Effect of Repetitive Peripheral Magnetic Stimulation in Patients with Neck Myofascial Pain: A Randomized Sham-Controlled Crossover Trial
Abstract
1. Introduction
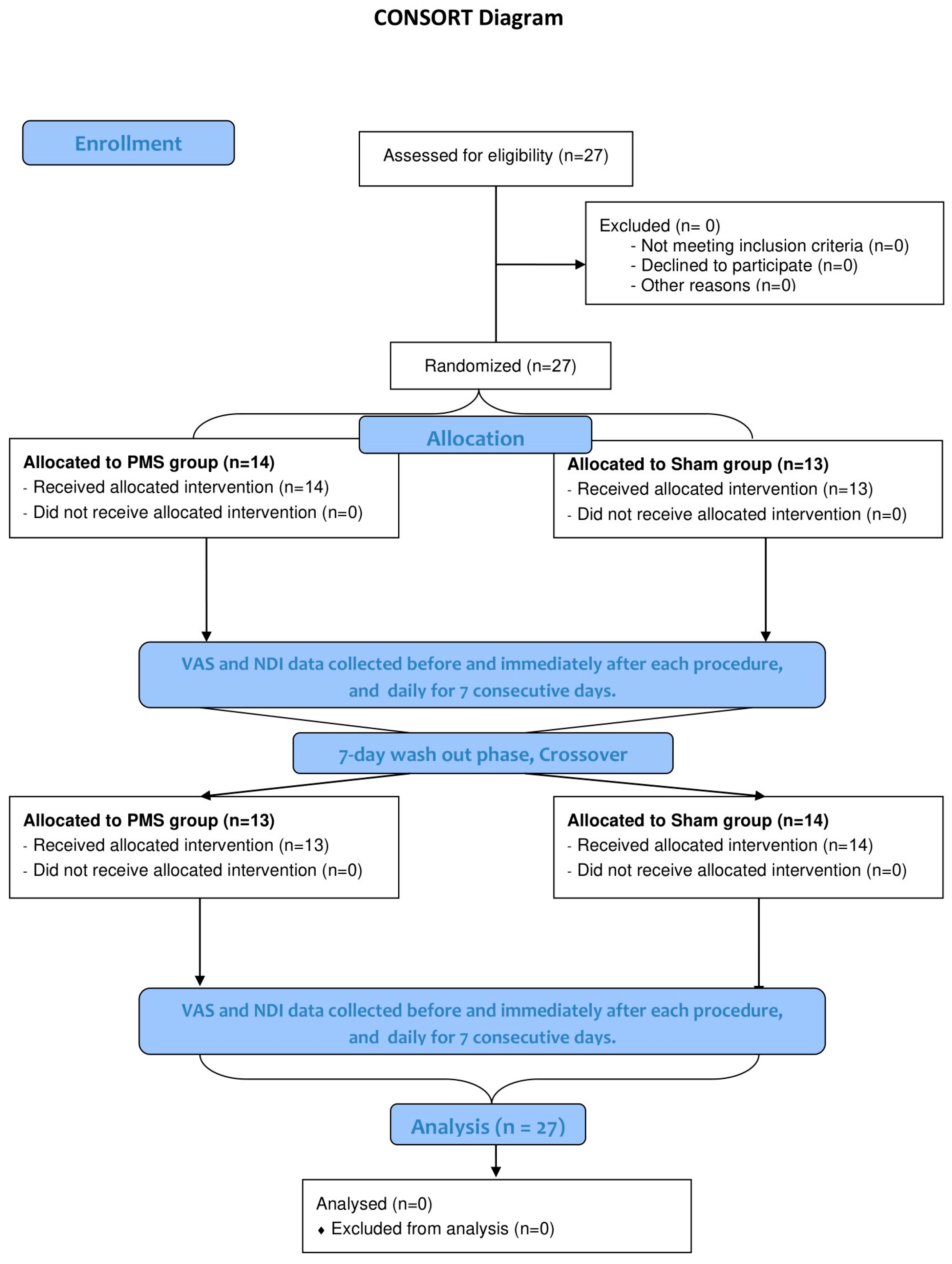
2. Materials and Methods
2.1. Procedures
- Scan phase: 5 Hz continuous stimulation for 3 min (total of 900 pulses).
- Therapeutic phase: 10 Hz stimulation with a train duration of 4 s and an intertrain interval of 1 s (total of 2400 pulses) over 5 min. The intensity was adjusted to produce visible muscle contractions while ensuring patient comfort.
- Cool down phase: 5 Hz continuous stimulation for 2 min (total of 600 pulses).
