenterol. Clin. N. Am. 2020, 49, 499–517. [Google Scholar] [CrossRef]
- Alshammari, M.T.; Alyami, A.S.; Wilkinson-Smith, V.; Spiller, R.C.; Gowland, P.; Marciani, L.; Moran, G.W.; Hoad, C.L. MRI tagging of colonic chyme mixing in healthy subjects: Inter-observer variability and reliability of the measurement with time. Neurogastroenterol. Motil. 2023, 35, e14610. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, D.; Mark, E.B.; Liao, D.; Okdahl, T.; Nauser, S.; Daugberg, L.H.; Brock, C.; Brock, B.; Knop, F.K.; Krogh, K.; et al. MRI-based quantification of pan-alimentary function and motility in subjects with diabetes and gastrointestinal symptoms. J. Clin. Med. 2023, 12, 5968. [Google Scholar] [CrossRef] [PubMed]
- Gunn, D.; Murthy, R.; Major, G.; Wilkinson-Smith, V.; Hoad, C.; Marciani, L.; Remes-Troche, J.; Gill, S.; Rossi, M.; Harris, H.; et al. Contrasting effects of viscous and particulate fibers on colonic fermentation in vitro and in vivo, and their impact on intestinal water studied by MRI in a randomized trial. Am. J. Clin. Nutr. 2020, 112, 595–602. [Google Scholar] [CrossRef]
- Sharif, H.; Devadason, D.; Abrehart, N.; Stevenson, R.; Marciani, L. Imaging measurement of whole gut transit time in paediatric and adult functional gastrointestinal disorders: A systematic review and narrative synthesis. Diagnostics 2019, 9, 221. [Google Scholar] [CrossRef]
- O’Farrell, C.; Hoad, C.L.; Stamatopoulos, K.; Marciani, L.; Sulaiman, S.; Simmons, M.J.H.; Batchelor, H.K. Luminal fluid motion inside an in vitro dissolution model of the human ascending colon assessed using magnetic resonance imaging. Pharmaceutics 2021, 13, 1545. [Google Scholar] [CrossRef]
- Langhorst, J.; Schöls, M.; Cinar, Z.; Eilert, R.; Kofink, K.; Paul, A.; Zempel, C.; Elsenbruch, S.; Lauche, R.; Ahmed, M.; et al. Comprehensive lifestyle-modification in patients with ulcerative colitis-a randomized controlled trial. J. Clin. Med. 2020, 9, 3087. [Google Scholar] [CrossRef]
- Varni, J.W.; Shulman, R.J.; Self, M.M.; Saeed, S.A.; Patel, A.S.; Nurko, S.; Neigut, D.A.; Saps, M.; Franciosi, J.P.; Denham, J.M.; et al. Gastrointestinal symptoms predictors of health-related quality of life in patients with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2016, 63, e186–e192. [Google Scholar] [CrossRef]
- Hyams, J.; Davis, P.; Lerer, T.; Colletti, R.B.; Bousvaros, A.; Leichtner, A.; Benkov, K.; Justinich, C.; Markowitz, J. Clinical outcome of ulcerative proctitis in children. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 149–152. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassotti, G.; Bologna, S.; Antonelli, E. Constipation in Ulcerative Colitis: An Underestimated Problem. J. Clin. Med. 2025, 14, 5428. https://doi.org/10.3390/jcm14155428
Bassotti G, Bologna S, Antonelli E. Constipation in Ulcerative Colitis: An Underestimated Problem. Journal of Clinical Medicine. 2025; 14(15):5428. https://doi.org/10.3390/jcm14155428
Chicago/Turabian StyleBassotti, Gabrio, Sara Bologna, and Elisabetta Antonelli. 2025. "Constipation in Ulcerative Colitis: An Underestimated Problem" Journal of Clinical Medicine 14, no. 15: 5428. https://doi.org/10.3390/jcm14155428
APA StyleBassotti, G., Bologna, S., & Antonelli, E. (2025). Constipation in Ulcerative Colitis: An Underestimated Problem. Journal of Clinical Medicine, 14(15), 5428. https://doi.org/10.3390/jcm14155428