2.2. Outcome Measurement
2.3. Sample Size Calculation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, A.M.; Cross, M.; Elliott, J.M.; Culbreth, G.T.; Haile, L.M.; Steinmetz, J.D.; Hagins, H.; Kopec, J.A.; Brooks, P.M.; Woolf, A.D.; et al. Global, regional, and national burden of neck pain, 1990-2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2024, 6, e142–e155. [Google Scholar] [CrossRef]
- Ezzati, K.; Ravarian, B.; Saberi, A.; Salari, A.; Reihanian, Z.; Khakpour, M.; Chabok, S.Y. Prevalence of Cervical Myofascial Pain Syndrome and its Correlation with the Severity of Pain and Disability in Patients with Chronic Non-specific Neck Pain. Arch. Bone Jt. Surg. 2021, 9, 230–234. [Google Scholar]
- Cerezo-Téllez, E.; Torres-Lacomba, M.; Mayoral-Del Moral, O.; Sánchez-Sánchez, B.; Dommerholt, J.; Gutiérrez-Ortega, C. Prevalence of Myofascial Pain Syndrome in Chronic Non-Specific Neck Pain: A Population-Based Cross-Sectional Descriptive Study. Pain. Med. 2016, 17, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Steen, J.P.; Jaiswal, K.S.; Kumbhare, D. Myofascial Pain Syndrome: An Update on Clinical Characteristics, Etiopathogenesis, Diagnosis, and Treatment. Muscle Nerve 2025, 71, 889–910. [Google Scholar] [CrossRef] [PubMed]
- Annemarie, G.; Ivan, U.; An, D.; Diep, N.; Matthew, B.; Cyrus, Y.; Laxmaiah, M.; Kaye, R.J.; Kaye, A.D.; Mancuso, K.F.; et al. A Comprehensive Review of the Treatment and Management of Myofascial Pain Syndrome. Curr. Pain. Headache Rep. 2020, 24, 43. [Google Scholar]
- Anwar, N.; Wei, X.; Jie, Y.; Hongbo, Z.; Jin, H.; Zhu, Z. Current advances in the treatment of myofascial pain syndrome with trigger point injections: A review. Medicine 2024, 103, e39885. [Google Scholar] [CrossRef]
- Criscuolo, C.M. Interventional approaches to the management of myofascial pain syndrome. Curr. Pain. Headache Rep. 2001, 5, 407–411. [Google Scholar] [CrossRef]
- Lim, Y.H.; Song, J.M.; Choi, E.H.; Lee, J.W. Effects of Repetitive Peripheral Magnetic Stimulation on Patients With Acute Low Back Pain: A Pilot Study. Ann. Rehabil. Med. 2018, 42, 229–238. [Google Scholar] [CrossRef]
- Khedr, E.M.; Ahmed, M.A.; Alkady, E.A.; Mostafa, M.G.; Said, H.G. Therapeutic effects of peripheral magnetic stimulation on traumatic brachial plexopathy: Clinical and neurophysiological study. Neurophysiol. Clin. 2012, 42, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Smania, N.; Corato, E.; Fiaschi, A.; Pietropoli, P.; Aglioti, S.M.; Tinazzi, M. Repetitive magnetic stimulation: A novel therapeutic approach for myofascial pain syndrome. J. Neurol. 2005, 252, 307–314. [Google Scholar] [CrossRef]
- Smania, N.; Corato, E.; Fiaschi, A.; Pietropoli, P.; Aglioti, S.M.; Tinazzi, M. Therapeutic effects of peripheral repetitive magnetic stimulation on myofascial pain syndrome. Clin. Neurophysiol. 2003, 114, 350–358. [Google Scholar] [CrossRef]
- Kanjanapanang, N.; Chang, K.V. Peripheral Magnetic Stimulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Diao, Y.; Pan, J.; Xie, Y.; Liao, M.; Wu, D.; Liu, H.; Liao, L. Effect of Repetitive Peripheral Magnetic Stimulation on Patients With Low Back Pain: A Meta-analysis of Randomized Controlled Trials. Arch. Phys. Med. Rehabil. 2023, 104, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Renner, T.; Sollmann, N.; Heinen, F.; Albers, L.; Trepte-Freisleder, F.; Klose, B.; König, H.; Krieg, S.M.; Bonfert, M.V.; Landgraf, M.N. Alleviation of migraine symptoms by application of repetitive peripheral magnetic stimulation to myofascial trigger points of neck and shoulder muscles—A randomized trial. Sci. Rep. 2020, 10, 5954. [Google Scholar] [CrossRef]
- Banyat, N. Effectiveness of Peripheral Magnetic Stimulation and Dry Needling in Patients with Myofascial Pain Syndrome of Neck. J. Health Sci. 2023, 32 (Suppl. S2), S310–S319. [Google Scholar]
- Pujol, J.; Pascual-Leone, A.; Dolz, C.; Delgado, E.; Dolz, J.L.; Aldomà, J. The effect of repetitive magnetic stimulation on localized musculoskeletal pain. NeuroReport 1998, 9, 1745–1748. [Google Scholar] [CrossRef]
- Travell, J.G.; Simons, D.G. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual; Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Haefeli, M.; Elfering, A. Pain assessment. Eur Spine J. 2006, 15 (Suppl. S1), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Luksanapruksa, P.; Wathana-apisit, T.; Wanasinthop, S.; Sanpakit, S.; Chavasiri, C. Reliability and validity study of a Thai version of the Neck Disability Index in patients with neck pain. J. Med. Assoc. Thail. 2012, 95, 681–688. [Google Scholar]
- Siyasinghe, N.; Sooriyarachchi, M. Guidelines for calculating sample size in 2x2 crossover trials: A simulation study. J. Nat. Sci. Found. Sri Lanka 2011, 39, 77–89. [Google Scholar] [CrossRef]
- Bahreini, M.; Safaie, A.; Mirfazaelian, H.; Jalili, M. How much change in pain score does really matter to patients? Am. J. Emerg. Med. 2020, 38, 1641–1646. [Google Scholar] [CrossRef]
- Jiravichitchai, T.; Khamket, C.; Ruthiraphong, P. Short-term efficacy of repetitive peripheral magnetic stimulation for chronic low back pain: A double-blinded randomized control trial. J. Med. Assoc. Thail. 2024, 107, 894–901. [Google Scholar]
- Keesukphan, A.; Phakdepiboon, T.; Iamchaimongkol, A. Short-Term Efficacy of Peripheral Magnetic Stimulation in Reducing Pain in Knee Osteoarthritis: A Randomized Controlled Trial. ASEAN J. Rehabil. Med. 2023, 33, 57. [Google Scholar]
- Beaulieu, L.D.; Schneider, C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: A literature review on parameters of application and afferents recruitment. Neurophysiol. Clin. 2015, 45, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Young, I.A.; Dunning, J.; Butts, R.; Mourad, F.; Cleland, J.A. Reliability, construct validity, and responsiveness of the neck disability index and numeric pain rating scale in patients with mechanical neck pain without upper extremity symptoms. Physiother. Theory Pract. 2019, 35, 1328–1335. [Google Scholar] [CrossRef] [PubMed]

| Variables | N (%) |
|---|---|
| Male/Female | 10 (37.0)/17 (63.0) |
| Age (years), mean (SD) | 43.8 (9.1) |
| Marital status | |
| Married | 16 (59.3) |
| Single | 5 (18.5) |
| Divorce/Widow/Separated | 6 (32.2) |
| Educational level | |
| Lower or equal to secondary school | 18 (66.7) |
| Higher than secondary school | 9 (33.3) |
| Body mass index (kg/m2), mean (SD) | 24.5 (2.6) |
| Smoking | 3 (11.1) |
| Alcoholic drinking | 8 (29.6) |
| Underlying disease | |
| Hypertension | 2 (7.4) |
| Diabetes Mellitus | 1 (3.7) |
| Dyslipidemia | 1 (3.7) |
| Duration of neck pain (months), median (p25, p75) | 12 (4, 12) |
| Baseline VAS, mean (SD) | 61.8 (10.5) |
| Baseline NDI, mean (SD) | 26.0 (6.3) |
| Pain medication | 15 (55.6) |
| NSAIDs | 6 (22.2) |
| Muscle relaxant | 4 (14.8) |
| Acetaminophen | 10 (37.0) |
| Concomitant treatment | |
| Physical therapy | 4 (14.8) |
| Massage | 7 (25.9) |
| Variables | Sham Group Mean (95% CI) | PMS Group Mean (95% CI) | Adjusted Differences (95% CI) * | p-Value * |
|---|---|---|---|---|
| VAS | ||||
| Pretreatment | 58.3 (54.6–61.9) | 61.9 (57.7–66.2) | ||
| Day 1 | 55.3 (51.5–59.0) | 47.5 (40.9–54.1) | −11.5 (−16.7 to −6.3) | <0.001 |
| Day 2 | 48.8 (44.4–53.1) | 37.1 (29.9–44.3) | −15.4 (−20.7 to −10.1) | <0.001 |
| Day 3 | 50.7 (46.7–54.6) | 32.1 (25.8–38.4) | −22.2 (−27.7 to −16.8) | <0.001 |
| Day 4 | 54.9 (51.4–58.4) | 34.5 (28.7–40.4) | −24.1 (−29.7 to −18.4) | <0.001 |
| Day 5 | 56.0 (52.1–59.9) | 40.3 (34.5–46.0) | −19.4 (−25.3 to −13.6) | <0.001 |
| Day 6 | 56.6 (52.2–60.7) | 44.0 (38.5–49.4) | −16.3 (−22.5 to −10.1) | <0.001 |
| Day 7 | 57.0 (53.0–60.9) | 51.6 (46.6–56.6) | −9.1 (−15.5 to −2.6) | <0.001 |
| NDI | ||||
| Pretreatment | 25.3 (23.0–27.8) | 26.9 (24.3–29.5) | ||
| Day 1 | 25.4 (23.0–27.8) | 26.3 (23.7–28.9) | −0.6 (−2.6 to 1.3) | 0.53 |
| Day 2 | 22.2 (20.1–24.4) | 17.7 (15.2–20.2) | −6.1 (−8.1 to −4.1) | <0.001 |
| Day 3 | 22.5 (20.4–24.5) | 16.5 (13.9–19.1) | −7.6 (−9.6 to −5.5) | <0.001 |
| Day 4 | 23.3 (21.1–25.6) | 16.4 (13.7–19.1) | −8.5 (−10.6 to −6.4) | <0.001 |
| Day 5 | 24.3 (21.8–26.8) | 19.1 (15.6–22.5) | −6.8 (−9.0 to −4.6) | <0.001 |
| Day 6 | 24.7 (22.3–27.2) | 21.2 (17.9–24.5) | −5.1 (−7.3 to −2.8) | <0.001 |
| Day 7 | 24.9 (22.5–27.4) | 24.5 (21.8–27.3) | −2.0 (−4.3 to 0.4) | 0.11 |
| Diclofenac used (Tabs) | ||||
| Day 1 | 0.00 (−0.03–0.04) | −0.0 (−0.17–0.01) | −0.01 (−0.05 to 0.03) | 0.70 |
| Day 2 | 0.04 (−0.03–0.12) | 0.07 (−0.07–0.21) | 0.02 (−0.13 to 0.18) | 0.76 |
| Day 3 | 0.00 (−0.03–0.04) | 0.03 (−0.04–0.10) | 0.03 (−0.05 to 0.10) | 0.48 |
| Day 4 | 0.20 (−0.11–0.50) | 0.07 (−0.08–0.21) | −0.13 (−0.48 to 0.22) | 0.46 |
| Day 5 | 0.24 (−0.09–0.56) | 0.07 (−0.03–0.16) | −0.17 (−0.52 to 0.18) | 0.34 |
| Day 6 | 0.08 (−0.03–0.19) | 0.21 (−0.07–0.49) | 0.12 (−0.17 to 0.42) | 0.39 |
| Day 7 | 0.20 (−0.02–0.41) | 0.24 (−0.05–0.54) | 0.05 (−0.31 to 0.41) | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahisanun, T.; Saengsuwan, J. Effect of Repetitive Peripheral Magnetic Stimulation in Patients with Neck Myofascial Pain: A Randomized Sham-Controlled Crossover Trial. J. Clin. Med. 2025, 14, 5410. https://doi.org/10.3390/jcm14155410
Mahisanun T, Saengsuwan J. Effect of Repetitive Peripheral Magnetic Stimulation in Patients with Neck Myofascial Pain: A Randomized Sham-Controlled Crossover Trial. Journal of Clinical Medicine. 2025; 14(15):5410. https://doi.org/10.3390/jcm14155410
Chicago/Turabian StyleMahisanun, Thapanun, and Jittima Saengsuwan. 2025. "Effect of Repetitive Peripheral Magnetic Stimulation in Patients with Neck Myofascial Pain: A Randomized Sham-Controlled Crossover Trial" Journal of Clinical Medicine 14, no. 15: 5410. https://doi.org/10.3390/jcm14155410
APA StyleMahisanun, T., & Saengsuwan, J. (2025). Effect of Repetitive Peripheral Magnetic Stimulation in Patients with Neck Myofascial Pain: A Randomized Sham-Controlled Crossover Trial. Journal of Clinical Medicine, 14(15), 5410. https://doi.org/10.3390/jcm14155410





